Abstract
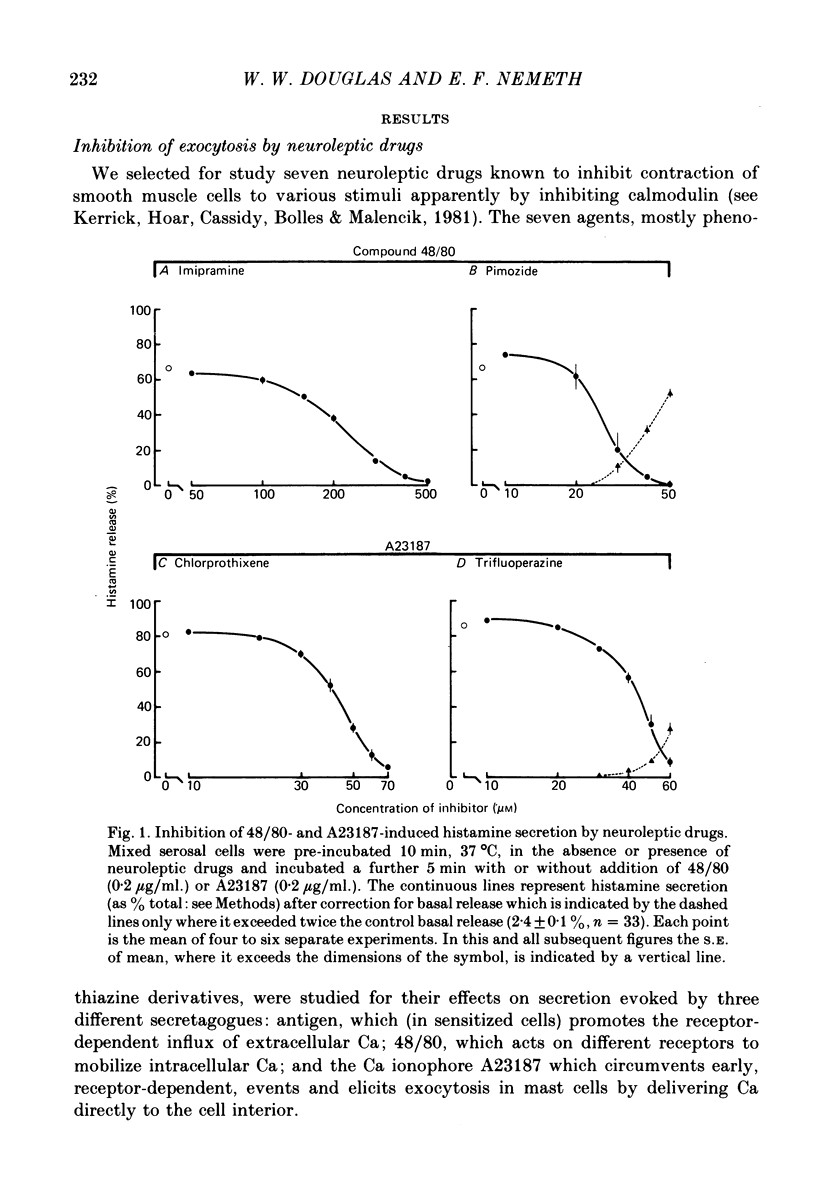
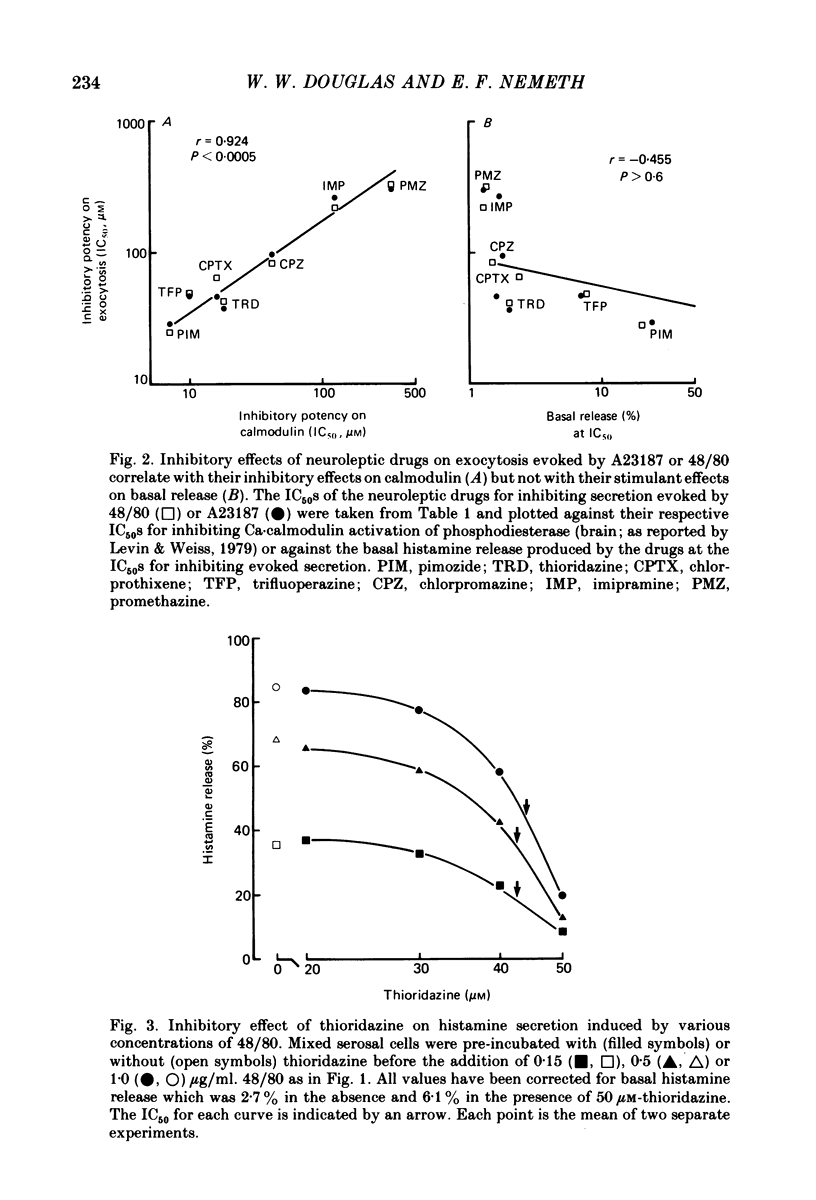
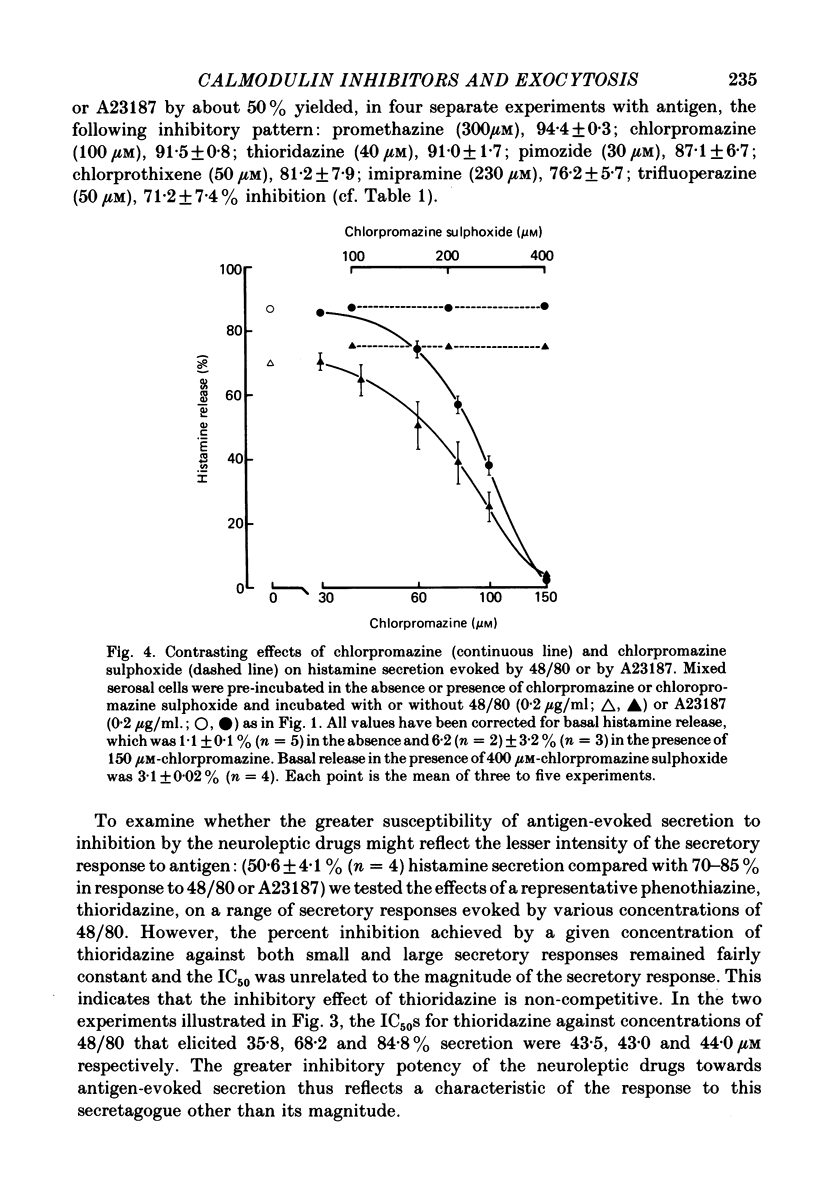
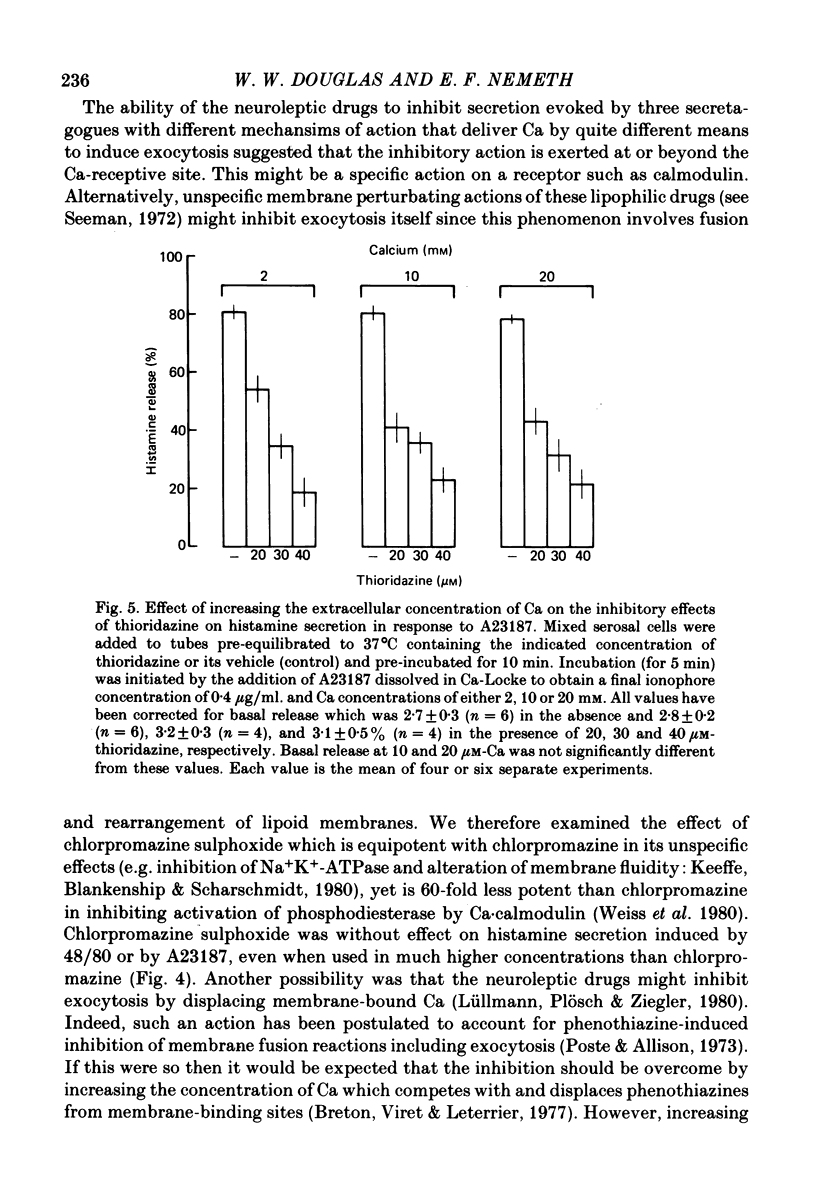
1. A series of neuroleptic drugs (five phenothiazines, imipramine, and pimozide) and the smooth muscle relaxant W-7, which all inhibit calcium-calmodulin-activated processes inhibited rat mast cell secretion elicited by antigen, by 48/80, and by the calcium ionophore A23187. 2. Neither the phenothiazines nor W-7 reduced 45Ca uptake in response to A23187. The drugs thus exert an inhibitory action distal to the rise in intracellular Ca ions that activates exocytosis. 3. Chlorpromazine sulphoxide, which shares several membrane-perturbing actions of the phenothiazines but is a weak inhibitor of calmodulin, did not inhibit secretion. Moreover, the inhibitory effects of the phenothiazines were not overcome by a 5- or 10-fold increase in the concentration of calcium, which should counter unspecific membrane effects. 4. The inhibitory effects of the various neuroleptic drugs appeared to be related to their ability to inhibit calmodulin because the individual potencies of these compounds on secretion evoked by 48/80 or A23187 correlated significantly with their reported potencies in inhibiting calmodulin-activated processes. (The greater potency and different rank order of these compounds on secretion evoked by antigen suggests an additional inhibitory action, perhaps involving Ca entry.) 5. These results, which parallel those obtained with drugs of this sort in smooth muscle where calmodulin seemingly functions as the Ca receptor activating contraction, strengthen the view that calmodulin, or some calmodulin-like protein, is the Ca receptor activating exocytosis.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Eisenberg E. Regulation and kinetics of the actin-myosin-ATP interaction. Annu Rev Biochem. 1980;49:921–956. doi: 10.1146/annurev.bi.49.070180.004421. [DOI] [PubMed] [Google Scholar]
- Andersson K. E. Effects of chlorpromazine, imipramine, and quinidine on the mechanical activity of single skeletal muscle fibres of the frog. Acta Physiol Scand. 1972 Aug;85(4):532–546. doi: 10.1111/j.1748-1716.1971.tb05292.x. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Knight D. E. Gaining access to the site of exocytosis in bovine adrenal medullary cells. J Physiol (Paris) 1980 Sep;76(5):497–504. [PubMed] [Google Scholar]
- Baker P. F., Whitaker M. J. Trifluoperazine inhibits exocytosis in sea-urchin eggs [proceedings]. J Physiol. 1980 Jan;298:55P–55P. [PubMed] [Google Scholar]
- Breton J., Viret J., Leterrier F. Calcium and chlorpromazine interactions in rat synaptic plasma membranes. A spin-label and fluorescence probe study. Arch Biochem Biophys. 1977 Mar;179(2):625–633. doi: 10.1016/0003-9861(77)90151-5. [DOI] [PubMed] [Google Scholar]
- Cassidy P., Hoar P. E., Kerrick W. G. Inhibition of Ca2+-activated tension and myosin light chain phosphorylation in skinned smooth muscle strips by the phenothiazines. Pflugers Arch. 1980 Sep;387(2):115–120. doi: 10.1007/BF00584261. [DOI] [PubMed] [Google Scholar]
- Chaturvedi A. K., Landon E. J., Rama Sastry B. V. Influence of chlorpromazine on calcium movements and contractile responses of guinea-pig ileum longitudinal smooth muscle to agonists. Arch Int Pharmacodyn Ther. 1978 Nov;236(1):109–124. [PubMed] [Google Scholar]
- Cochrane D. E., Douglas W. W. Calcium-induced extrusion of secretory granules (exocytosis) in mast cells exposed to 48-80 or the ionophores A-23187 and X-537A. Proc Natl Acad Sci U S A. 1974 Feb;71(2):408–412. doi: 10.1073/pnas.71.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutts S. M., Nehring R. E., Jr, Jariwala N. U. Purification of rat peritoneal mast cells: occupation of IgE-receptors by IgE prevents loss of the receptors. J Immunol. 1980 May;124(5):2309–2315. [PubMed] [Google Scholar]
- Crosby N. D., Diamond J. Effects of phenothiazines on calcium induced contractions of chemically skinned smooth muscle. Proc West Pharmacol Soc. 1980;23:335–338. [PubMed] [Google Scholar]
- Diamant B. Energy production in rat mast cells and its role for histamine release. Int Arch Allergy Appl Immunol. 1975;49(1-2):155–171. doi: 10.1159/000231391. [DOI] [PubMed] [Google Scholar]
- Douglas W. W. Involvement of calcium in exocytosis and the exocytosis--vesiculation sequence. Biochem Soc Symp. 1974;(39):1–28. [PubMed] [Google Scholar]
- Douglas W. W., Kagayama M. Calcium and stimulus-secretion coupling in the mast cell: stimulant and inhibitory effects of calcium-rich media on exocytosis. J Physiol. 1977 Sep;270(3):691–703. doi: 10.1113/jphysiol.1977.sp011976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Ueda Y. Proceedings: Mast cell secretion (histamine release) induced by 48-80: calcium-dependent exocytosis inhibited strongly by cytochalasin only when glycolysis is rate-limiting. J Physiol. 1973 Oct;234(2):97P–98P. [PubMed] [Google Scholar]
- Elferink J. G. Chlorpromazine inhibits phagocytosis and exocytosis in rabbit polymorphonuclear leukocytes. Biochem Pharmacol. 1979 Apr 1;28(7):965–968. doi: 10.1016/0006-2952(79)90287-9. [DOI] [PubMed] [Google Scholar]
- Fleckman A., Erlichman J., Schubart U. K., Fleischer N. Effect of trifluoperazine, D600, and phenytoin on depolarization- and thyrotropin-releasing hormone-induced thyrotropin release from rat pituitary tissue. Endocrinology. 1981 Jun;108(6):2072–2077. doi: 10.1210/endo-108-6-2072. [DOI] [PubMed] [Google Scholar]
- Foreman J. C., Hallett M. B., Mongar J. L. The relationship between histamine secretion and 45calcium uptake by mast cells. J Physiol. 1977 Sep;271(1):193–214. doi: 10.1113/jphysiol.1977.sp011996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L., Gomperts B. D. Calcium ionophores and movement of calcium ions following the physiological stimulus to a secretory process. Nature. 1973 Oct 5;245(5423):249–251. doi: 10.1038/245249a0. [DOI] [PubMed] [Google Scholar]
- Foreman J. C. The pharmacological control of immediate hypersensitivity. Annu Rev Pharmacol Toxicol. 1981;21:63–81. doi: 10.1146/annurev.pa.21.040181.000431. [DOI] [PubMed] [Google Scholar]
- Gagliardino J. J., Harrison D. E., Christie M. R., Gagliardino E. E., Ashcroft S. J. Evidence for the participation of calmodulin in stimulus-secretion coupling in the pancreatic beta-cell. Biochem J. 1980 Dec 15;192(3):919–927. doi: 10.1042/bj1920919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland L. G., Payne A. N. The role of cell-fixed calcium in histamine release by compound 48/80. Br J Pharmacol. 1979 Apr;65(4):609–613. doi: 10.1111/j.1476-5381.1979.tb07871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorne D. J., Siemankowski R. F. Regulation of smooth muscle actomyosin. Annu Rev Physiol. 1981;43:519–530. doi: 10.1146/annurev.ph.43.030181.002511. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Asano M., Iwadare S., Matsumoto I., Totsuka T., Aoki N. A novel vascular relaxing agent, N-(6--aminohexyl)-5-chloro-1-naphthalensulfonamide which affects vascular smooth muscle actomyosin. J Pharmacol Exp Ther. 1978 Oct;207(1):8–15. [PubMed] [Google Scholar]
- Hidaka H., Yamaki T., Naka M., Tanaka T., Hayashi H., Kobayashi R. Calcium-regulated modulator protein interacting agents inhibit smooth muscle calcium-stimulated protein kinase and ATPase. Mol Pharmacol. 1980 Jan;17(1):66–72. [PubMed] [Google Scholar]
- Hidaka H., Yamaki T., Totsuka T., Asano M. Selective inhibitors of Ca2+-binding modulator of phosphodiesterase produce vascular relaxation and inhibit actin-myosin interaction. Mol Pharmacol. 1979 Jan;15(1):49–59. [PubMed] [Google Scholar]
- Jansson S. E. Effect of chlorpromazine, mepyramine, prenylamine and reserpine of 5-hydroxytryptamine content and fine structure of rat peritoneal mast cells incubated in vitro. Acta Physiol Scand. 1970 Mar;78(3):420–430. doi: 10.1111/j.1748-1716.1970.tb04678.x. [DOI] [PubMed] [Google Scholar]
- Johansen T. Further observations on the utilization of adenosine triphosphate in rat mast cells during histamine release induced by the ionophore A23187. Br J Pharmacol. 1980 Aug;69(4):657–662. doi: 10.1111/j.1476-5381.1980.tb07918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen T. Mechanism of histamine release from rat mast cells induced by the ionophore A23187: effects of calcium and temperature. Br J Pharmacol. 1978 Aug;63(4):643–649. doi: 10.1111/j.1476-5381.1978.tb17277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagayama M., Douglas W. W. Electron microscope evidence of calcium-induced exocytosis in mast cells treated with 48-80 or the ionophores A-23187 and X-537A. J Cell Biol. 1974 Aug;62(2):519–526. doi: 10.1083/jcb.62.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno T., Cochrane D. E., Douglas W. W. Exocytosis (secretory granule extrusion) induced by injection of calcium into mast cells. Can J Physiol Pharmacol. 1973 Dec;51(12):1001–1004. doi: 10.1139/y73-153. [DOI] [PubMed] [Google Scholar]
- Keeffe E. B., Blankenship N. M., Scharschmidt B. F. Alteration of rat liver plasma membrane fluidity and ATPase activity by chlorpromazine hydrochloride and its metabolites. Gastroenterology. 1980 Aug;79(2):222–231. [PubMed] [Google Scholar]
- Kerrick W. G., Hoar P. E., Cassidy P. S., Bolles L., Malencik D. A. Calcium-regulatory mechanisms. Functional classification using skinned fibers. J Gen Physiol. 1981 Feb;77(2):177–190. doi: 10.1085/jgp.77.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi R., Tawata M., Hidaka H. Ca2+ regulated modulator protein interacting agents: inhibition of Ca2+-Mg2+-ATPase of human erythrocyte ghost. Biochem Biophys Res Commun. 1979 Jun 13;88(3):1037–1045. doi: 10.1016/0006-291x(79)91513-4. [DOI] [PubMed] [Google Scholar]
- Krausz Y., Wollheim C. B., Siegel E., Sharp G. W. Possible role for calmodulin in insulin release. Studies with trifluoperazine in rat pancreatic islets. J Clin Invest. 1980 Sep;66(3):603–607. doi: 10.1172/JCI109893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Selective binding of antipsychotics and other psychoactive agents to the calcium-dependent activator of cyclic nucleotide phosphodiesterase. J Pharmacol Exp Ther. 1979 Mar;208(3):454–459. [PubMed] [Google Scholar]
- Lüllmann H., Plösch H., Ziegler A. Ca replacement by cationic amphiphilic drugs from lipid monolayers. Biochem Pharmacol. 1980 Nov 1;29(21):2969–2974. doi: 10.1016/0006-2952(80)90046-5. [DOI] [PubMed] [Google Scholar]
- Means A. R., Dedman J. R. Calmodulin--an intracellular calcium receptor. Nature. 1980 May 8;285(5760):73–77. doi: 10.1038/285073a0. [DOI] [PubMed] [Google Scholar]
- Naccache P. H., Molski T. F., Alobaidi T., Becker E. L., Showell H. J., Sha'afi R. I. Calmodulin inhibitors block neutrophil degranulation at a step distal from the mobilization of calcium. Biochem Biophys Res Commun. 1980 Nov 17;97(1):62–68. doi: 10.1016/s0006-291x(80)80134-3. [DOI] [PubMed] [Google Scholar]
- Nemeth E. F., Douglas W. W. Differential inhibitory effects of the arachidonic acid analog ETYA on rat mast cell exocytosis evoked by secretagogues utilizing cellular or extracellular calcium. Eur J Pharmacol. 1980 Oct 31;67(4):439–450. doi: 10.1016/0014-2999(80)90185-5. [DOI] [PubMed] [Google Scholar]
- Nemeth E. F., Douglas W. W. Effects of microfilament-active drugs, phalloidin and the cytochalasins A and B, on exocytosis in mast cells evoked by 48/80 or A23187. Naunyn Schmiedebergs Arch Pharmacol. 1978 Apr;302(2):153–163. doi: 10.1007/BF00517982. [DOI] [PubMed] [Google Scholar]
- Nishikawa M., Tanaka T., Hidaka H. Ca2+-calmodulin-dependent phosphorylation and platelet secretion. Nature. 1980 Oct 30;287(5785):863–865. doi: 10.1038/287863a0. [DOI] [PubMed] [Google Scholar]
- Poste G., Allison A. C. Membrane fusion. Biochim Biophys Acta. 1973 Dec 28;300(4):421–465. doi: 10.1016/0304-4157(73)90015-4. [DOI] [PubMed] [Google Scholar]
- Schubart U. K., Erlichman J., Fleischer N. The role of calmodulin in the regulation of protein phosphorylation and insulin release in hamster insulinoma cells. J Biol Chem. 1980 May 10;255(9):4120–4124. [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Sieghart W., Theoharides T. C., Alper S. L., Douglas W. W., Greengard P. Calcium-dependent protein phosphorylation during secretion by exocytosis in the mast cell. Nature. 1978 Sep 28;275(5678):329–331. doi: 10.1038/275329a0. [DOI] [PubMed] [Google Scholar]
- Singh M. Amylase release from dissociated mouse pancreatic acinar cells stimulated by glucagon: effect of membrane stabilizers. J Physiol. 1980 Dec;309:81–91. doi: 10.1113/jphysiol.1980.sp013495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden M. C., Christie M. R., Ashcroft S. J. Presence and possible role of calcium-dependent regulator (calmodulin) in rat islets of Langerhans. FEBS Lett. 1979 Sep 1;105(1):95–100. doi: 10.1016/0014-5793(79)80894-7. [DOI] [PubMed] [Google Scholar]
- Theoharides T. C., Douglas W. W. Secretion in mast cells induced by calcium entrapped within phospholipid vesicles. Science. 1978 Sep 22;201(4361):1143–1145. doi: 10.1126/science.684435. [DOI] [PubMed] [Google Scholar]
- Valverde I., Sener A., Lebrun P., Herchuelz A., Malaisse W. J. The stimulus-secretion coupling of glucose-induced insulin release. XLVII. The possible role of calmodulin. Endocrinology. 1981 Apr;108(4):1305–1312. doi: 10.1210/endo-108-4-1305. [DOI] [PubMed] [Google Scholar]
- Weiss B., Prozialeck W., Cimino M., Barnette M. S., Wallace T. L. Pharmacological regulation of calmodulin. Ann N Y Acad Sci. 1980;356:319–345. doi: 10.1111/j.1749-6632.1980.tb29621.x. [DOI] [PubMed] [Google Scholar]
- White G. C., 2nd, Raynor S. T. The effects of trifluoperazine, an inhibitor of calmodulin, on platelet function. Thromb Res. 1980 Apr 1;18(1-2):279–284. doi: 10.1016/0049-3848(80)90194-2. [DOI] [PubMed] [Google Scholar]
- Williams J. A., Poulsen J. H., Lee M. Effects of membrane stabilizers on pancreatic amylase release. J Membr Biol. 1977 May 6;33(1-2):185–195. doi: 10.1007/BF01869515. [DOI] [PubMed] [Google Scholar]
- Yurt R. W., Leid R. W., Jr, Austen K. F. Native heparin from rat peritoneal mast cells. J Biol Chem. 1977 Jan 25;252(2):518–521. [PubMed] [Google Scholar]


