Abstract
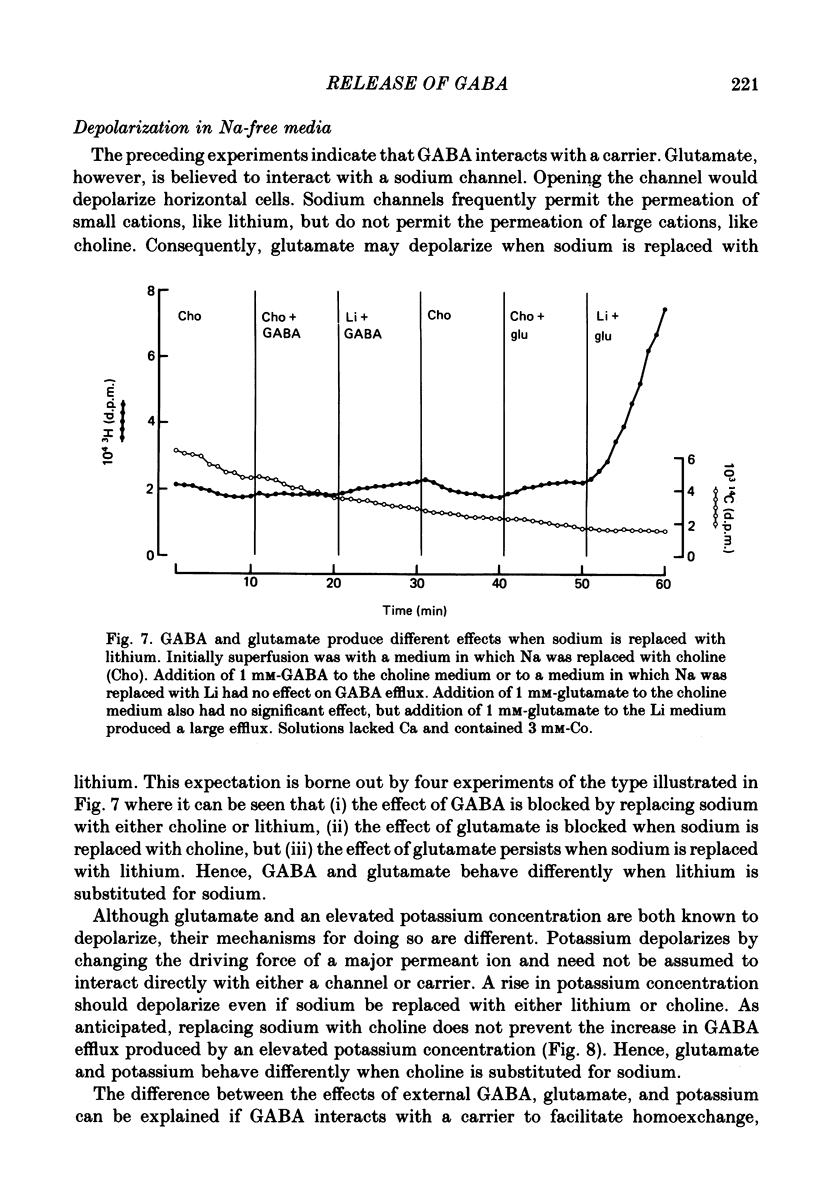
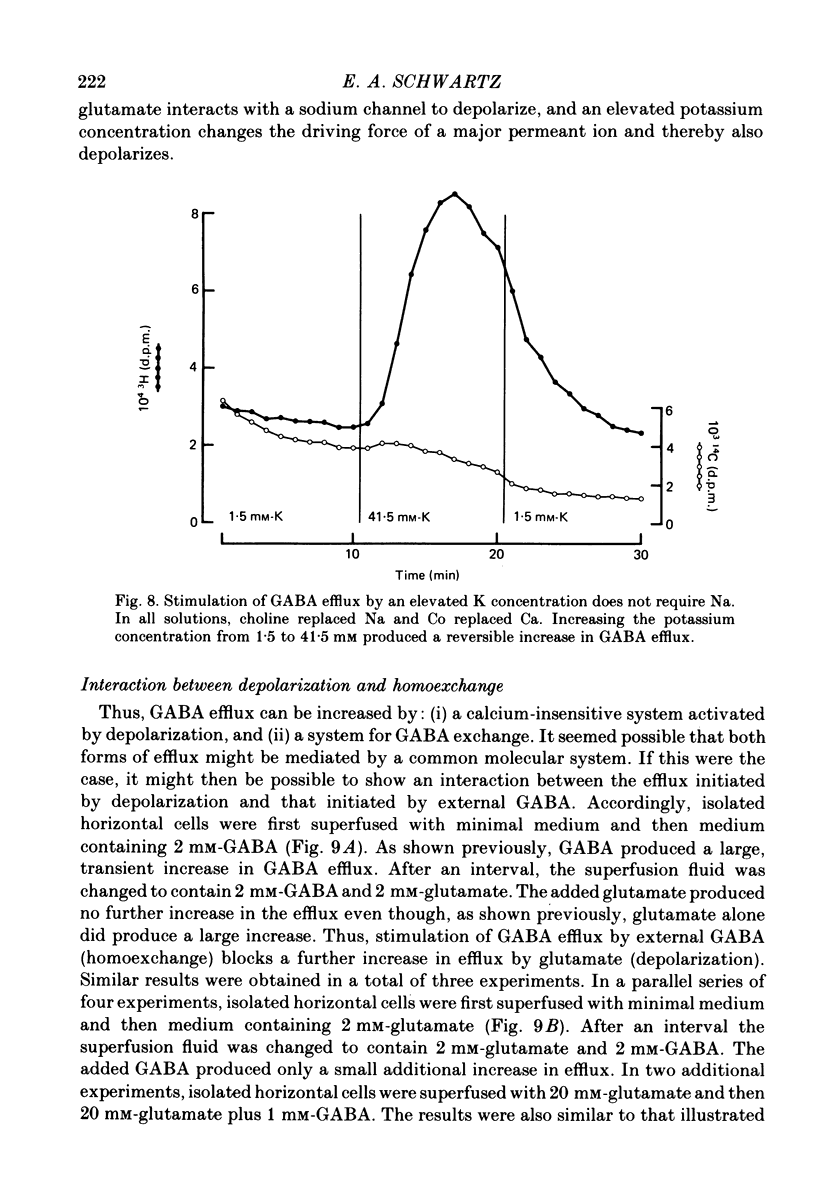
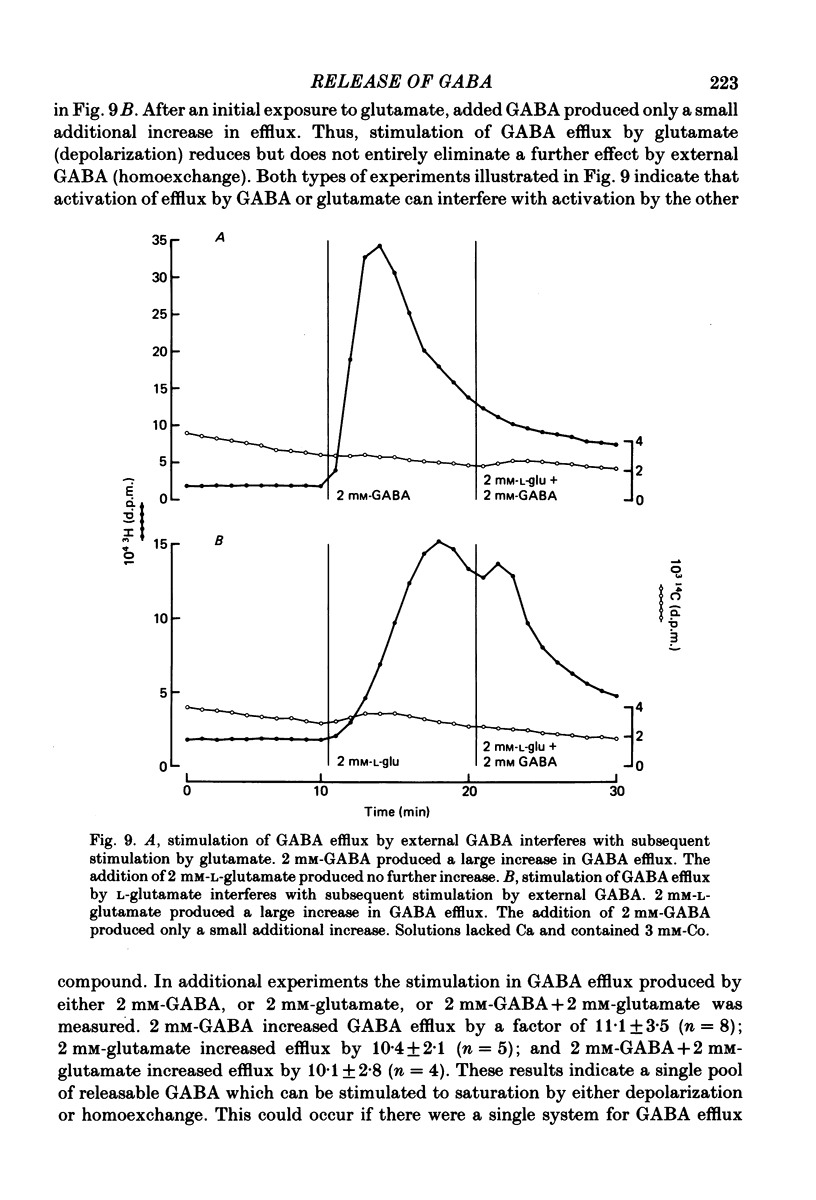
1. When toad retinae were incubated first with veratrine, then with antibodies that reacted with the outer segments of photoreceptors, and finally with complement, horizontal cells survived and most other neurones died. This preparation of 'isolated' horizontal cells accumulated radioactive GABA from the incubation medium. The subsequent release of radioactive GABA could then be measured. 2. The efflux of GABA was increased by exposure to an elevated potassium concentration or added glutamate. Both procedures are known to depolarize horizontal cells. 3. GABA in the external medium also increased the efflux of GABA. 4. The increase in GABA efflux produced by an elevated potassium concentration was unaffected with calcium in the external medium was replaced with cobalt and when sodium was replaced with choline or lithium. 5. The increase in GABA efflux produced by glutamate was unaffected when calcium was replaced with cobalt and when sodium was replaced with lithium, but was inhibited when sodium was replaced with choline. 6. The increase in GABA efflux produced by external GABA was unaffected when calcium was replaced with cobalt but required sodium. Neither choline nor lithium would substitute for sodium. 7. An increase in GABA efflux was accompanied by an increase in sodium efflux. 8. After a high concentration of GABA (2-20 mM) had produced a maximal increase in GABA efflux, the addition of glutamate produced no further effect. Conversely, after a high concentration of glutamate (2-20 mM) had produced a maximal increase in efflux, the addition of external GABA produced only a small further increase. These and the preceding results could occur if GABA release were mediated by a carrier system which could be activated by either depolarization or homoexchange.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruun A., Ehinger B. Uptake of certain possible neurotransmitters into retinal neurons of some mammals. Exp Eye Res. 1974 Nov;19(5):435–447. doi: 10.1016/0014-4835(74)90052-9. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu Rev Pharmacol Toxicol. 1980;20:15–43. doi: 10.1146/annurev.pa.20.040180.000311. [DOI] [PubMed] [Google Scholar]
- Cervetto L., MacNichol E. F., Jr Inactivation of horizontal cells in turtle retina by glutamate and aspartate. Science. 1972 Nov 17;178(4062):767–768. doi: 10.1126/science.178.4062.767. [DOI] [PubMed] [Google Scholar]
- Crnic D. M., Hammerstad J. P., Cutler R. W. Accelerated efflux of ( 14 C) and ( 3 H) amino acids from superfused slices of rat brain. J Neurochem. 1973 Jan;20(1):203–209. doi: 10.1111/j.1471-4159.1973.tb12117.x. [DOI] [PubMed] [Google Scholar]
- Fagg G. E., Lane J. D. The uptake and release of putative amino acid neurotransmitters. Neuroscience. 1979;4(8):1015–1036. doi: 10.1016/0306-4522(79)90185-4. [DOI] [PubMed] [Google Scholar]
- Geduldig D., Junge D. Sodium and calcium components of action potentials in the Aplysia giant neurone. J Physiol. 1968 Dec;199(2):347–365. doi: 10.1113/jphysiol.1968.sp008657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S. Ca-dependent action potential. Membranes. 1975;3:359–381. [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. Surface density of calcium ions and calcium spikes in the barnacle muscle fiber membrane. J Gen Physiol. 1967 Jan;50(3):583–601. doi: 10.1085/jgp.50.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Dennis M. J., Jan Y., Jan L., Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979 May;81(2):275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J., Miledi R. Effects of lanthanum ions on function and structure of frog neuromuscular junctions. Proc R Soc Lond B Biol Sci. 1971 Dec 14;179(1056):247–260. doi: 10.1098/rspb.1971.0096. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy A. J., Voaden M. J. Factors affecting the spontaneous release of (3H) gamma-aminobutyric acid from the frog retina in vitro. J Neurochem. 1974 Jan;22(1):63–71. doi: 10.1111/j.1471-4159.1974.tb12179.x. [DOI] [PubMed] [Google Scholar]
- Lam D. M. Biosynthesis of gamma-aminobutyric acid by isolated axons of cone horizontal cells in the goldfish retina. Nature. 1975 Mar 27;254(5498):345–347. doi: 10.1038/254345a0. [DOI] [PubMed] [Google Scholar]
- Lam D. M., Lasater E. M., Naka K. I. gamma-Aminobutyric acid: a neurotransmitter candidate for cone horizontal cells of the catfish retina. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6310–6313. doi: 10.1073/pnas.75.12.6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam D. M., Steinman L. The uptake of ( - 3 H) aminobutyric acid in the goldfish retina. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2777–2781. doi: 10.1073/pnas.68.11.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam D. M., Su Y. Y., Swain L., Marc R. E., Brandon C., Wu J. Y. Immunocytochemical localisation of L-glutamic acid decarboxylase in the goldfish retina. Nature. 1979 Apr 5;278(5704):565–567. doi: 10.1038/278565a0. [DOI] [PubMed] [Google Scholar]
- Lasansky A. Lateral contacts and interactions of horizontal cell dendrites in the retina of the larval tiger salamander. J Physiol. 1980 Apr;301:59–68. doi: 10.1113/jphysiol.1980.sp013188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc R. E., Stell W. K., Bok D., Lam D. M. GABA-ergic pathways in the goldfish retina. J Comp Neurol. 1978 Nov 15;182(2):221–244. doi: 10.1002/cne.901820204. [DOI] [PubMed] [Google Scholar]
- Marshall J., Voaden M. An investigation of the cells incorporating (3H)GABA and (3H)glycine in the isolated retina of the rat. Exp Eye Res. 1974 Apr;18(4):367–370. doi: 10.1016/0014-4835(74)90113-4. [DOI] [PubMed] [Google Scholar]
- Miledi R. Lanthanum ions abolish the "calcium response" of nerve terminals. Nature. 1971 Feb 5;229(5284):410–411. doi: 10.1038/229410a0. [DOI] [PubMed] [Google Scholar]
- Miledi R. Transmitter release induced by injection of calcium ions into nerve terminals. Proc R Soc Lond B Biol Sci. 1973 Jul 3;183(1073):421–425. doi: 10.1098/rspb.1973.0026. [DOI] [PubMed] [Google Scholar]
- Murakami M., Otsu K., Otsuka T. Effects of chemicals on receptors and horizontal cells in the retina. J Physiol. 1972 Dec;227(3):899–913. doi: 10.1113/jphysiol.1972.sp010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal M. J., Iversen L. L. Autoradiographic localization of 3 H-GABA in rat retina. Nat New Biol. 1972 Feb 16;235(59):217–218. doi: 10.1038/newbio235217a0. [DOI] [PubMed] [Google Scholar]
- Papermaster D. S., Dreyer W. J. Rhodopsin content in the outer segment membranes of bovine and frog retinal rods. Biochemistry. 1974 May 21;13(11):2438–2444. doi: 10.1021/bi00708a031. [DOI] [PubMed] [Google Scholar]
- Raiteri M., Federico R., Coletti A., Levi G. Release and exchange studies relating to the synaptosomal uptake of GABA. J Neurochem. 1975 Jun;24(6):1243–1250. doi: 10.1111/j.1471-4159.1975.tb03905.x. [DOI] [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F. Coupled transport of sodium and organic solutes. Physiol Rev. 1970 Oct;50(4):637–718. doi: 10.1152/physrev.1970.50.4.637. [DOI] [PubMed] [Google Scholar]
- Simon E. J. Two types of luminosity horizontal cells in the retina of the turtle. J Physiol. 1973 Apr;230(1):199–211. doi: 10.1113/jphysiol.1973.sp010183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J. R., Martin D. L., Kroll M. Sodium-dependent efflux and exchange of GABA in synaptosomes. J Neurochem. 1974 Nov;23(5):981–991. doi: 10.1111/j.1471-4159.1974.tb10750.x. [DOI] [PubMed] [Google Scholar]
- Sugawara K., Negishi K. Effects of some amino acids on the horizontal cell membrane potential in the isolated carp retina. Vision Res. 1973 May;13(5):977–981. doi: 10.1016/0042-6989(73)90076-x. [DOI] [PubMed] [Google Scholar]
- Voaden M. J., Marshall J., Murani N. The uptake of [3H]gamma-amino butyric acid and [3H]glycine by the isolated retina of the frog. Brain Res. 1974 Feb 15;67(1):115–132. doi: 10.1016/0006-8993(74)90302-3. [DOI] [PubMed] [Google Scholar]
- Weakly J. N. The action of cobalt ions on neuromuscular transmission in the frog. J Physiol. 1973 Nov;234(3):597–612. doi: 10.1113/jphysiol.1973.sp010363. [DOI] [PMC free article] [PubMed] [Google Scholar]