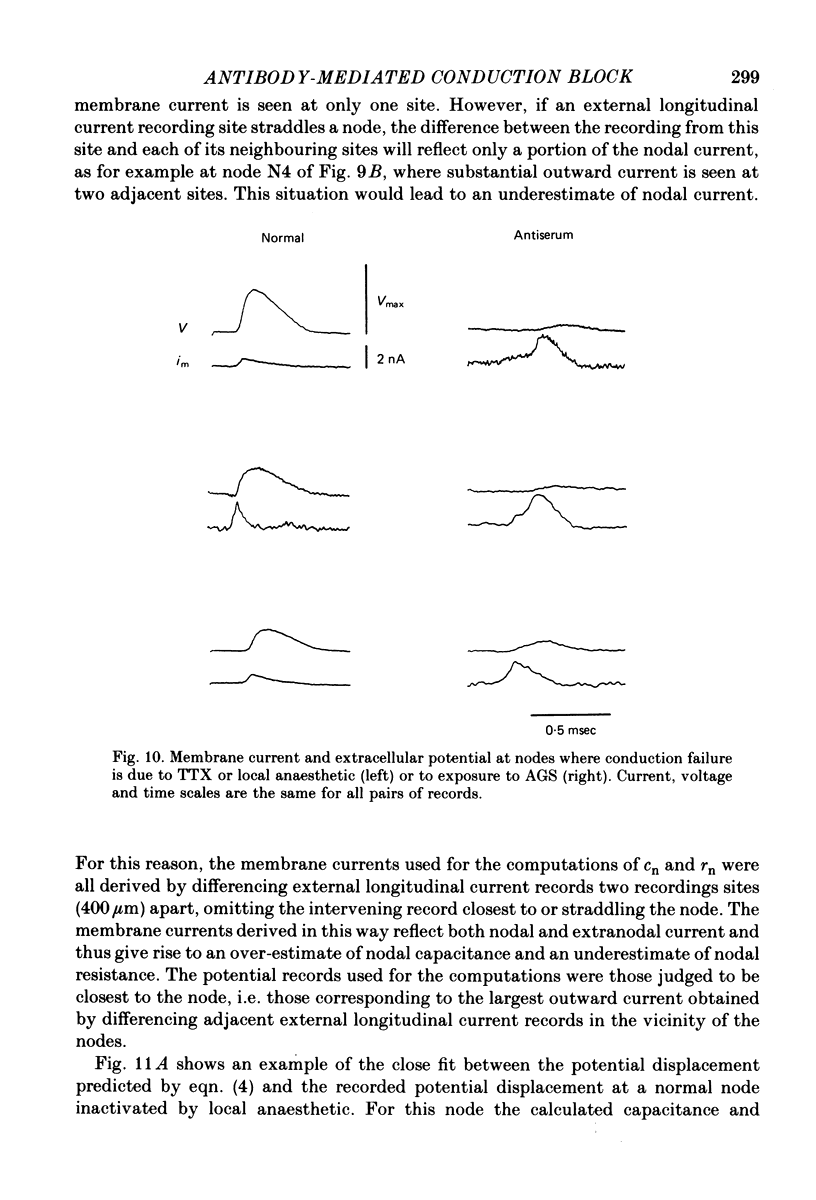
Abstract
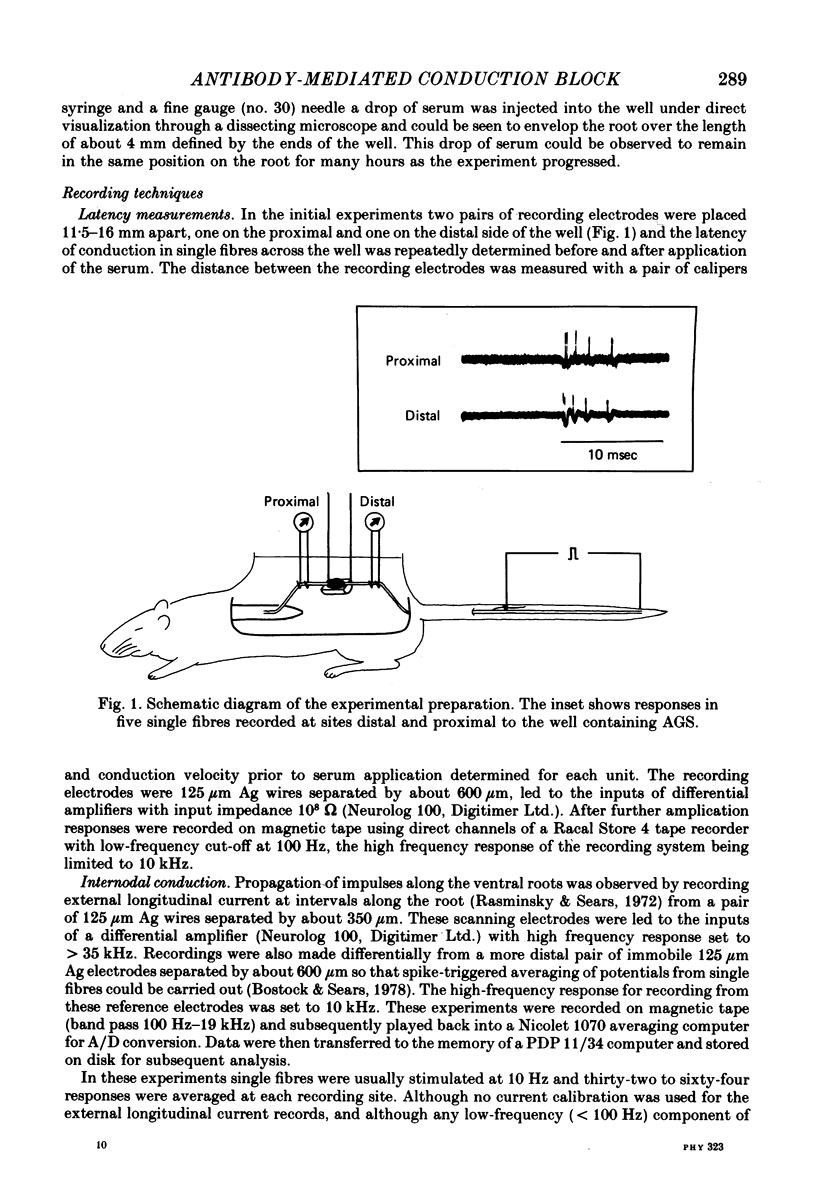
1. We have observed conduction in single rat spinal ventral root nerve fibres following acute topical application of anti-galactocerebroside serum.
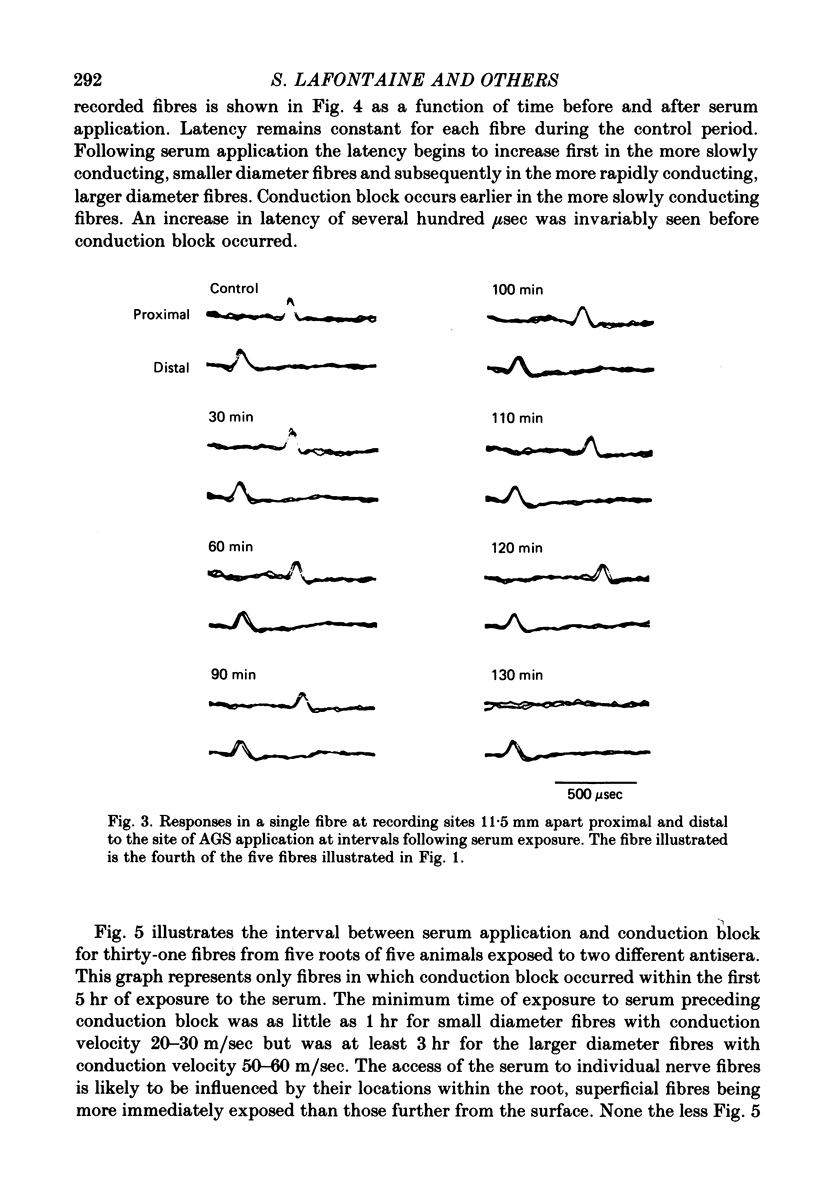
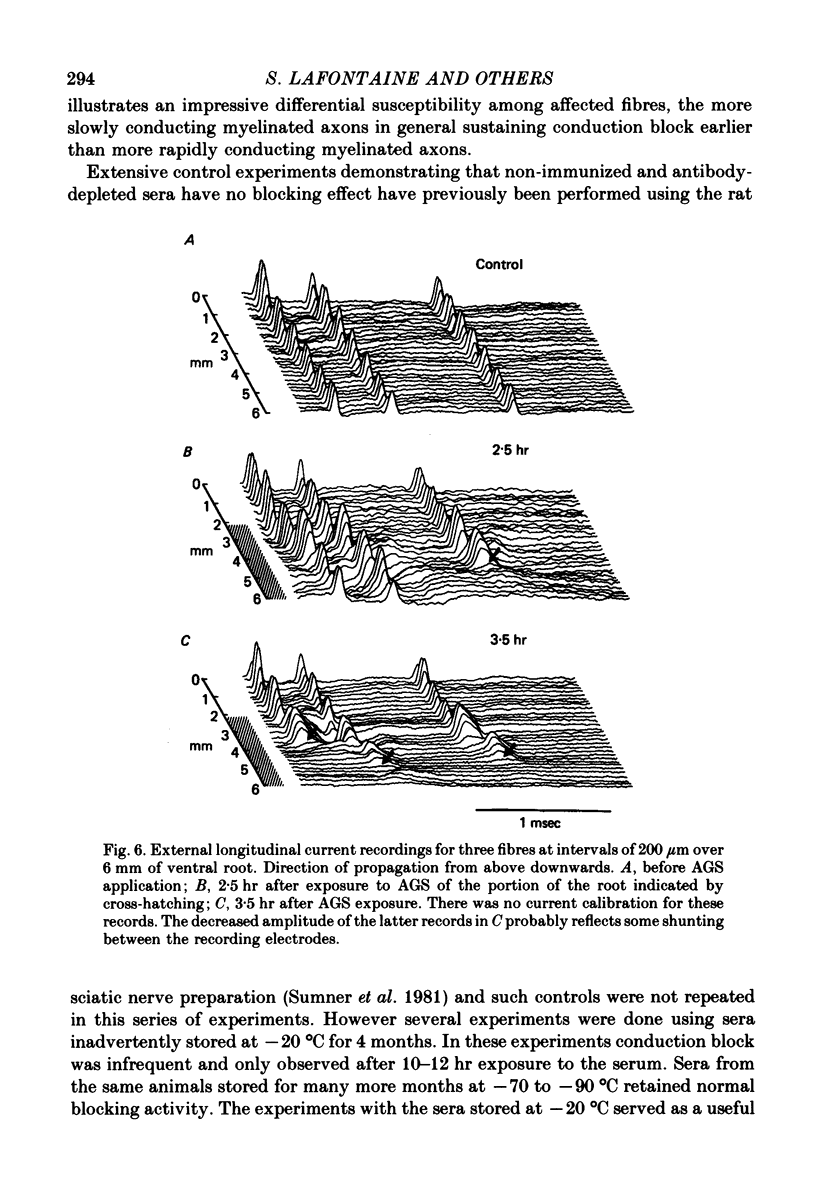
2. Conduction of nerve impulses was initially slowed and subsequently blocked at the site of serum exposure.
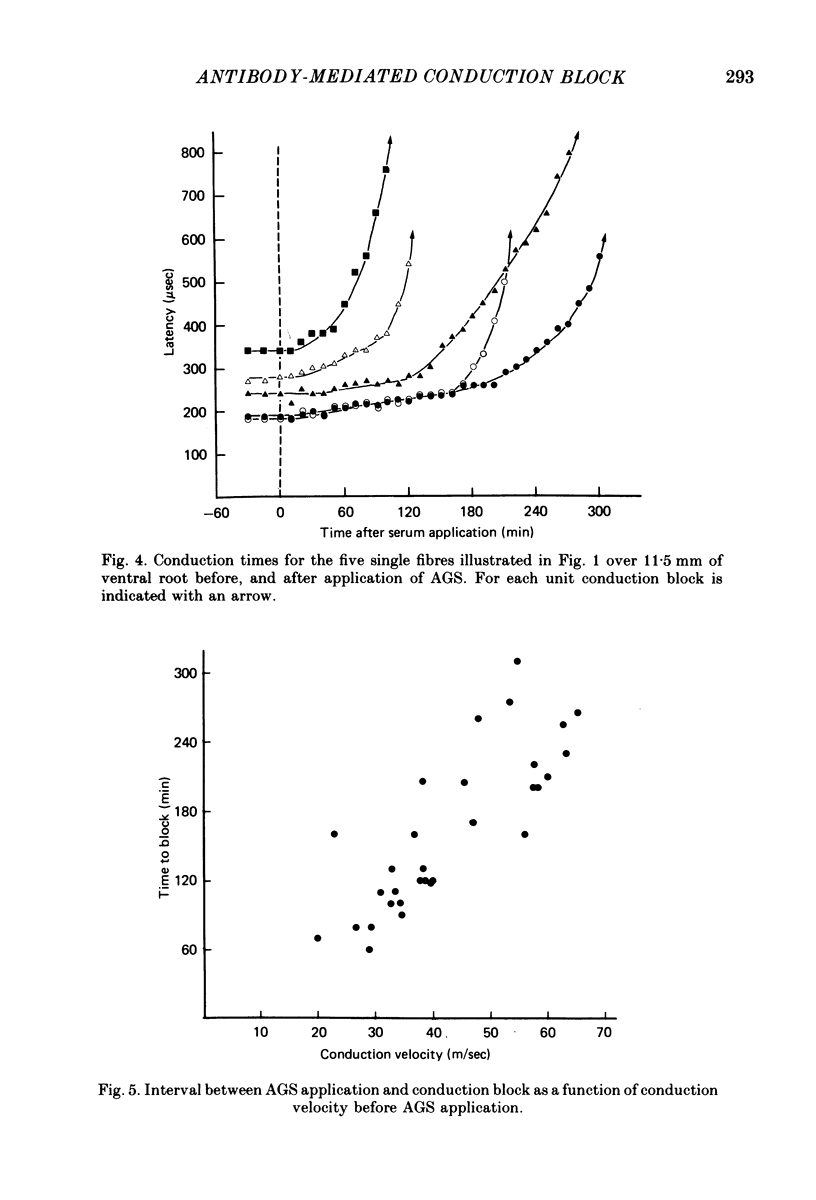
3. Conduction block occurred within as little as 1 hr in more slowly conducting (20-30 m/sec) myelinated fibres but occurred later in fibres conducting more rapidly.
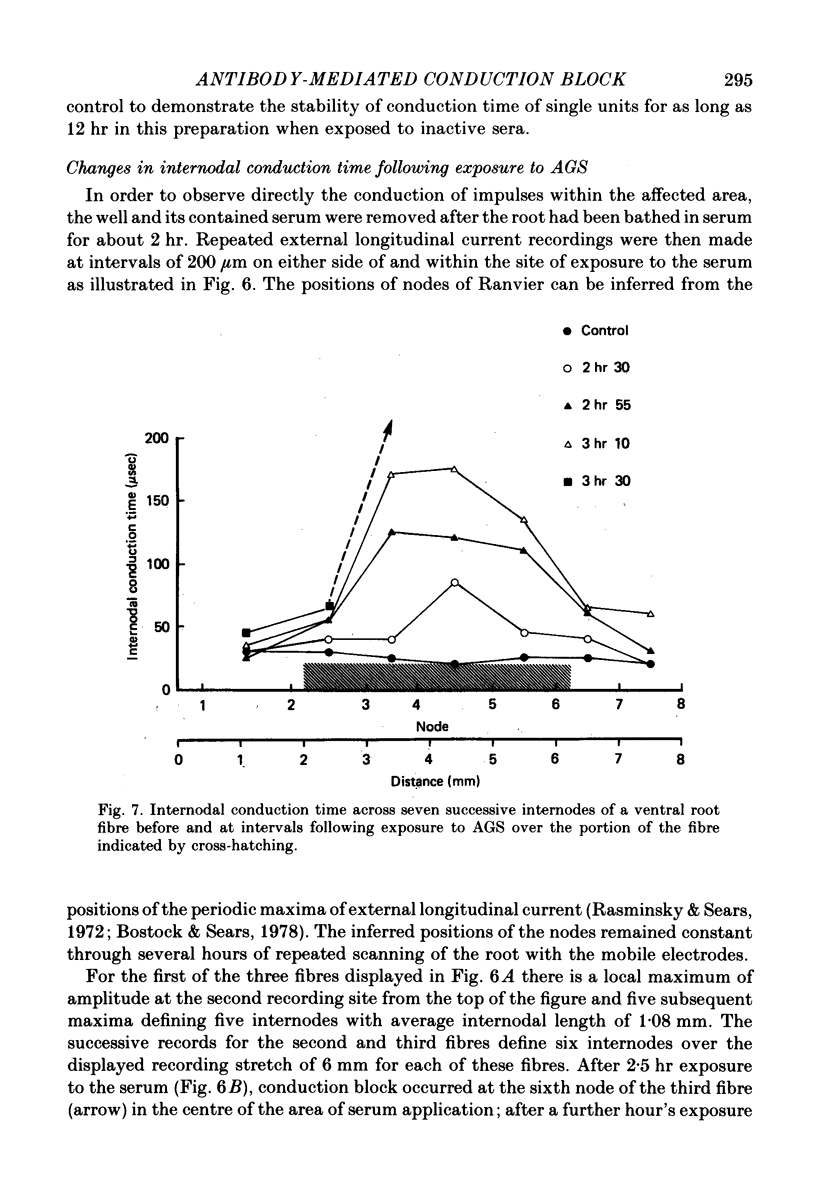
4. Conduction block was preceded by a rise in internodal conduction time from the normal 20 μsec to about 200 μsec.
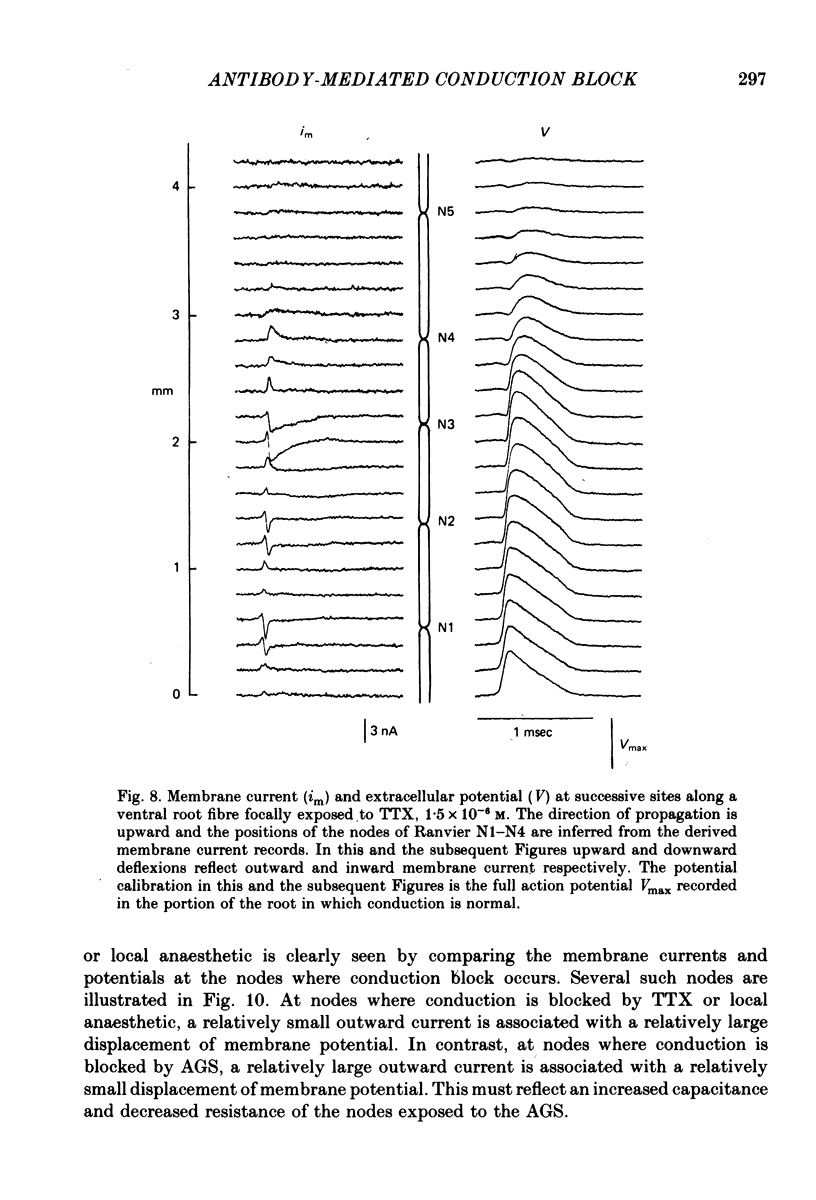
5. At nodes exposed to serum, conduction block was invariably associated with greatly decreased depolarization; this was contrasted with nodes exposed to local anaesthetic or tetrodotoxin where conduction block occurred despite nodal depolarization well beyond threshold potential.
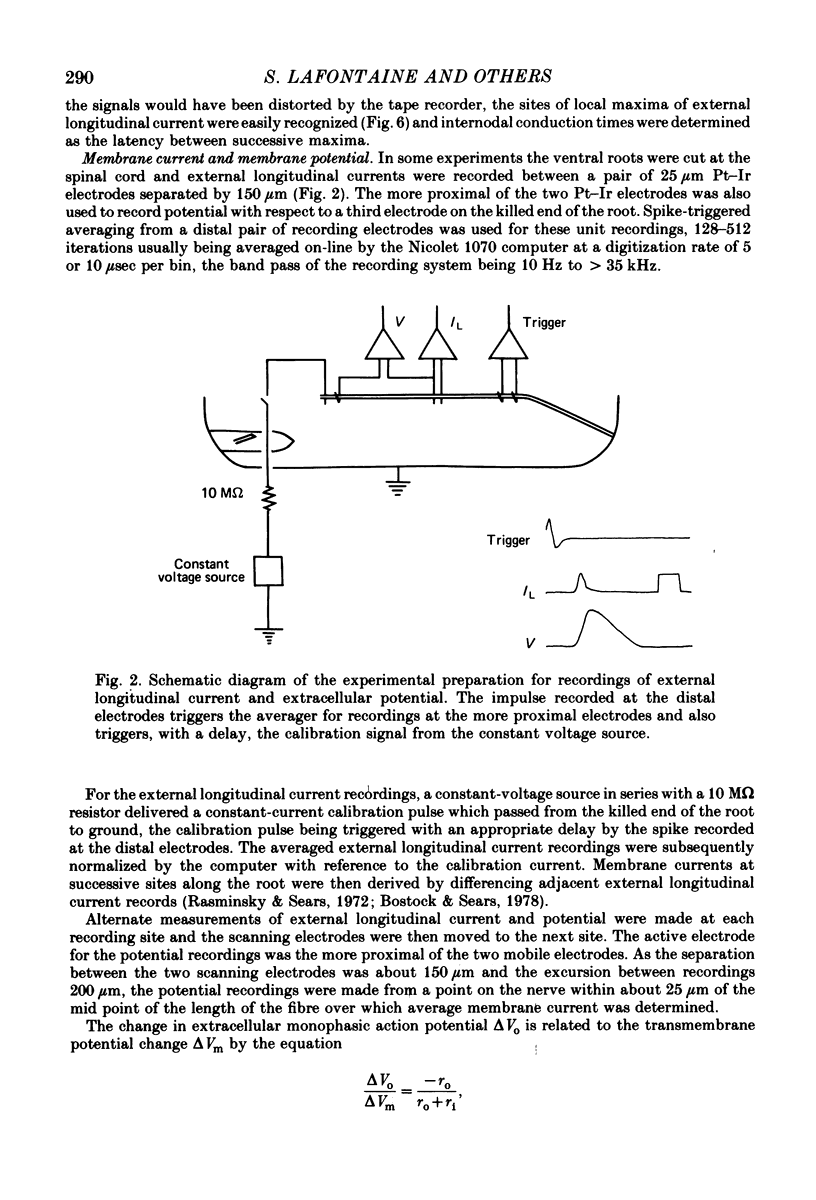
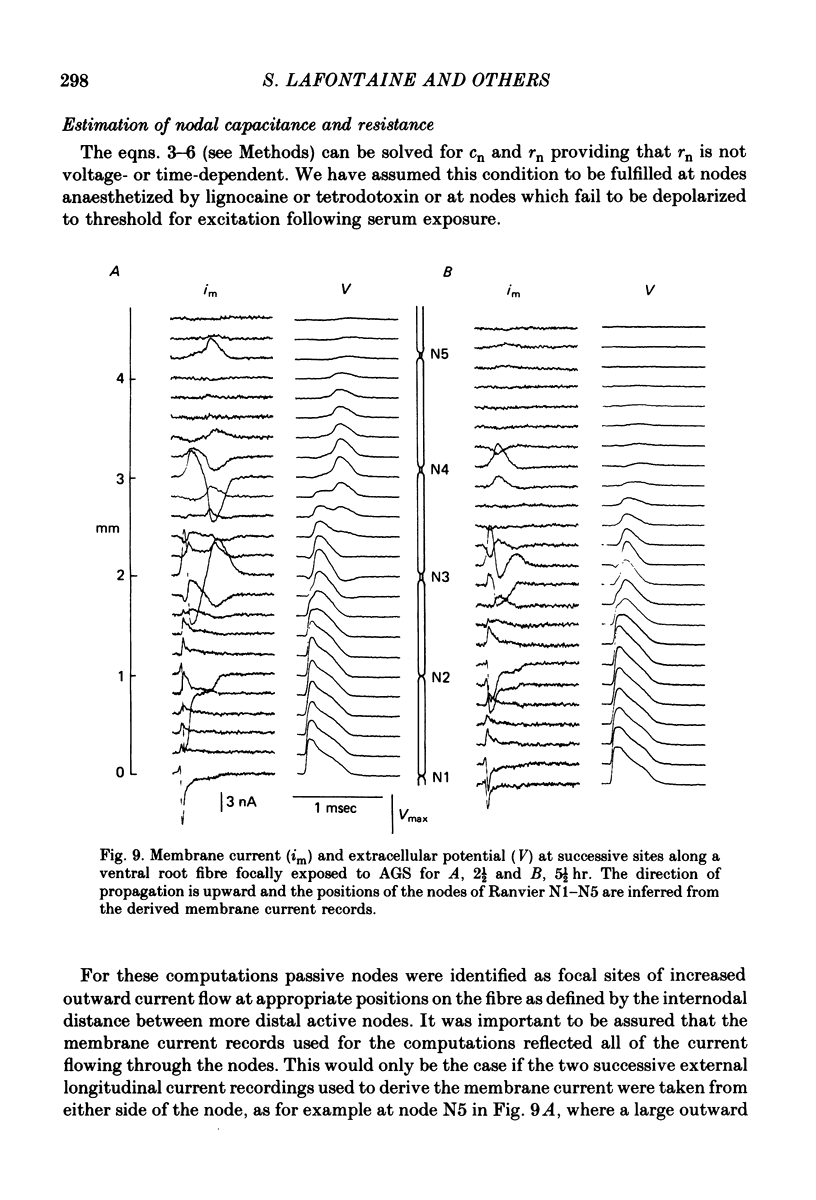
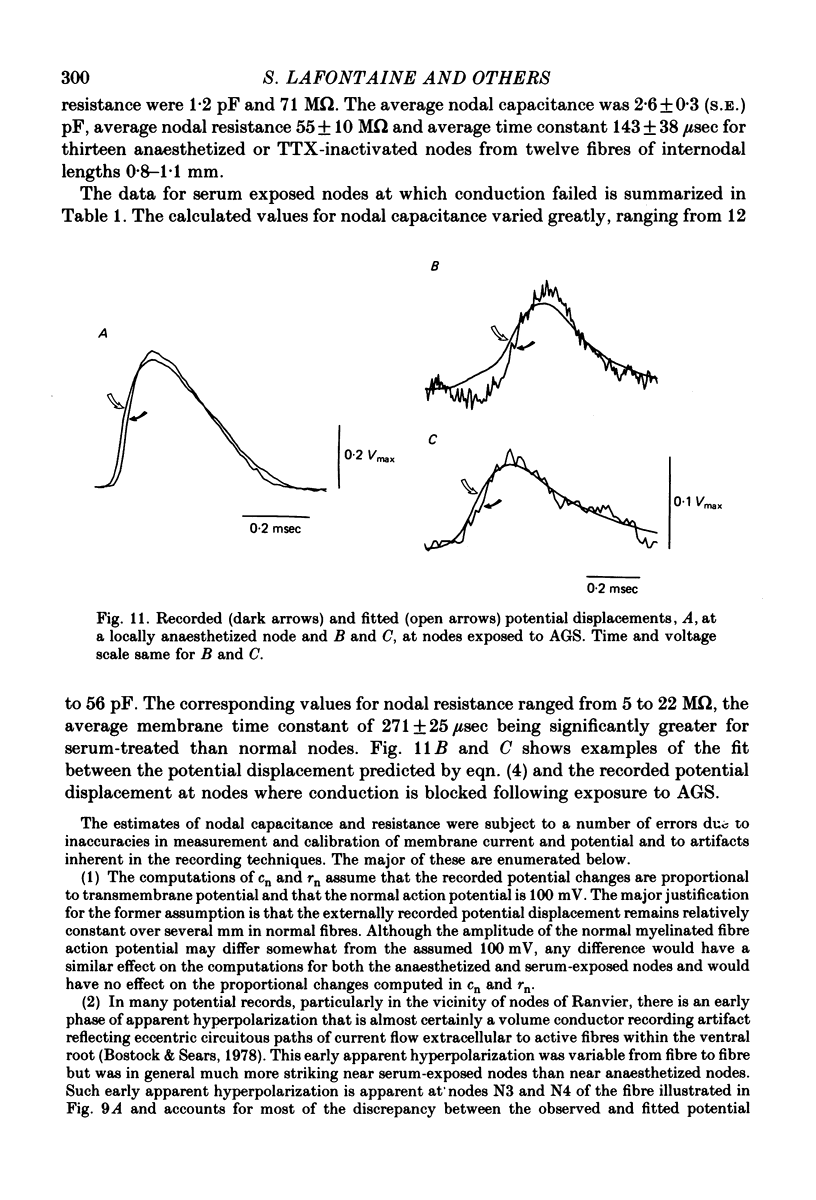
6. Nodal capacitance and resistance were estimated from simultaneous recordings of membrane current and extracellular potential at blocked nodes exposed to local anaesthetic or tetrodotoxin (normal nodes) and at blocked nodes exposed to anti-galactocerebroside serum.
7. For normal fibres of internodal length 0·8-1·1 mm, an upper limit estimate for average nodal capacitance was 2·6 ± 0·3 pF and a lower limit estimate for average nodal resistance was 55 ± 10 MΩ. There was an order of magnitude increase in the capacitance of nodes at which conduction block occurred following exposure to anti-galactocerebroside serum.
8. We conclude that the early conduction block caused by anti-galactocerebroside serum is due to paranodal demyelination and that acute paranodal demyelination is sufficient to cause conduction block.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bostock H., Sears T. A. Continuous conduction in demyelinated mammalian nerve fibers. Nature. 1976 Oct 28;263(5580):786–787. doi: 10.1038/263786a0. [DOI] [PubMed] [Google Scholar]
- Bostock H., Sears T. A. The internodal axon membrane: electrical excitability and continuous conduction in segmental demyelination. J Physiol. 1978 Jul;280:273–301. doi: 10.1113/jphysiol.1978.sp012384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H., Sherratt R. M., Sears T. A. Overcoming conduction failure in demyelinated nerve fibres by prolonging action potentials. Nature. 1978 Jul 27;274(5669):385–387. doi: 10.1038/274385a0. [DOI] [PubMed] [Google Scholar]
- Brismar T. Potential clamp analysis of membrane currents in rat myelinated nerve fibres. J Physiol. 1980 Jan;298:171–184. doi: 10.1113/jphysiol.1980.sp013074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. Y., Ritchie J. M. Evidence for the presence of potassium channels in the paranodal region of acutely demyelinated mammalian single nerve fibres. J Physiol. 1981;313:415–437. doi: 10.1113/jphysiol.1981.sp013674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. Y., Ritchie J. M. Potassium channels in nodal and internodal axonal membrane of mammalian myelinated fibres. Nature. 1980 Mar 13;284(5752):170–171. doi: 10.1038/284170a0. [DOI] [PubMed] [Google Scholar]
- Chiu S. Y., Ritchie J. M., Rogart R. B., Stagg D. A quantitative description of membrane currents in rabbit myelinated nerve. J Physiol. 1979 Jul;292:149–166. doi: 10.1113/jphysiol.1979.sp012843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koles Z. J., Rasminsky M. A computer simulation of conduction in demyelinated nerve fibres. J Physiol. 1972 Dec;227(2):351–364. doi: 10.1113/jphysiol.1972.sp010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. W., Joyner R. W., Brill M. H., Waxman S. D., Najar-Joa M. Simulations of conduction in uniform myelinated fibers. Relative sensitivity to changes in nodal and internodal parameters. Biophys J. 1978 Feb;21(2):147–160. doi: 10.1016/S0006-3495(78)85515-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasminsky M., Sears T. A. Internodal conduction in undissected demyelinated nerve fibres. J Physiol. 1972 Dec;227(2):323–350. doi: 10.1113/jphysiol.1972.sp010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasminsky M. The effects of temperature on conduction in demyelinated single nerve fibers. Arch Neurol. 1973 May;28(5):287–292. doi: 10.1001/archneur.1973.00490230023001. [DOI] [PubMed] [Google Scholar]
- Saida K., Saida T., Brown M. J., Silberberg D. H. In vivo demyelination induced by intraneural injection of anti-galactocerebroside serum: a morphologic study. Am J Pathol. 1979 Apr;95(1):99–116. [PMC free article] [PubMed] [Google Scholar]
- Saida K., Sumner A. J., Saida T., Brown M. J., Silberberg D. H. Antiserum-mediated demyelination: relationship between remyelination and functional recovery. Ann Neurol. 1980 Jul;8(1):12–24. doi: 10.1002/ana.410080103. [DOI] [PubMed] [Google Scholar]
- Sherratt R. M., Bostock H., Sears T. A. Effects of 4-aminopyridine on normal and demyelinated mammalian nerve fibres. Nature. 1980 Feb 7;283(5747):570–572. doi: 10.1038/283570a0. [DOI] [PubMed] [Google Scholar]
- Sumner A. J. The physiological basis for symptoms in Guillain-Barré syndrome. Ann Neurol. 1981;9 (Suppl):28–30. doi: 10.1002/ana.410090706. [DOI] [PubMed] [Google Scholar]


