Abstract
1. Vibratory stimuli applied to the hand of a monkey evoke phase locked impulse trains in the three classes of low threshold mechanoreceptive afferents which innervate the area. The responses of each class of afferent (slowly adapting (SA), rapidly adapting (RA), and Pacinian (PC) vary in a systematic but complex way across the range of frequencies and intensities to which they are sensitive. The receptors are not accessible for electrophysiological recording. The aim in this study was to infer the mechanisms underlying their responses from detailed examination of the statistical properties of the impulse trains.
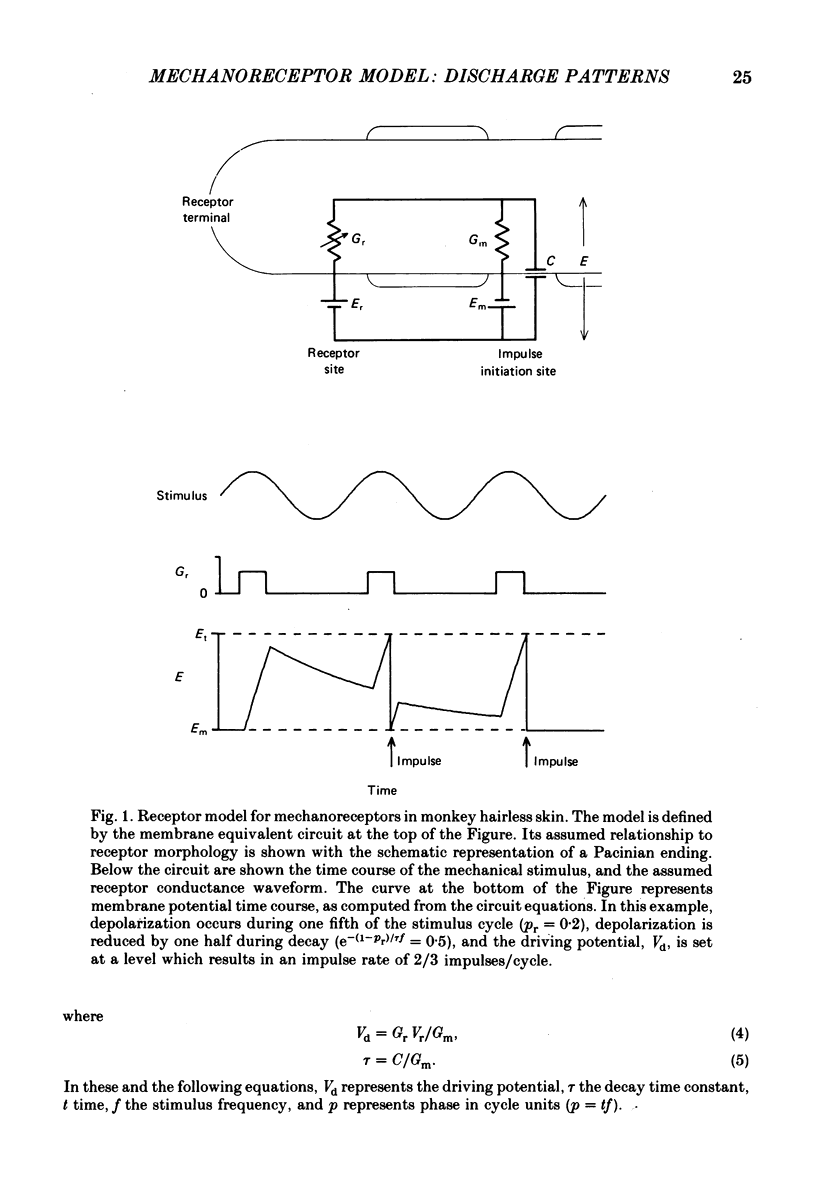
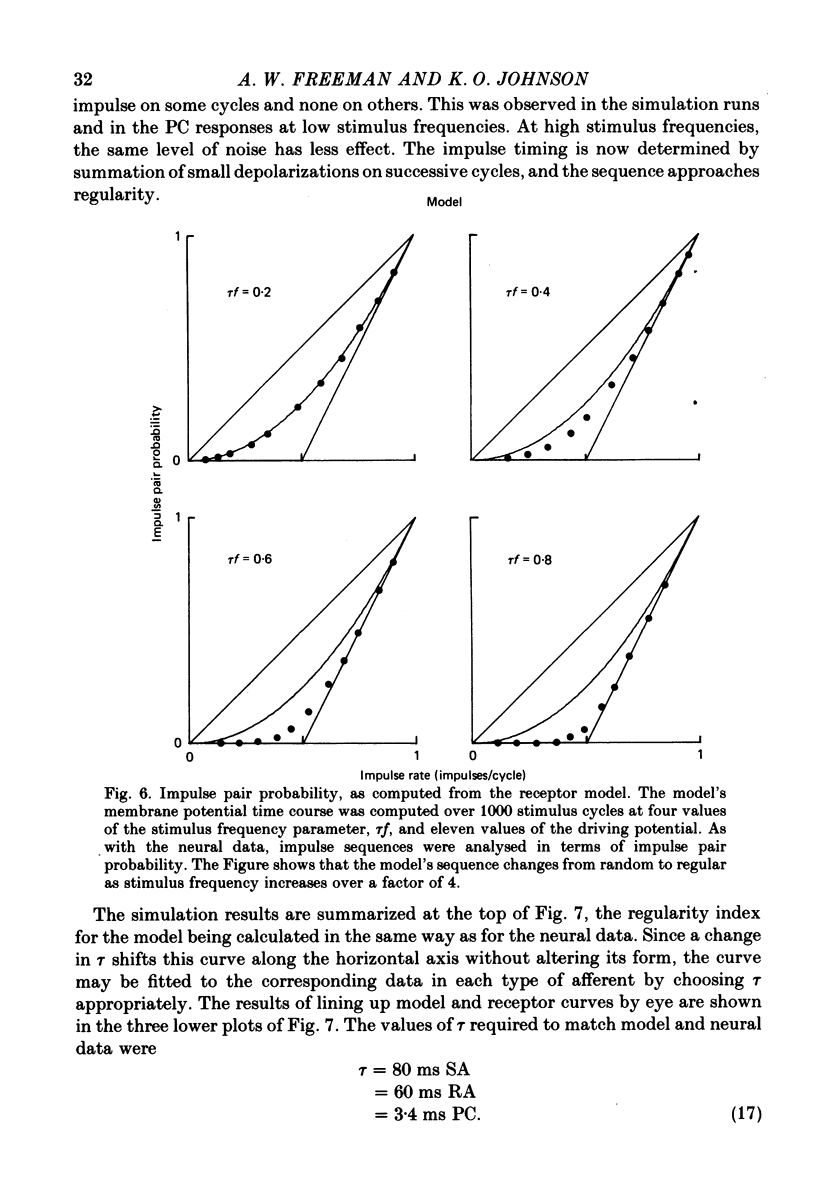
2. A very simple receptor model with four degrees of freedom was chosen as a starting point. The independent variables consisted of the resting membrane time constant, τ, a variable membrane conductance, Gr, the fraction of each sinusoidal stimulus cycle producing depolarization, pr, and the noise level, σ, which was assigned to the impulse threshold. The aim was to use the deviations between observed data and predictions from the basic model to construct a more effective model. In fact, the deviations were minor and were mostly explained by periods of increased excitability in the wake of each action potential. Almost all of the differences between the responses of the three mechanoreceptive classes examined in this paper were accounted for by differences in time constants.
3. The temporal structure of the responses from each mechanoreceptive class was examined at two levels of resolution, a coarse level where the resolution unit was a full cycle, and a fine level where the unit was 0·1 ms.
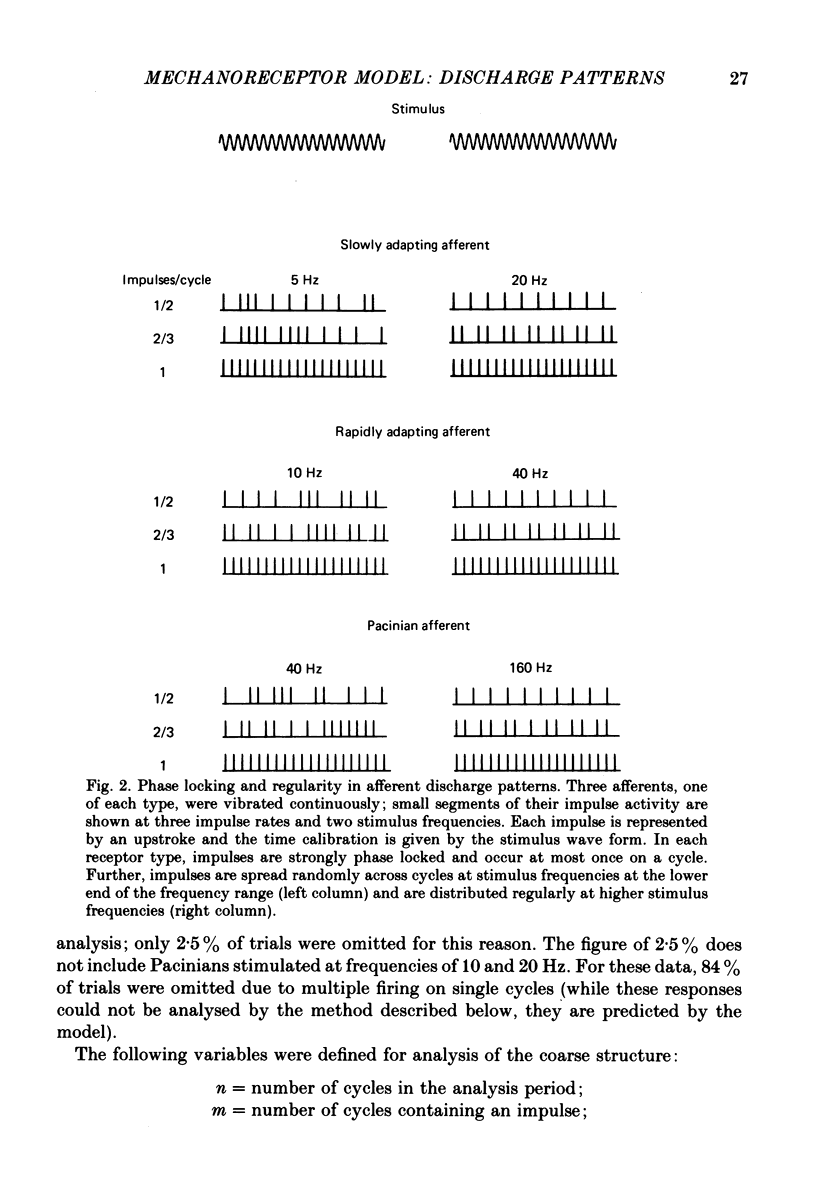
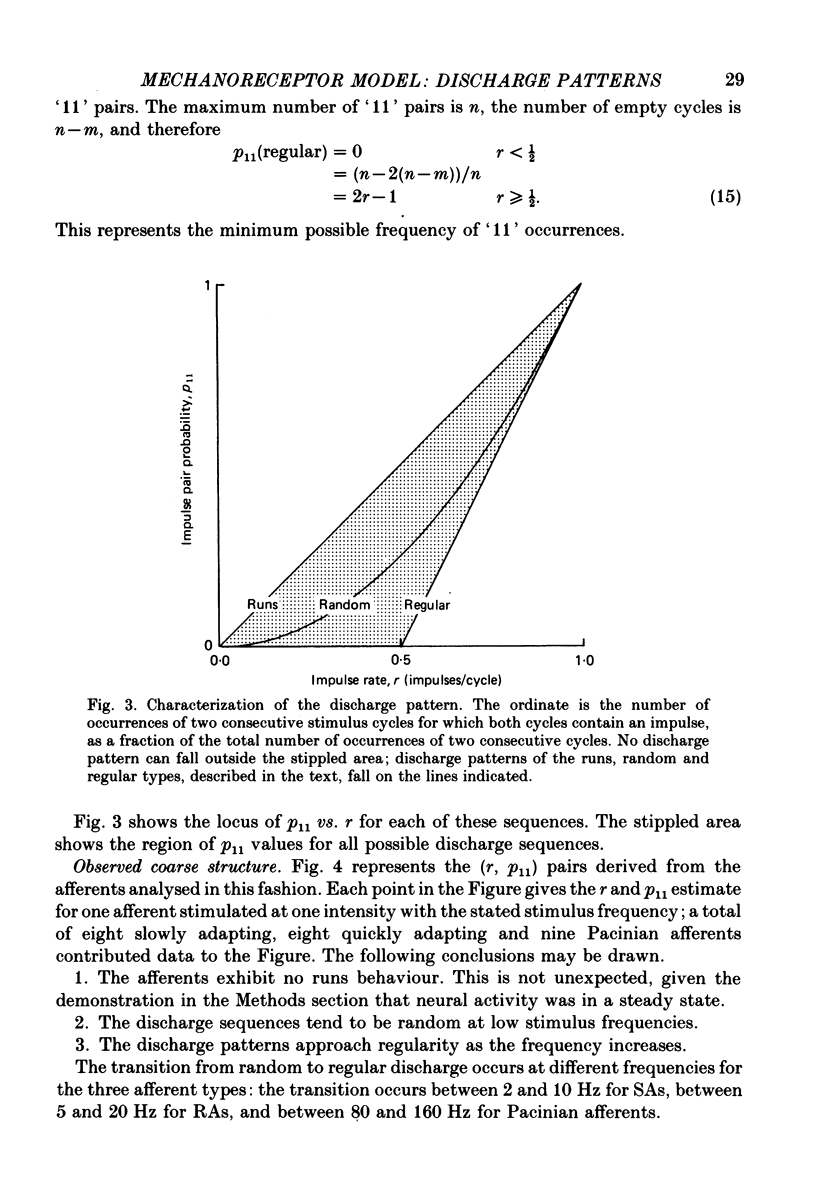
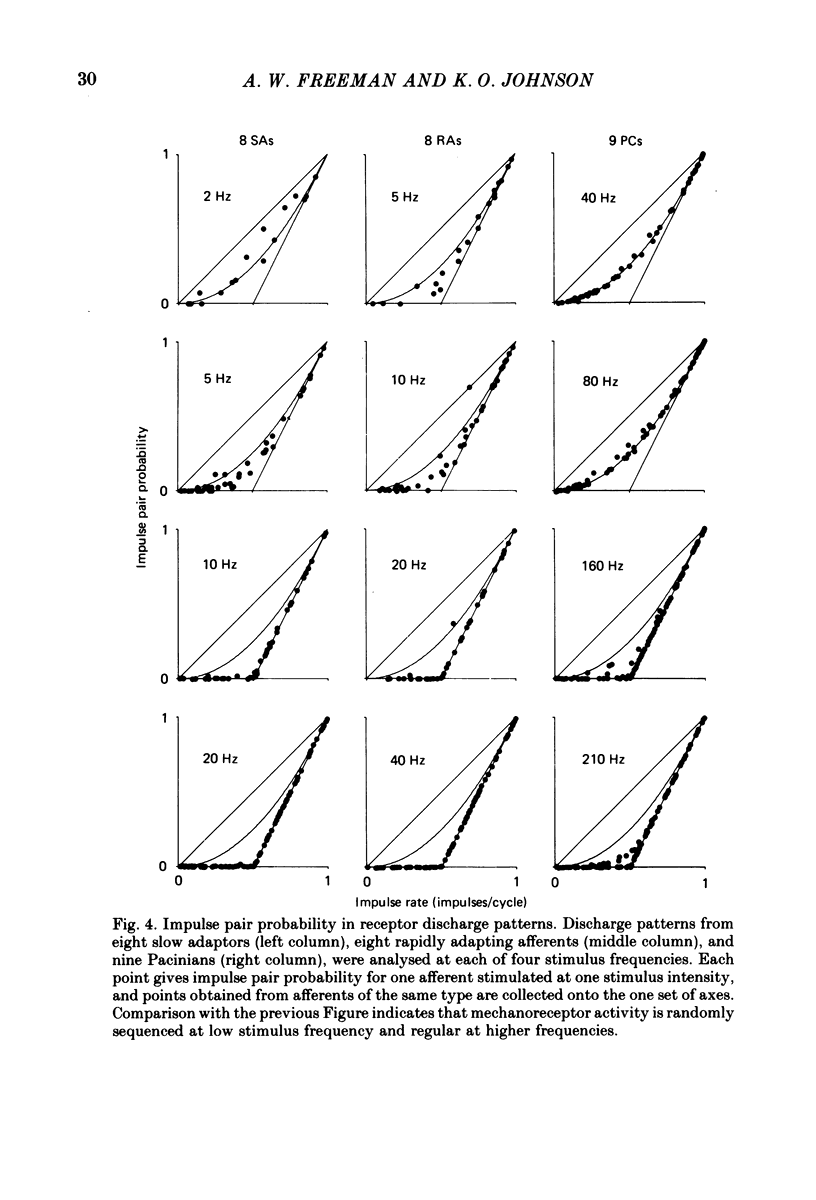
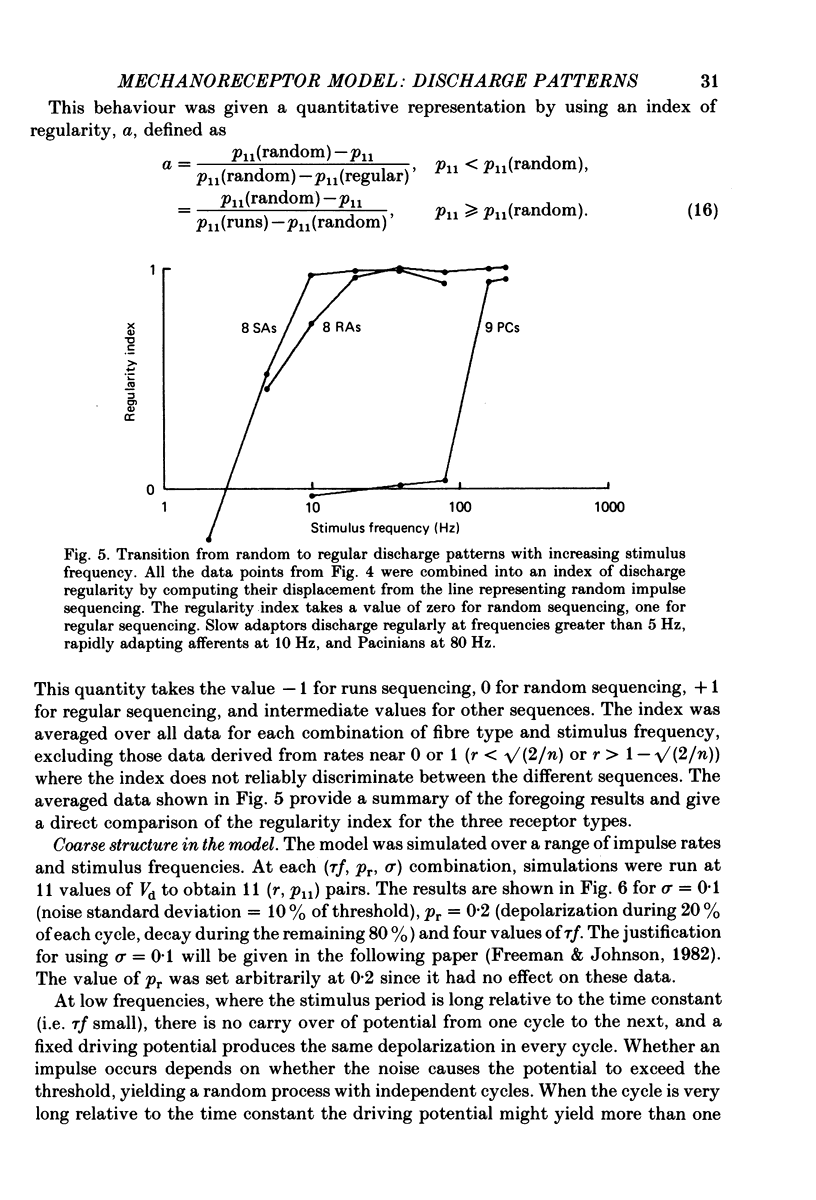
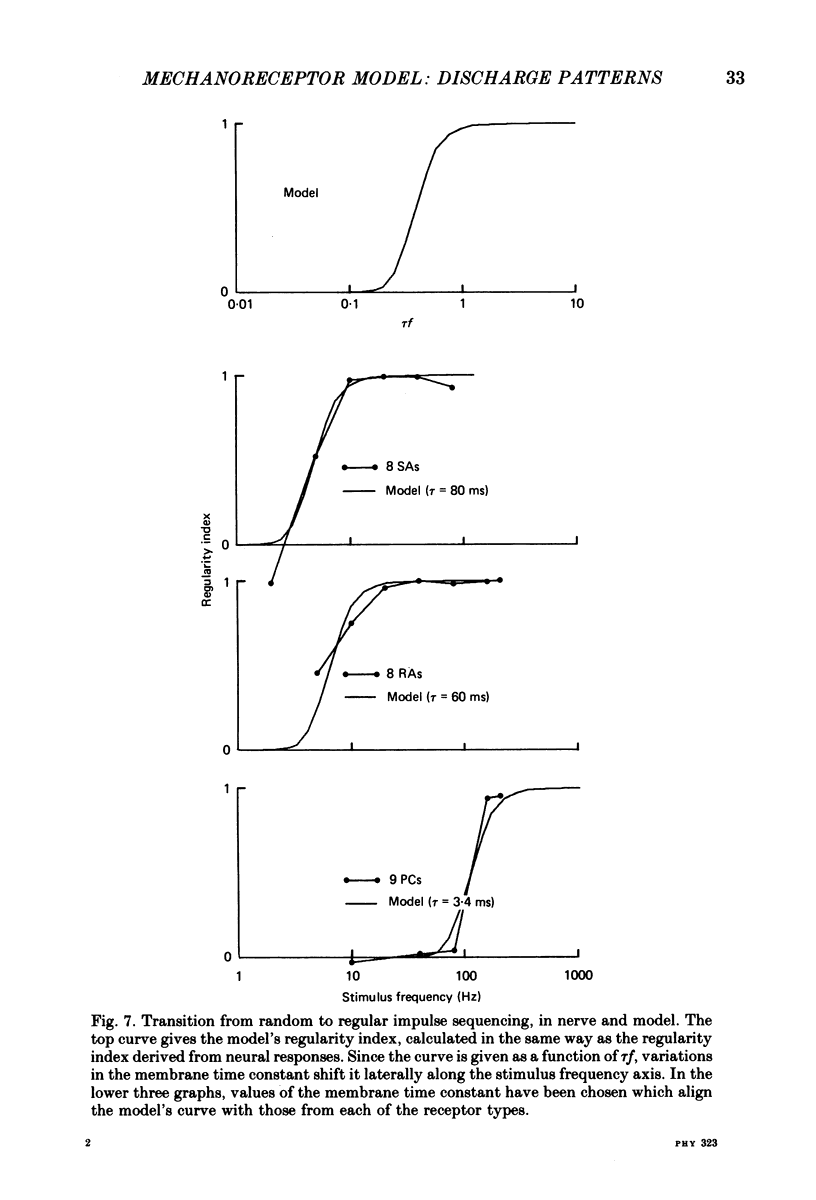
4. The coarse structure of each response was represented by the presence or absence of an impulse on each stimulus cycle. In each mechanoreceptive class, the impulse sequences were random at low stimulus frequencies and regular at high frequencies. The transition frequencies were roughly 5 Hz for the slowly adapting afferents, 7 Hz for the rapidly adapting afferents, and 110 Hz for the Pacinian afferents. The model matched these data closely when the time constants were set at 80, 60 and 3·4 ms for SAs, RAs and PCs, respectively.
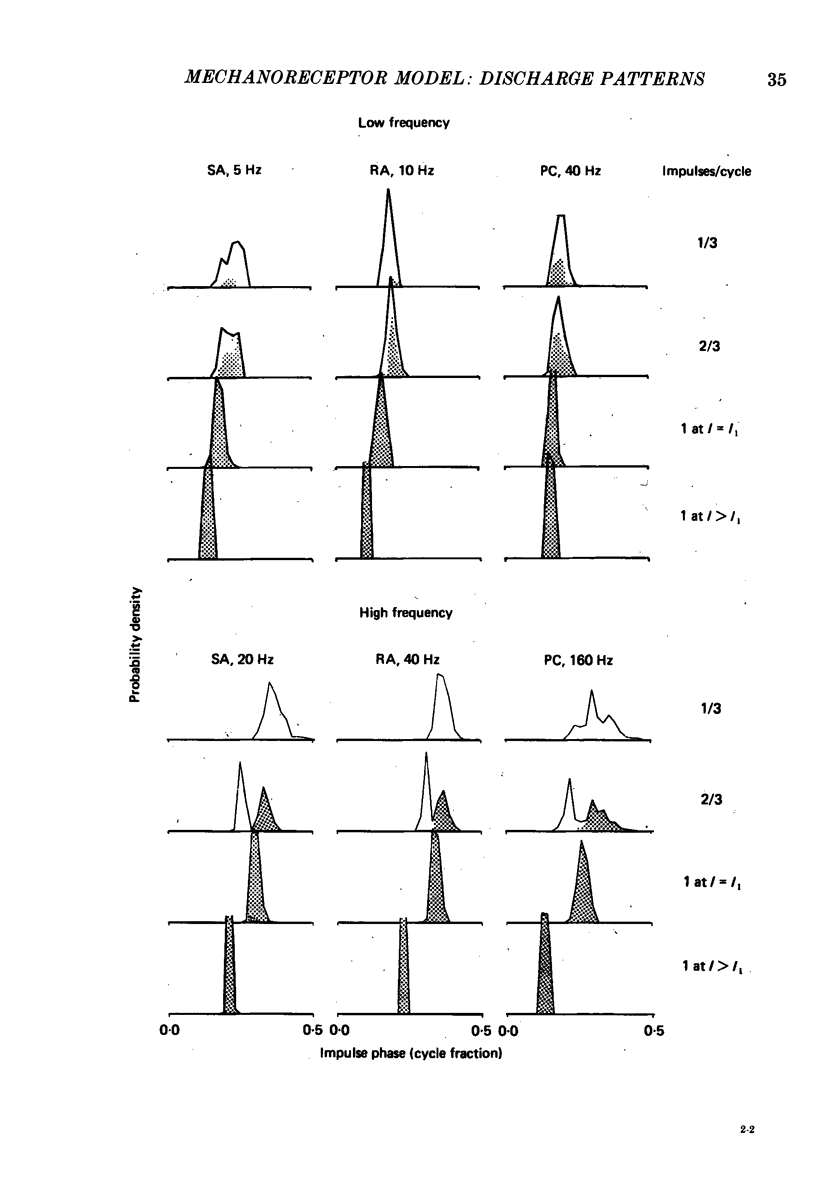
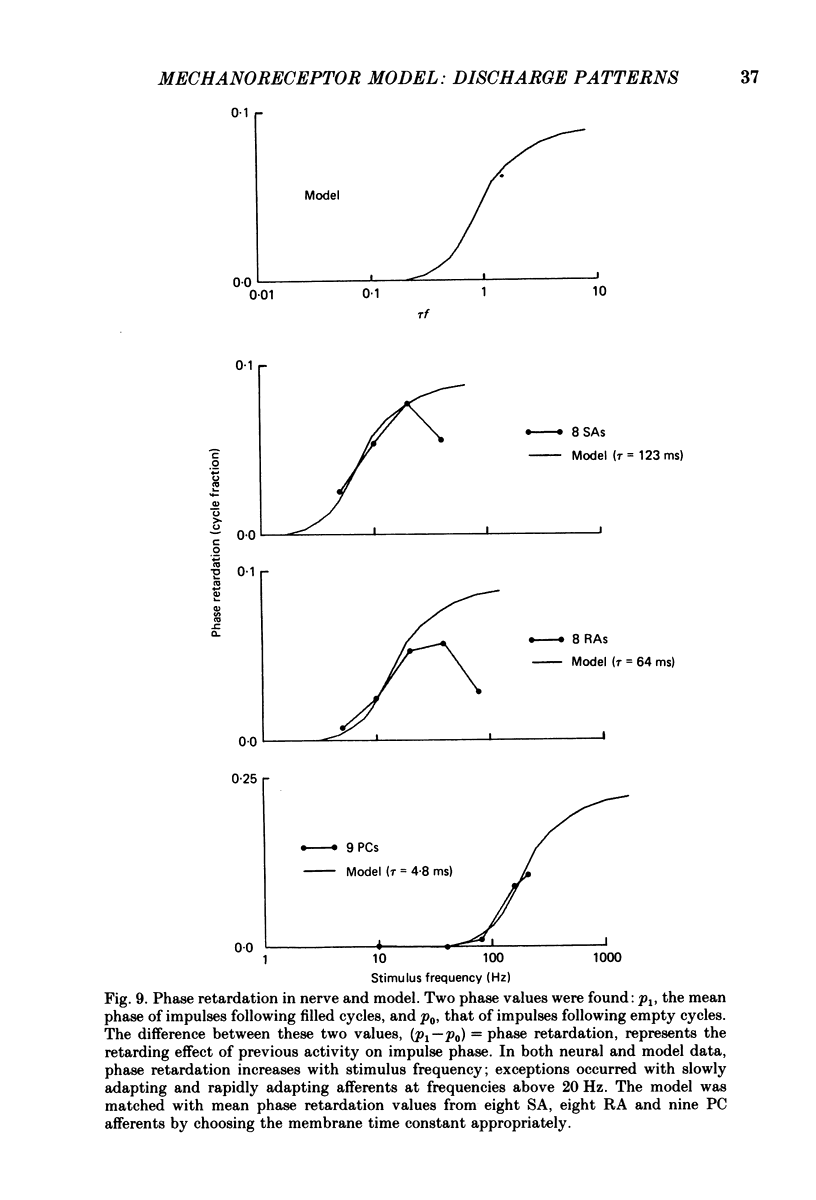
5. The fine structure of the responses of each mechanoreceptive class exhibited impulse phase locking, phase advance with increasing intensity, and bimodal phase distributions at higher frequencies. Impulses contributing to the first mode of bimodal distributions always occurred in cycles following cycles in which no impulse occurred. Impulses contributing to the retarded mode always occurred in cycles following filled cycles. The mean phase differences between the two modes was called phase retardation. Phase retardation grew with stimulus frequency for both the receptors and the model; the time constants required to match the model against neural phase retardation curves were 123, 64 and 4·8 ms for SAs, RAs and PCs, respectively.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buller A. J. A model illustrating some aspects of muscle spindle physiology. J Physiol. 1965 Aug;179(3):402–416. doi: 10.1113/jphysiol.1965.sp007669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND J., GRAY J. A., INMAN D. R. The relation between receptor potentials and the concentration of sodium ions. J Physiol. 1958 Jul 14;142(2):382–394. doi: 10.1113/jphysiol.1958.sp006024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND J., GRAY J. A., SATO M. The site of initiation of impulses in Pacinian corpuscles. J Physiol. 1956 Jul 27;133(1):54–67. doi: 10.1113/jphysiol.1956.sp005566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman A. W., Johnson K. O. A model accounting for effects of vibratory amplitude on responses of cutaneous mechanoreceptors in macaque monkey. J Physiol. 1982 Feb;323:43–64. doi: 10.1113/jphysiol.1982.sp014060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY J. A., SATO M. Properties of the receptor potential in Pacinian corpuscles. J Physiol. 1953 Dec 29;122(3):610–636. doi: 10.1113/jphysiol.1953.sp005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C., TAKEUCHI A. Responses of the nerve terminal of the Pacinian corpuscle. J Physiol. 1962 Jan;160:1–21. doi: 10.1113/jphysiol.1962.sp006829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden A. V. The response of excitable membrane models to a cyclic input. Biol Cybern. 1976 Jan 2;21(1):1–7. doi: 10.1007/BF00326666. [DOI] [PubMed] [Google Scholar]
- Johnson K. O. Reconstruction of population response to a vibratory stimulus in quickly adapting mechanoreceptive afferent fiber population innervating glabrous skin of the monkey. J Neurophysiol. 1974 Jan;37(1):48–72. doi: 10.1152/jn.1974.37.1.48. [DOI] [PubMed] [Google Scholar]
- KATZ B. Depolarization of sensory terminals and the initiation of impulses in the muscle spindle. J Physiol. 1950 Oct 16;111(3-4):261–282. doi: 10.1113/jphysiol.1950.sp004479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOEWENSTEIN W. R., RATHKAMP R. The sites for mechano-electric conversion in a Pacinian corpuscle. J Gen Physiol. 1958 Jul 20;41(6):1245–1265. doi: 10.1085/jgp.41.6.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein W. R., Skalak R. Mechanical transmission in a Pacinian corpuscle. An analysis and a theory. J Physiol. 1966 Jan;182(2):346–378. doi: 10.1113/jphysiol.1966.sp007827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto I., Miyazaki S., Saito M., Utsunomiya T. Behavior of solutions of the Hodgkin-Huxley equations and its relation to properties of mechanoreceptors. Biophys J. 2009 Jan 1;15(5):469–479. doi: 10.1016/S0006-3495(75)85831-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OZEKI M., SATO M. INITIATION OF IMPULSES AT THE NON-MYELINATED NERVE TERMINAL IN PACINIAN CORPUSCLES. J Physiol. 1964 Jan;170:167–185. doi: 10.1113/jphysiol.1964.sp007321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescigno A., Stein R. B., Purple R. L., Poppele R. E. A neuronal model for the discharge patterns produced by cyclic inputs. Bull Math Biophys. 1970 Sep;32(3):337–353. doi: 10.1007/BF02476873. [DOI] [PubMed] [Google Scholar]
- Talbot W. H., Darian-Smith I., Kornhuber H. H., Mountcastle V. B. The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J Neurophysiol. 1968 Mar;31(2):301–334. doi: 10.1152/jn.1968.31.2.301. [DOI] [PubMed] [Google Scholar]


