Abstract
It is important to know whether the human population includes genetically predisposed radiosensitive subsets. In vitro studies have shown that cells from individuals homozygous for ataxia telangiectasia (A-T) are much more radiosensitive than cells from unaffected individuals. Although cells heterozygous for the ATM gene (ATM+/−) may be slightly more radiosensitive in vitro, it remained to be determined whether the greater susceptibility of ATM+/− cells translates into an increased sensitivity for late effects in vivo, though there is a suggestion that radiotherapy patients that are heterozygous for the ATM gene may be more at risk of developing late normal tissue damage. We chose cataractogenesis in the lens as a means to assay for the effects of ATM deficiency in a late-responding tissue. One eye of wild-type, Atm heterozygous and homozygous knockout mice was exposed to 0.5-, 1.0-, 2.0-, or 4.0-Gy x rays. The animals were followed weekly for cataract development by conventional slit-lamp biomicroscopy. Cataract development in the animals of all three groups was strongly dependent on dose. The lenses of homozygous mice were the first to opacify at any given dose. Most important in the present context is that cataracts appeared earlier in the heterozygous versus wild-type animals. The data suggest that ATM heterozygotes in the human population may also be radiosensitive. This may influence the choice of individuals destined to be exposed to higher than normal doses of radiation, such as astronauts, and may also suggest that radiotherapy patients who are ATM heterozygotes could be predisposed to increased late normal tissue damage.
Current radiation safety guidelines are predicated on the assumption that the human population is homogeneous as far as radiosensitivity is concerned. There is, however, some evidence to the contrary. For example, a few percent of cancer patients receiving radiation therapy show unexpected normal tissue damage (1), and there is the small unexplained group of survivors of the A-bomb who developed breast cancer early in life (2). It has long been suspected that there might be a genetic component to this radiosensitivity.
A well known but relatively rare autosomal recessive disorder, ataxia telangiectasia (A-T), has been shown to be associated with increased radiation sensitivity when mutations in both alleles of the ATM (A-T mutated) gene are present. In addition to elevated radiation sensitivity, individuals homozygous for ATM express varied neuropathies, immunological anomalies, and cancer predisposition (3). More common are individuals with mutations in only one allele (ATM+/−). These individuals compose 1–3% of the human population (4). Recent studies have shown that, although phenotypically indistinguishable from the rest of the population, individuals heterozygous for ATM gene may have increased risk of developing cancer (5, 6). This being the case, one must consider that the same population may also exhibit greater sensitivity to radiation exposure. This predilection may not only express as an elevated risk to radiogenic cancers but also in exacerbations of normal tissue responses to radiation; indeed, there are preliminary reports suggesting that radiotherapy patients who are heterozygous for the ATM gene may be at greater risk of developing late normal tissue damage (1, 7). Being able to identify radiosensitive persons would be of tremendous medico-social value. That knowledge would avoid exposure of sensitive individuals to unusually high radiation doses.
Cataractogenesis was chosen as a model to test for radiation sensitivity in Atm gene deficient vs. wild-type mice. The radiation cataract model has enjoyed a long history as a means to determine radiation damage in a late responding tissue. Its amenability to noninvasive assessment over extended periods and the predictability of the pathology underscore its utility as a means to monitor radiation exposure. The fact that the pathology itself is one that is of great concern to those responsible for formulating risk guidelines adds to its appeal for radiation studies.
Materials and Methods
Descendents of breeding Atm knockouts kindly provided by Philip Leder (Harvard University, Cambridge, MA) were used throughout the study. The genetic background of the mice is a cross of the 129SvEv and Black Swiss strains. The Atm gene was disrupted by inserting a neo cassette within an exon at a site corresponding to nucleotide number 5460 of the human ATM. There was no presence of full-length or truncated protein in the Atm knockout mice (8). The Atm-deficient mice display many characteristics associated with A-T, such as retarded growth, defective lymphocytic differentiation, neurological dysfunction, and hypersensitivity to ionizing radiation. Most Atm−/− mice develop thymic lymphomas and rarely live beyond 5 months. Also, pup yield for homozygotes appear non-Mendelian, ostensibly because of reduced embryo survivability. Heterozygotes, on the other hand, appear healthy. It is on the heterozygotes that we focused our attention because 1–3% of the human population are ATM heterozygotes and are clinically indistinguishable from the general population.
Before irradiation at 28 days (±0.5 day) postpartum the mice were genotyped as described elsewhere (8). Each treatment group contained approximately equal numbers of males and females. Animals were anesthetized, and one eye of each animal was irradiated while the other was shielded. Shielding was accomplished by using a collimator on the x-ray machine, as well as a contoured 5-mm-thick lead covering for the contralateral eye. Measurements indicate that the shielded eye receives less than 2% of the dose delivered to the unshielded eye. The shielded eye served as an intra-animal control. In addition, mice with both eyes left unirradiated but otherwise treated in the same manner as those exposed served as inter-animal and group controls. A subsequent analysis showed no difference in cataract formation between the intra-animal and inter-animal controls. The treated eyes were exposed to 250 kilovolt peak X-rays at a dose-rate of 0.5 Gy per min. Each mouse was examined weekly by two independent experienced observers, using conventional slit-lamp biomicroscopy as described below until the mouse died or developed a 3+ cataract stage. The animals were coded so that the radiation dose and genotype were unknown to the observers. The outcomes were compared with wild-type mice of the same strain and treated similarly.
Cataract Analyses.
Cataract assessment used a frequently used modification to the well-defined and widely used Merriam/Focht radiation cataract scoring method (9). The technique involved using a conventional slit-lamp biomicroscope to follow changes in lens transparency. The earliest changes consist of vacuoles or diffuse opacities around the suture in the central posterior subcapsular region; these are gauged as 1+. If fewer than four vacuoles are noted, a 0.5+ cataract is scored. Continued cataract development leads to progression of the posterior changes and the involvement of the anterior subepithelial region, the 2+ stage. Fewer than four vacuoles observed anteriorly are recorded as a 1.5+ cataract. A 3+ stage is scored when the anterior opacities progress and the density of the cataract posteriorly prevents the slit beam from passing into the vitreous. If however, the entire cortex is involved, but the posterior capsule can still be discerned, a 2.5+ cataract is noted. A 4+ cataract stage is one with complete anterior opacification preventing visualization of the remainder of the lens. If the opacity has not become severe enough to prevent passage of the slit beam into the posterior region, but detailed visualization is impossible, a 3.5+ stage is scored. The pluses after the score indicate the reality that a particular score at some given examination time reflects a cataract stage that was reached during the interval between the previous and current examinations.
Data Analyses.
It has been customary for those using semiquantitative techniques to simply plot linear averages of cataract stage as a function of time after irradiation. These data, as similar analyses have shown in the past, provide us with general information regarding trends in cataract development. However, this type of analysis is greatly limited by the nonlinearity of the scoring technique. It is obviously misleading to take linear averages of cataract stages when a relationship, linear or otherwise, does not exist between cataract stages and therefore cataract severity. For example, in the scoring system a 2+ cataract is not twice as severe as a 1+ cataract and not half as extensive as a 4+ cataract.
These limitations, further amplified by the subjective nature of the analysis, and of the need to account for competing risks—i.e., variable exclusions of individuals from the analysis (because of occasional corneal injury or death of the animal)—call for a more robust statistical treatment of the data. One method used successfully in our studies (10–13) is the application of the Kaplan–Meier technique (14) to make nonparametric maximum likelihood estimates of grade-specific cataract prevalence as a function of time after exposure.
Our observations are right-censored in the sense that if a cataract appears, we know (within reasonable limits) when it appeared; however, if an animal died from any cause before showing any or complete lens opacification, we do not know what the subsequent history of the lens would have been. Our aim is to estimate the prevalence, P(t), the probability that an animal eye will develop a cataract of a particular stage by a given time, t. For our right-censored data, the nonparametric maximum-likelihood estimate of P(t) is given by the Kaplan–Meier (product limit) estimate (14).
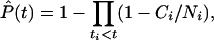 |
1 |
where Ni are the number of eyes at risk just before time ti, and Ci are the number of eyes that develop a given stage of cataract at time ti. Use of Eq. 1 presupposes that cataract development is independent of death. The variances of the nonparametric maximum-likelihood estimates of the prevalences were calculated by using Greenwood's formula (15):
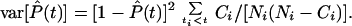 |
2 |
Results
Throughout this study the development of radiation cataracts qualitatively followed the pathogenic sequence characteristic of radiogenic cataracts in all mammalian species, including man. After a latent period dependent on dose, the initial opacities appeared in the posterior subcapsular (posterior superficial cortex) of the lens. At a rate also correlated with dose, the posterior cortex became more involved and anterior cortical changes appeared and progressed. Those animals whose lenses reached a stage wherein the cortex was in an advanced state of opacification (2.5+ to 3.0+) were killed.
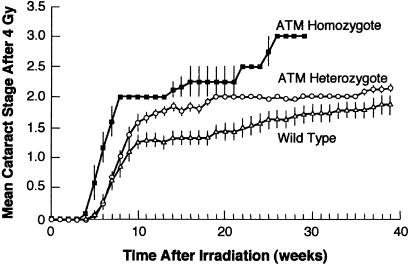
Fig. 1 shows the increase with time of the mean cataract stage after exposure to 4 Gy. Clearly, Atm knockouts (homozygotes) are exquisitely sensitive to radiation, whereas the heterozygotes exhibit a sensitivity intermediate between the homozygotes and their wild-type littermates. Figs. 2–4 show the cataract prevalence, as a function of time for Atm knockouts, heterozygous, and wild-type animals for doses of 0.5, 2, or 4 Gy, respectively.
Figure 1.
Mean cataract stage as a function of time after exposure to a dose of 4 Gy for wild-type mice and for animals homozygous or heterozygous for the ATM gene. The vertical bars represent the standard deviation of the means, where they are larger than the data point.
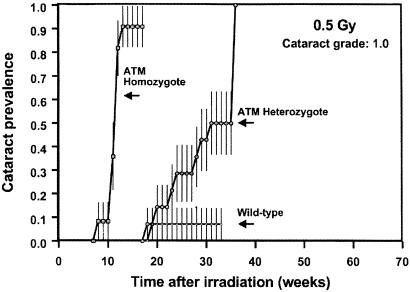
Figure 2.
Cataract prevalence (grade 1) as a function of time after exposure to 0.5 Gy in wild-type mice or in animals homozygous or heterozygous for the ATM gene. Note that at this dose, the lowest used in this study, wild-type animals are essentially unaffected, whereas half of the A-T heterozygotes develop a grade 1.0 cataract. The vertical bars are standard errors, calculated by using Greenwood's formula (15).
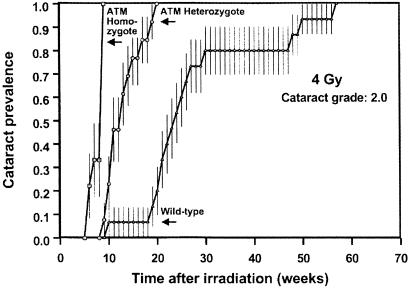
Figure 4.
Prevalence of cataracts of grade 2 (vision impairing) as a function of time after exposure to 4 Gy in wild-type mice and in animals homozygous or heterozygous for the ATM gene. The heterozygous animals develop grade 2 (vision impairing) cataracts about 10 weeks earlier than wild-type animals. The vertical bars are standard errors, calculated by using Greenwood's formula (15).
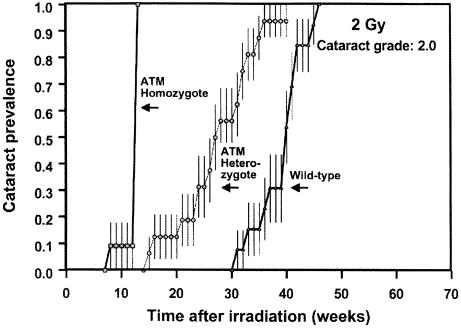
The Atm knockouts all developed cataracts rapidly, but this was no surprise because it is well documented that cells homozygous for the Atm gene are very radiosensitive (3). The results for the Atm heterozygotes are of more interest. The lowest dose (0.5 Gy) produces essentially no opacification in wild-type animals, whereas half of the heterozygotes develop a grade 1.0 cataract. At the higher doses, the principal difference is that potentially vision-impairing cataracts (grade 2) develop 10–15 weeks earlier in Atm heterozygotes than in their wild-type litter-mates. Based on relative life-spans, 10–15 weeks in a mouse corresponds to 8–10 years in a human.
Discussion
The findings clearly show that mice heterozygous for the Atm gene are more sensitive than wild-type animals to the cataractogenic effects of ionizing radiation. Radiation-induced cataracts have been studied in cancer patients (16), patients undergoing computed tomography scans (17), and survivors of the atomic bombings during World War II (ref. 18; for a review see ref. 19). Radiation cataracts result from the accumulation of abnormally differentiated progeny of the germinative cells of the lens epithelium (20, 21). Cataract severity and time of onset are directly related to the number of genomically damaged cells attempting differentiation (fibergenesis) (22, 23). The mechanisms of cataract formation are linked to DNA damage of lens epithelial cells, which can result in defective cell cycle controls and apoptotic pathways (24). ATM is involved in both processes, and as our results indicate, animals deficient in ATM protein are very susceptible to cataract formation. In the case of heterozygous organisms, one of the Atm alleles is normal and codes active ATM protein. Although the background cancer rate in ATM heterozygotes has been extensively studied (25), much less is known about their sensitivity to radiation and radiation-induced cancers. A positive relation was shown in obligate heterozygotes who had undergone medical irradiation (26). In addition, Atm heterozygous prevalence in breast cancer survivors who had received large radiotherapy doses to the breast was examined by three different groups (27–29). No excess of A-T heterozygotes in the breast cancer cases were detected, but a further test for Atm heterozygosity (27) did yield the presence of “functional” A-T heterozygotes in 9% of the breast cancer cases. These contradictory results point to the need for a model system in which extensive analysis can be done. In our study we used a mouse model because it is a quantitative model and results could be obtained in a relatively short term period. As it was noticed in the analysis of Atm heterozygous individuals, the amount of the ATM protein is remarkably reduced in some cases and leads to defective apoptotic control and mitotic spindle checkpoint (30, 31). According to current models, the ATM protein, is a sensor protein that detects DNA double strand breaks and directly regulates multiple cell signaling pathways involved in the response to DNA damage and repair. Primary targets for the ATM protein are the p53, Chk1, and Chk2/hCds1 kinases, cAbl, RPA, RAD 51, and BRCA1 and BRCA2 complex (32, 33). Our results show clearly that haploinsufficiency of the ATM protein is an important factor in the development of radiation induced cataracts in mice.
These data assume additional significance in the light of a recent publication (34) linking an increased risk of cataracts for astronauts with higher lens doses (>8 mSv) of space radiation relative to other astronauts with lower lens doses (<8 mSv). In addition, there was a significant association between cataracts and high inclinations or lunar missions, with 35 of the 39 cases observed after space flight occurring in astronauts participating in these missions. It is speculated that the cause is the much higher flux of heaving ions associated with high inclination and lunar missions, with lower inclination missions having a large fraction of the dose from low linear energy transfer trapped protons. Although the report emphasizes nuclear cataracts, the data on the prevalence of posterior subcapsular and cortical lens opacities provide more highly suggestive evidence to support a radiogenic etiology. As shown in the present ATM study and consistently throughout the history of radiation cataract research, opacification in these areas, although not pathognomonic, are highly and definably characteristic of radiation cataractogenesis. In fact, the Merriam/Focht radiation cataract scoring system is fully defined by opacities appearing in these regions (9). In the case of the astronauts, among the 295 flight personnel examined, 40 of the 48 recorded cataracts involved opacities of the cortical and/or posterior subcapsular variety. Overall, 3 of the 295 astronauts that were followed developed vision-impairing cataracts requiring surgery relatively early in life, even though the accumulated doses were quite low; it is unfortunate that the report does not indicate whether these three cases were of a type consistent with a radiation etiology, but the number possibly suggests a genetically predisposed radiosensitivity in some individuals.
Figure 3.
Prevalence of cataracts of grade 2 (vision impairing) as a function of time after exposure to 2 Gy in wild-type mice and in animals homozygous or heterozygous for the ATM gene. The heterozygous animals develop grade 2 cataracts (vision impairing) about 10 weeks earlier than wild-type animals. The vertical bars are standard errors, calculated by using Greenwood's formula (15).
Acknowledgments
We thank Dr. Philip Leder for providing a breeding pair of Atm knockout mice, Dr. Tej Pandita for much useful advice, Dr. Shang Xu for her ophthalmic input, and Ronald Baker for skilled technical assistance. This research was supported by National Aeronautics and Space Administration Grant NAG 9-1148 and support from Research to Prevent Blindness, Inc.
Abbreviations
- A-T
ataxia telangiectasia
- ATM
A-T mutated
References
- 1.Hall E J, Schiff P B, Hanks G E, Brenner D J, Russo J, Chen J, Sawant S G, Pandita T K. Cancer J. 1998;4:385–389. [PubMed] [Google Scholar]
- 2.Tokunaga M, Lande C E, Tokuoka S, Nishimori I, Soda M, Akiba S. Radiat Res. 1994;138:209–223. [PubMed] [Google Scholar]
- 3.Swift M. Immunodefic Rev. 1990;2:67–81. [PubMed] [Google Scholar]
- 4.Swift M, Morrell D, Massey R B, Chase C L. N Eng J Med. 1991;325:1831–1836. doi: 10.1056/NEJM199112263252602. [DOI] [PubMed] [Google Scholar]
- 5.Bay J O, Uhrhammer N, Pernin D, Presneau N, Tchirkov A, Vuillaume M, Laplace V, Grancho M, Verrelle P, Hall J, Bignon Y J. Hum Mutat. 1999;14:485–492. doi: 10.1002/(SICI)1098-1004(199912)14:6<485::AID-HUMU7>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 6.Broeks A, Urbanus J H, Floore A N, Dahler E C, Klijin J G, Rutgers E J, Devilee P, Russell N S, van Leeuwen F E, van't Veer L J. Am J Hum Genet. 2000;66:494–500. doi: 10.1086/302746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iannuzzi C M, Atenico D P, Green S, Stock R G, Rosenstein B S. Int J Radiation Oncology Biol Phys. 2002;52:606–613. doi: 10.1016/s0360-3016(01)02684-0. [DOI] [PubMed] [Google Scholar]
- 8.Elson A, Wang Y, Daugherty C J, Morton C C, Zhou F, Campos-Torres J, Leder P. Proc Natl Acad Sci USA. 1996;93:13084–13089. doi: 10.1073/pnas.93.23.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merriam G R, Jr, Focht E F. Tr Am Ophthal Soc. 1962;60:35–52. [PMC free article] [PubMed] [Google Scholar]
- 10.Worgul B V, Merriam G R, Jr, Medvedovsky C, Brenner D J. Radiat Res. 1989;118:93–100. [PubMed] [Google Scholar]
- 11.Brenner D J, Medvedovsky C, Huang Y, Merriam G R, Jr, Worgul B V. Radiat Res. 1991;128:73–81. [PubMed] [Google Scholar]
- 12.Brenner D J, Medvedovsky C, Huang Y, Worgul B V. Radiat Res. 1993;133:198–203. [PubMed] [Google Scholar]
- 13.Worgul B V, Brenner D J, Medvedovsky C, Merriam G R, Jr, Huang Y. Invest Ophthal Vis Sci. 1993;34:184–193. [PubMed] [Google Scholar]
- 14.Kaplan E L, Meier P. J Am Stat Assoc. 1957;53:457–481. [Google Scholar]
- 15.Greenwood M. Rep Pub Health Med Sub. 1926;33:1–26. [Google Scholar]
- 16.Gragoudas E S, Egan K M, Walsh S M, Regan S, Munzenrider J E, Tarututa V. Am J Ophthalmol. 1995;119:157–164. doi: 10.1016/s0002-9394(14)73868-1. [DOI] [PubMed] [Google Scholar]
- 17.Klein B E K, Klein I, Linton K L P, Franke T. Am J Public Health. 1993;83:588–590. doi: 10.2105/ajph.83.4.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otake M, Neriishi K, Schull W J. Radiat Res. 1996;146:339–348. [PubMed] [Google Scholar]
- 19.Junk A K, Kundiev Y, Vitte P, Worgul B V. Ocular Radiaton Risk Assessment in Populations Exposed to Environmental Radiation Contamination. Dordrecht, The Netherlands: Kluwer Academic; 1999. [Google Scholar]
- 20.Worgul B V, Rothstein H. Ophthalmic Res. 1975;7:21–32. [Google Scholar]
- 21.Worgul B V, Merriam G R, Jr, Szechter A, Srinivasan B D. Arch Ophthal. 1976;94:996–999. doi: 10.1001/archopht.1976.03910030506013. [DOI] [PubMed] [Google Scholar]
- 22.Worgul B V, Medvedovsky C M, Merriam G R. Lens Eye Tox Res. 1989;6:559–571. [PubMed] [Google Scholar]
- 23.Holsclaw D S, Rothstein H, Medvedovsky C, Worgul B V. Exp Eye Res. 1994;59:291–296. doi: 10.1006/exer.1994.1110. [DOI] [PubMed] [Google Scholar]
- 24.Harocopas G J, Alvares K M, Kolker A E, Beebe D C. Invest Ophthalmol Visual Sci. 1998;39:2696–2706. [PubMed] [Google Scholar]
- 25.Khanna K K. J Natl Cancer Inst. 2000;92:795–802. doi: 10.1093/jnci/92.10.795. [DOI] [PubMed] [Google Scholar]
- 26.Swift M, Reitnauer P J, Morrell D, Chase C L. N Engl J Med. 1987;316:1289–1294. doi: 10.1056/NEJM198705213162101. [DOI] [PubMed] [Google Scholar]
- 27.Broeks A, Russell N S, Floore A N, Urbanus J H, Dahler E C, van't Veer M B, Hagenbeek A, Noordijk E M, Crommelin M A, van Leeuwen F E, van't Veer L J. Int J Radiat Biol. 2000;76:693–698. doi: 10.1080/095530000138367. [DOI] [PubMed] [Google Scholar]
- 28.Nichols K E, Levitz S, Shannon K E, Wahrer D C, Bell D W, Chang G, Hegde S, Neuberg D, Shafman T, Tarbell N J, et al. J Clin Oncol. 1999;17:1259–1266. doi: 10.1200/JCO.1999.17.4.1259. [DOI] [PubMed] [Google Scholar]
- 29.Shafman T D, Levitz S, Nixon A J, Gibans L A, Nichols K E, Bell D W, Ishioka C, Isselbacher K J, Gelman R, Garber J, Harris J R, Haber D A. Genes Chromosomes Cancer. 2000;27:124–129. [PubMed] [Google Scholar]
- 30.Kairouz R, Clarke R A, Marr P J, Watters D, Lavin M F, Kearsley J H, Lee C S. Mol Pathol. 1999;52:252–256. doi: 10.1136/mp.52.5.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shigeta T, Takagi M, Delia D, Chessa L, Iwata S, Kanke Y, Asada M, Eguchi M, Mizutani S. Cancer Res. 1999;59:2602–2607. [PubMed] [Google Scholar]
- 32.Abraham R T. Genes Dev Sep. 2001;15:2177–2196. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- 33.Khanna K K, Lavin M F, Jackson S P, Mulhern T D. Cell Death Differ. 2001;11:1052–1065. doi: 10.1038/sj.cdd.4400874. [DOI] [PubMed] [Google Scholar]
- 34.Cucinotta F A, Manuel F K, Jones J, Iszard G, Murrey J, Djojonegro B, Wear M. Radiat Res. 2001;156:460–466. doi: 10.1667/0033-7587(2001)156[0460:sracia]2.0.co;2. [DOI] [PubMed] [Google Scholar]