Abstract
Topographic mapping of axon terminals is a general principle of neural architecture that underlies the interconnections among many neural structures. The Eph family tyrosine kinase receptors and their ligands, the ephrins, have been implicated in the formation of topographic projection maps. We show that multiple Eph receptors and ligands are expressed in the hippocampus and its major subcortical projection target, the lateral septum, and that expression of a truncated Eph receptor in the mouse brain results in a pronounced alteration of the hippocamposeptal topographic map. Our observations provide strong support for a critical role of Eph family guidance factors in regulating ontogeny of hippocampal projections.
Information encoding as topographic maps, which reflects the spatial organization of sensory surfaces and body effectors, is a fundamental mechanism of nervous system function (1, 2). Such topographic representation is critically dependent on precise ordering of axon projections, which maintains the spatial organization of neurons in synaptic connections with target neurons. It has been suggested that topographic axon connections allow transfer of spatial information in the sensory systems (3). In the eye, retinal axons form an inverted projection map in the tectum, with dorsal axons projecting to the ventral tectum, and ventral axons to the dorsal tectum. Similarly, retinal ganglion neurons in the temporal and nasal sides send axons to the anterior and posterior regions of the tectum, respectively (4). Comparable mechanisms may also operate in the limbic circuits, where learning, memory, and emotional responses are elaborated. For example, the hippocampus sends axons topographically to the lateral septum, with dorsomedial neurons projecting to the dorsomedial area of the target, and ventrolateral neurons projecting to the ventrolateral septal region (5, 6). Similarity in organizational principles between limbic circuits and sensory systems suggests that learning, memory, and emotions may be governed by the same spatial constraints as sensory information processing.
Based on analyses of regenerating retinal axons, Roger Sperry (7) proposed that axons and target neurons carry specific cytochemical identification tags, and that each axon makes synaptic connections only with neurons carrying matching affinity tags. These tags may be distributed as gradients in the projecting and target fields to specify topographic maps. Recent expression and functional studies suggest that ephrins and their receptors serve as the postulated chemoaffinity guidance tags. The ephrins are a family of cell surface molecules that are ligands of the Eph family tyrosine kinase receptors (8). The Eph receptors, EphA3 in the chicken and EphA5/A6 in the mouse retina, are expressed in a nasal (low) to temporal (high) gradient (3, 9, 10). Complementary to the receptor gradient, the ligands ephrin-A2 and -A5 constitute an anterior (low) to posterior (high) gradient in the optic tectum (9, 11–13). Disruption of the ligand gradient by retrovirus-mediated gene expression or by gene manipulation techniques resulted in mistargeting of retinal axons (3, 14–19). These analyses suggest critical roles of ephrins and their receptors in regulating development of retinotectal topographic map.
We have shown previously that in the hippocamposeptal topographic map, EphA5 is expressed in a lateral (low) to medial (high) gradient, whereas ephrin-A2, -A3, and -A5 form a complementary dorsomedial (low) to ventrolateral (high) gradient in the lateral septum (12, 20). The expression patterns parallel that of EphA3–ephrin-A2/-A5 in the retinotectal map (reviewed in ref. 21), suggesting that topographic projections in different neural systems may share similar developmental mechanisms. To elucidate how ephrins and receptors may regulate the development of the hippocamposeptal map, we examined the expression of EphA receptors and ligands during development and generated transgenic mice expressing a dominant-negative EphA5 mutant receptor. We report here that the hippocamposeptal map may be specified by a combination of multiple Eph receptors and ligands expressed in the projecting and target fields, and that inhibition of the EphA receptor function induces mistargeting of hippocampal axons. These observations indicate that the ephrins and Eph receptors play fundamental roles in topographic map formation in multiple neural systems.
Materials and Methods
In Situ Hybridization and Semiquantitative Analysis.
Localization of transcripts encoding the Eph family receptors and ligands was performed by using in situ hybridization methods as described (22). Anti-sense riboprobes were used for EphA3, EphA4, EphA5, EphA7, EphA8, and ephrin-A5. Antisense oligonucleotide probes were used for EphA6, ephrin-A2, -A3, and -A4. The hybridization conditions have been shown previously to provide specific signals (12, 23). Corresponding sense probes were used as controls.
Analysis of Receptor Tyrosine Kinase Activation.
COS-1 cells in 60-mm tissue culture dishes were transfected with 2 μg of wild-type EphA3 or EphA5 in pcDNA1amp vector (Invitrogen), under the transcriptional control of a cytomegalovirus promoter, along with equal amount of kinase deficient EphA5 mutant [EphA5(K−)] DNA in the same vector as indicated in Fig. 3. Two sets of parallel transfections were performed. One set was used to examine levels of protein expression of wild-type EphA5 under these conditions, and the remaining set was used to characterize EphA5 tyrosine kinase activation. To examine protein expression, transfected COS-1 cells were first cultured in methionine/cysteine-free medium containing 1% dialyzed FBS (GIBCO/BRL) for 30 min, and then in the same medium containing 100 μCi/ml [35S]methionine and cysteine (>1,000 Ci/mmol, NEN Life Science Products; 1 Ci = 37 GBq) overnight at 37°C with 5% CO2. The cells were washed three times with ice-cold PBS, scrubbed off, and collected by centrifugation at 2,000 rpm in a clinical centrifuge (International Equipment) at 4°C. The cell pellet was then resuspended in lysis buffer (1% Nonidet P-40/150 mM NaCl/50 mM Tris⋅Cl, pH8.0/1 μg/ml aprotinin/10 μg/ml Leupeptin/20 μg/ml Phenylmethane-sulfonyl fluoride/1 mM sodium orthovanodate). Wild-type EphA receptor proteins were immunoprecipitated with anti-EphA3 (Santa Cruz Biotechnology) or EphA5 (against a C-terminal peptide) antibodies, fractionated on SDS/PAGE, and exposed to x-ray film.
Fig 3.
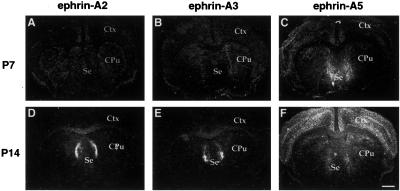
Spatial and temporal patterns of expression of three A-ephrins in the developing septum. (A–C) Expression of ephrin-A2, -A3, and -A5 in the P7 mouse septum. Note that at this stage, no ephrin-A2 and -A3 were detected. (D–F) Expression of ephrin-A2, -A3, and -A5 in the P14 septum. Ctx, cerebral cortex; Cpu, caudate putamen; Se, septum. (Scale bar: 1.5 mm.)
To examine the effect of EphA5(K−) on wild-type receptor activation, transfected cells were treated with 500 ng/ml crosslinked ephrin-A5-Fc for 30 min at 37°C 2 days after transfection. The ligand was crosslinked by incubating ephrin-A5-Fc with anti-human Fc anti-body in a 10:1 ratio (30 min at room temperature). After ligand stimulation, the cells were lysed and immunoprecipitated as described above. For analysis of Eph receptor activation in transgenic mice, dissected hippocampi were similarly lysed and immunoprecipitated. The immunoprecipitates were fractionated on SDS/PAGE, and analyzed with a Western blot technique using anti-phosphotyrosine monoclonal antibody 4G10 (Upstate Biotechnology, Lake Placid, NY) in conjunction with alkaline phosphatase-conjugated anti-mouse IgG secondary antibody.
Generation of Transgenic Mice Expressing EphA5 Dominant-Negative Mutant Receptor.
A dominant-negative mutant of EphA5 was constructed by PCR using an upstream and a downstream primer. The upstream primer, 5′-GC GCA TCG ATC GCC ACC ATG GCA CGG GGC TCC GGG CCC CGC GGT GC-3′, contains a ClaI restriction site and a consensus Kozak sequence for translation (25, 26). The oligonucleotide sequence has homology with EphA5 from position 418–443 and includes the translation initiation codon ATG (24). The downstream primer, 5′-GGCC CTT AAT TAA GCA TGA AGC TTC AAT CTC CTT CGC AAA TTC-3′, contains a PacI restriction site, and is homologous with mouse EphA5 from nucleotide position 1927–1956 (24). The PCR fragment was then cloned into pEGFP-N1 vector (CLONTECH), to fuse in frame with the green fluorescence protein (GFP) at the BamH1 site (102 nt downstream of the EphA5 transmembrane domain). The fusion protein contains the extracellular ligand-binding and transmembrane domains of mouse EphA5, as well as the GFP coding sequence. The entire fusion gene was then cloned into the P253 vector kindly provided by Freda Miller (Montreal Neurological Institute, Montreal) under the transcriptional control of a neuron-specific α-tubulin promoter (27).
To generate transgenic mice, the purified transgene fragment was microinjected into fertilized (C57BL/6J × C3H/HeJ) F1 mouse eggs as described (28). Tail DNA from mice born from injected embryos were screened for the presence of transgene by using Southern blot analysis. A GFP probe that was not present in wild-type mice was used in these screens. Eleven founders with the transgene integrated were obtained. Among these mice, two lines, Ag30-61 and Ag00-72, which express higher levels of transgene as judged by the fluorescence intensity in the brain, were chosen for further analyses. Mice were screened for presence of transgene initially by using PCR. The upstream primer, 5′-ATG GTG AGC AAG GGC GAG GAG-3′, is from the junction region of EphA5 and GFP. The downstream primer, 5′-GAA GAT GGT GCG CTC CTG GAC-3′, is 298 bp downstream of upstream primer. Southern blot analysis was also performed by using both probes from the EphA5 extracellular domain or from the GFP region (Fig. 4A).
Fig 4.
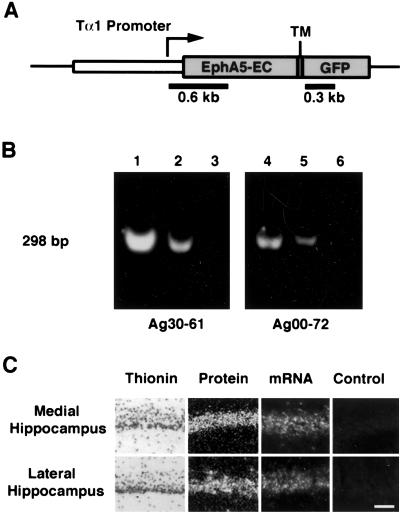
Expression of a truncated EphA5 receptor in the mouse brain. (A) Schematic illustration of the transgene construct. The extracellular (EC) and transmembrane domains (TM) of EphA5 was fused in frame to an enhanced GFP. The EphA5–GFP fusion gene is placed under the control of the neuron-specific α-tubulin promoter (Tα1). (B) Detection of transgene DNA. Two independent transgenic mouse lines express high levels of transgene, as shown by PCR. PCR analysis produced an expected 298-bp fragment that is highest in homozygous mice (lanes 1 and 4), intermediate in heterozygous mice (lanes 2 and 5). No PCR fragments were detected in the wild-type littermates (lanes 3 and 6). DNA probes from the EphA5 and GFP regions as indicated were also used in Southern blot analysis to confirm PCR results. (C) mRNA and protein expression of EphA5–GFP transgene in the hippocampus. The mRNA was detected with antisense probe specific to the GFP portion of the transgene. The fusion protein was visualized by using fluorescence microscopy. There are no differences in expression between the medial and lateral hippocampal pyramidal neurons. Control nontransgenic hippocampus showed only faint background fluorescence. (Scale bar: 50 μm.)
DiI Tracing.
DiI solution (10% in N,N-dimethylformamide, 40 nl) was injected into the medial or lateral hippocampus according to Franklin and Paxinos (29). Injected mice were allowed to survive for 2 days, and were then perfused with 4% paraformaldehyde in PBS. The brains were later dissected and placed in the same fixative solution at 37°C for 4 weeks. The brains were then sectioned at 100-μm thickness with a vibratome, and sections were examined with a Zeiss Axioskop equipped with a rhodamine filter.
Results
Multiple EphA Subfamily Tyrosine Kinase Receptors Are Expressed in the Developing Hippocampus.
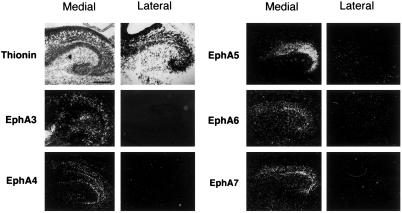
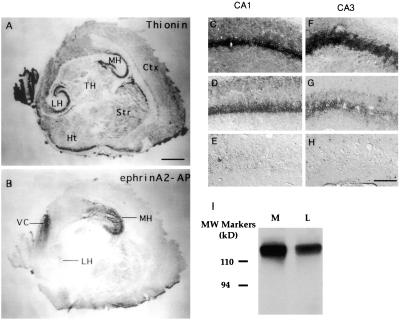
To examine the potential roles of ephrins and Eph receptors in the development of hippocamposeptal topographic maps, we examined the expression of the Eph receptors of the A-subfamily in the hippocampus. We observed that five receptors, EphA3, -A4, -A5, -A6, and -A7, are transcribed in lateral (low) to medial (high) gradients in late embryonic and early postnatal hippocampus (Fig. 1 and data not shown), a critical time for establishing hippocampal projection to the lateral septum (30). The expression gradients were found in the CA1 and CA3 neurons, both of which project topographically to the lateral septum. The expression gradients were maintained at later stages of development for all but EphA4. At postnatal day (P) 7 and later stages, EphA4 is expressed in both the lateral and the medial hippocampal neurons (data not shown). The presence of multiple Eph gradients in the same orientation suggests that these receptors may have redundant or interacting functions. To compare the relative overall levels of EphA receptors in the medial and lateral hippocampus, we used ephrin-A2–AP, a ligand of the EphA receptors fused to human placental alkaline phosphatase, to detect receptor protein (31). Consistent with the mRNA expression, significantly higher levels of Eph receptor proteins were found in the medial than the lateral hippocampus (Fig. 2 A and B). To examine the levels of individual receptor proteins in the hippocampus, we analyzed expression of a EphA5–lacZ fusion protein in a LacZ knock-in mouse strain. In these mice, β-gal gene was fused onto EphA5 extracellular domain, and the fusion protein is expressed under the control of the native EphA5 promoter (N.W.G. and G.D.Y., unpublished work). This analysis showed that EphA5 is expressed in a medial (high) to lateral (low) gradient in both CA1 and CA3 (Fig. 2 C–H). Western blot analysis with an anti-EphA3 antibody also showed higher levels of expression in medial than the lateral hippocampus (Fig. 2I). These observations strongly support a graded expression of the EphA receptors in the hippocampus.
Fig 1.
Differential expression of the Eph receptors in the medial and lateral hippocampus. Sagital mouse brain sections of different developmental stages were hybridized with radio-labeled anti-sense probes of 6 EphA receptors (EphA3-A8). The expression of EphA1 and -A2 was not examined because they lack significant expression in developing brain (8). Top two panels on the left show bright field photomicrographs of E18 medial and lateral hippocampus, respectively. The hybridized parasagittal sections were stained with thionin to show hippocampal cytoarchitecture. Other panels show darkfield images of the E18 brain sections hybridized with anti-sense receptor probes indicated. Sense controls showed no specific signals. Medial, medial hippocampus; Lateral, lateral hippocampus. (Scale bar: 250 μm.)
Fig 2.
EphA receptor protein expression in the hippocampus. (A and B) Ephrin-A2 binding revealed high EphA receptor protein levels in the medial hippocampus (MH), and low levels in the lateral hippocampus (LH). A neighboring section was stained with thionin to reveal major cytoarchitecture features (A). (C–H) EphA5-LacZ expression in the medial (C and F), intermediate (D and G), and lateral (E and H) CA1 (C–E) and CA3 (F–H) regions. (I) EphA3 protein levels in the medial (M) and lateral (L) hippocampal lysates. EphA3 protein was first precipitated from 200 μg of E18 medial or lateral hippocampal lysates, and then analyzed with Western blot using EphA3 antibody. (Scale bars: A, 500 μm; H, 62.5 μm.)
Three Ephrin-A Ligands Are Transcribed in the Lateral Septum in Spatially and Temporally Distinct Patterns.
We further defined the expression of the ephrin-A ligands in the subcortical hippocampal target, the lateral septum, during development of hippocamposeptal projections. Transcripts of ephrin-A5 began to appear as early as embryonic day (E) 16, although the levels were low (data not shown). By E18, a clear dorsal (low) to ventral (high) gradient of ephrin-A5 expression was detected (Fig. 3). This pattern of expression was maintained through P14, and decreased to a lower level in the adult (data not shown). Ephrin-A5 expression was found in most areas of the ventral lateral septum. Two other ephrins, A2 and A3, were also detected in the lateral septum (Fig. 3). Both ephrins were first detected around P9, and the expression was maintained through adulthood (12). The highest expression of these two ephrins was found along the lateral edge of the lateral septum, with lower levels in the dorsal and medial target (Fig. 3). These distinct spatial and temporal patterns of ephrin expression suggest that the hippocamposeptal map may form in different stages.
Construction of Transgenic Mice Expressing a Dominant-Negative Mutant EphA5 Receptor.
To critically examine the roles of the Eph family receptors and ligands in the development of the hippocamposeptal map, we studied the effect of expressing a truncated EphA5 receptor. Because there are at least five related EphA receptors expressed in similar lateral (low) to medial (high) gradients, knockout experiments inactivating single genes may not be informative because of potential functional compensations. Truncated receptors have been used widely to interfere with endogenous receptor tyrosine kinase functions (32–36). Thus, a truncated EphA5 receptor, EphA5(K−), containing only the extracellular and transmembrane domains, but lacking the active kinase domain, was generated. The truncated EphA5 was fused in frame to the enhanced GFP to facilitate detection (details in Materials and Methods). To express the EphA5(K−) gene in neurons, we placed the mutant receptor under the transcriptional control of the neuron-specific α-tubulin promoter (Fig. 4), and generated transgenic mouse lines. Among 11 founder mice, two lines, Ag30–61 and Ag00–72, which expressed relatively high levels of the transgene, were established (Fig. 4 B and C). Transgene expression was detected at high levels in the hippocampus (Fig. 4C), although expression in other brain regions, including the cerebral cortex, was also detected (data not shown).
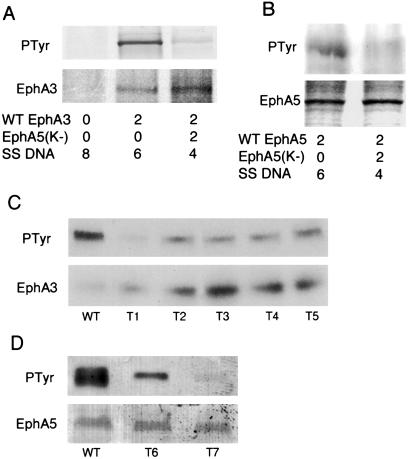
To examine whether EphA5(K−) inhibits wild-type EphA receptor function, we transfected the mutant together with wild-type EphA3 or EphA5 receptors into COS-1 cells. Two days after transfection, the cells were stimulated with crosslinked ephrin-A5-Fc protein, and then lysed and immunoprecipitated with anti-EphA3 or EphA5 antibodies. The immunoprecipitates were then examined for receptor tyrosine phosphorylation by using Western blot analysis. Expression of the EphA5(K−) dominant-negative construct suppressed the activation of wild-type receptors efficiently (Fig. 5). In the presence of equal amounts of wild-type and EphA5(K−) receptors, tyrosine phosphorylation on both EphA3 and EphA5 was greatly reduced. To examine whether EphA5(K−) inhibits endogenous Eph receptor activation in transgenic mice, EphA3 and EphA5 were immunoprecipitated from hippocampal lysates of individual normal or transgenic mice and similarly analyzed for receptor tyrosine kinase phosphorylation. Tyrosine phosphorylation was reduced for both EphA3 and EphA5 in all of the transgenic mice examined, though the extent of reduction varied (Fig. 5 C and D).
Fig 5.
Inhibition of wild-type EphA3 and A5 activation by a truncated EphA5 receptor. (A) Cotransfection of EphA5(K−) DNA inhibits activation of EphA3 receptor tyrosine kinase. (B) Cotransfection of EphA5(K−) DNA inhibits activation of EphA5 tyrosine kinase. (Top) Tyrosine kinase activation in the absence of EphA5(K−) and the lack of activation in the presence of Eph(K−), as indicated in Bottom (DNA transfected in μg). (Middle) Similar wild-type receptor protein synthesis in the presence and absence of EphA5(K−). The proteins were labeled with [35S]methionine and cysteine for analysis of expression levels (Middle). (C and D) Tyrosine phosphorylation of EphA3 (C) and EphA5 (D) is reduced in the trangenic hippocampus. Hippocampi from each wild-type control (WT) or transgenic (T1-T7) mice were analyzed separately. (Upper) Eph receptor immunoprecipitates analyzed with anti-phosphotyrosine antibody. (Lower) The blots were reprobed with anti-EphA3 (C) or EphA5 (D) antibody to show the protein levels of the receptors.
Hippocampal Axons Mistarget in EphA5(K−) Mice.
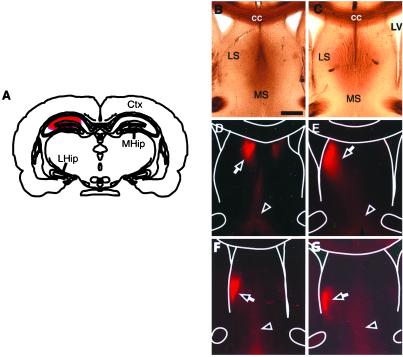
The homozygous mice with the transgene in both chromosomes develop to term normally, but a small percentage (around 5%) die between 1 and 2 months postnatally. However, the heterozygous mice (carrying the transgene in only one chromosome), developed normally, reproduced well, and lacked any apparent motor defects (data not shown). To examine the effects of transgene expression on hippocampal projections, we labeled medial or lateral hippocampal regions with DiI to trace the axon terminals in the lateral septum in the adult transgenic mice. The injected brains were then sectioned and analyzed for the position of hippocampal axon terminals in the septal target. In the wild-type littermates, medial hippocampal axons terminated in the medial dorsal corner of the lateral septum (n = 9) (Fig. 6). In contrast, in the transgenic mice, the terminal field of the medial axons moved ventrally and laterally in 6 of the 13 mice analyzed (Fig. 6), consistent with roles of Eph receptors/ligands in restricting receptor-rich medial hippocampal axons. However, tracing of the lateral hippocampal axons revealed no obvious differences between the transgenic (n = 7) and wild-type mice (n = 10). Thus, the function of Ephs/ephrins appears to be critical for the targeting of the medial hippocampal axons, but not for the lateral axons, a result consistent with the preferentially high levels of expression of EphA receptors in the medial neurons. In addition, the contralateral projection by the hippocampal axons is reduced (Fig. 6), suggesting that axon crossing of the midline is also affected.
Fig 6.
Mistargeting of hippocampal axons in the lateral septum. (A) Position of DiI injection in the medial hippocampus. (B and D) Bright-field and fluorescence pictures of septal sections from control mice with DiI injected in the medial hippocampus. (C and E–G) Bright-field and fluorescence photomicrographs of septal sections from three different transgenic mice with medial hippocampal DiI tracing. Note the shift of the terminal zone ventrally and laterally. Anatomic landmarks are marked with white lines in D–G for reference. Ctx, cerebral cortex; LHip, lateral hippocampus; MHip, medial hippocampus; LV, lateral ventricle; LS, lateral septum; MS, medial septum; cc, corpus callosum. Arrows indicate terminal area of hippocampal axons. Arrowheads denote retrograde labeling of septo-hippcampal axons and neurons. (Scale bar: 500 μm.)
Discussion
The ligands and receptors of the Eph family of tyrosine kinases have been shown to regulate development of topographic maps in the visual system (reviewed in refs. 8, 21, and 37–39). In this study, we provide evidence that multiple Eph receptors and ligands are expressed in opposing patterns in the hippocampus and the major subcortical target, the lateral septum, and that expression of a truncated EphA5 leads to mistargeting of medial hippocampal axons, suggesting that Eph receptors and ligands also regulate hippocamposeptal topographic map formation. Our observations, along with previous studies in other laboratories, point to a universal role of Eph family molecules in regulating topographic map formation in the nervous system.
Multiple Eph Receptors Mark Hippocampal Axons.
Our in situ hybridization analyses revealed that there were several EphA subfamily receptors, EphA3, EphA4, EphA5, EphA6, and EphA7, are expressed in lateral (low) to medial (high) gradients during embryonic development. It is particularly interesting that all receptor expression gradients exhibit the same orientation, suggesting functional redundancy. Indeed, analysis of mice with EphA5 deletion showed normal mapping of hippocampal axon terminals (Y.Y., N.W.G., and R.Z., unpublished results). It is unclear at present why multiple receptors are expressed in similar gradients. It is possible that these receptors also function to regulate intrahippocampal projections or projections to other hippocampal targets. Furthermore, different combinations of these receptors may confer distinct responses to the ligands. The patterns of EphA receptor expression is analogous to that in the retinal ganglion neurons, where EphA3 in the chicken or EphA5 and EphA6 in the mouse are expressed in low nasal to high temporal gradients (3). The graded expression of EphA receptors is indicative of a function of these receptors in regulating hippocamposeptal topographic map formation.
Analysis using an ephrin-A2-alkaline phosphatase (AP) fusion protein indicated that the composite receptor protein level is higher in the medial than the lateral hippocampus. The receptor gradient is further supported by analysis of a LacZ knock-in mutant of EphA5 and Western blot analysis of EphA3 protein levels in the medial and lateral hippocampus. The differential protein levels are consistent with data from in situ hybridization studies showing multiple receptors are expressed in lateral (low) to medial (high) gradients. Consequently, hippocampal neurons from different mediolateral positions are differentially marked by different levels of Eph receptors, consistent with a function as topographic positional tags (7).
Spatial and Temporal Dynamics of Ephrin-A Ligand Expression.
We reported previously that at least three different A-ephrins were expressed in the subcortical hippocampal target, the lateral septum in adult mice (22). However, whether ligand expression was regulated developmentally was undefined. We now show that only ephrin-A5 is expressed at E18, when hippocampal axons just arrive at the target (30). Ephrin-A2 and -A3 are transcribed only around P7. The differential expression of the ephrins in time and space suggest that the hippocamposeptal topographic map may be specified in two distinct stages. Because ephrin-A5 is expressed in a dorsal (low) to ventral (high) gradient, with only minor variation between medial and lateral target, hippocampal axons may be guided along the dorsoventral axis when they first arrive. A mediolateral topography may be established later by the expression of ephrin-A2 and -A3 along the lateral edge of the target. In the adult, ligand proteins detected by EphA5-AP binding appear as a composite gradient of all three ligands (22). We showed previously that these three ligands have similar activity on hippocampal axons (42, 50). In addition, in vitro binding studies suggest these ligands have similar biochemical activity (40). Thus, these three ligands may function in combination spatially or temporally to define the hippocampal axon target field.
Expression of a Truncated EphA Receptor Alters Hippocampal Axon Terminal Positions.
In an effort to critically assess the roles of ephrin-A ligands and receptors in hippocamposeptal topographic mapping, we generated transgenic mice expressing a truncated form of EphA5 receptor. By using this approach, we aim to circumvent the problem of functional compensation caused by the expression of multiple EphA receptor gradients in the hippocampus. We showed that this mutant receptor inhibited activation of wild-type EphA receptors both in vitro and in transgenic mice. This finding is consistent with several previous studies that kinase-null mutant receptors inhibit activation of wild-type receptors both in vitro and in transgenic animals (32–36). The truncated receptor may inhibit endogenous receptor function by several potential mechanisms. First, it may compete for ligand binding, and reduce the availability of ligands to the endogenous receptors. Second, it may form heterodimers with wild-type receptors, and inhibit activation of these receptors. It has been shown that heterodimerization occurs between two different Eph receptors (46). Third, it may be expressed in the target cells and mask ephrins expressed in the ventral lateral septum. It is also possible that the transgene serves as a ligand to regulate ephrin functions, which, in turn, leads to mistargeting through a yet undefined mechanism. Data presented in this study support the notion that the truncated receptor inhibits endogenous receptor function by either heterodimerization or ligand masking, but does not rule out the possibility of serving as a ligand. No matter what the mechanisms are, this study demonstrates an important role of EphA and ephrin-A in the specification of hippcomaposeptal topographic map formation.
Two transgenic lines expressing high levels of the transgene showed similar hippocampal projection defects, namely, medial hippocampal axon terminals shifted ventrally. This phenotype is only partially penetrant. In addition, varying degrees of targeting defects were observed. This variation is likely caused by differences in expression levels of the transgene, as indicated by the variation of the extent of inhibition of endogenous receptor activation. The ventroward shift of medial hippocampal axon terminals is consistent with an inhibitory function of the EphA receptors on the migration of these axons. Because medial hippocampal neurons express high levels of EphA receptors, they are normally restricted to the ligand-poor dorsomedial target. Expression of the truncated EphA5 inhibits endogenous receptor function, and consequently allows termination in a more ventral position. However, one would predict a wider spread of medial axon terminals throughout the dorsoventral space, if the ligands simply prevent axons from moving ventrally. Rather, our observations are consistent with the servomechanism model proposed by Honda (41). According to this model, ephrins may possess both positive and negative effects on axons. Axons terminate when the receptor (R)–ligand (L) interaction reaches a certain strength (S). When R–L < S, axons are attracted by the ligands and migrate in the direction of increasing ligand concentrations to increase the L value. When R–L > S, axons have moved beyond the proper target region, and are induced to grow toward the direction of decreasing ligand concentrations to reduce the L value. Evidence that ephrins may have positive effects on axons has been provided by recent studies using cortical and hippocampal neurons (42, 43, 49). Ephrin-A2, -A3, and -A5 all promoted growth and branching of medial hippocampal axons initially (42). But with prolonged exposure, axon retraction/fragmentation occurred. This dynamic pattern of initial growth, branching, and later degeneration is consistent with the servomechanism model. When medial axons are first exposed to ephrins, the axons are stimulated to grow and branch, because the signal strength is likely to be weak. With prolonged exposure, the signal strength may accumulate to high levels beyond the S value, and consequently axon retraction is induced. Branching of cortical layer 6 neurons is also promoted by ephrin-A5, though it is not known at present how stable these branches are (43). It is possible that under certain conditions, ephrins may have only positive effects. We showed previously that the A-ephrins have trophic activity on sympathetic neurons and promote early cortical axon growth in vitro (44, 49). An earlier study also showed that purified ephrin-A1 stimulated neurite outgrowth of spinal motor neurons (45). In addition, a truncated form of EphA7 mediates attractive interaction with ephrin-A5 in neural tube closure during development (47). The ventroward shift may reflect attractive interactions between EphA5(K−) transgene protein with the ligands in the septal target, in the absence of repulsive interactions caused by the inhibition of endogenous EphA receptors. In light of these findings, we speculate that with this type of dual activity, counter ephrin and Eph gradients are sufficient to specify topograhic maps, though other guidance cues are likely to participate (48).
Acknowledgments
We thank Elena Pasquale for her generous gifts of EphA3 cDNA and antibody, and Claudio Pikielny and Manny DiCicco-Bloom for critical readings of the manuscript. Research support from the National Institutes of Health and Center of Excellence for Autism, New Jersey Governor's Council on Autism (UMDNJ) (to R.Z.).
Abbreviations
GFP, green fluorescent protein
References
- 1.Kaas J. H. (1997) Brain Res. Bull. 44, 107-112. [DOI] [PubMed] [Google Scholar]
- 2.Kaas J. H. (1999) in The New Cognitive Neurosciences, ed. Gazzaniga, M. S. (MIT Press, Cambridge, MA), pp. 223–236.
- 3.Feldheim D. A., Kim, Y. I., Bergemann, A. D., Frisen, J., Barbacid, M. & Flanagan, J. G. (2000) Neuron 25, 563-574. [DOI] [PubMed] [Google Scholar]
- 4.Udin S. B. & Fawcett, J. W. (1988) Annu. Rev. Neurosci. 11, 289-327. [DOI] [PubMed] [Google Scholar]
- 5.Swanson L. W. & Cowan, W. M. (1977) J. Comp. Neurol. 172, 49-84. [DOI] [PubMed] [Google Scholar]
- 6.Swanson L. W., Kohler, C. & Bjorklund, A. (1987) in Handbook of Chemical Neuroanatomy, eds. Bjorklund, A., Hökfelt, T. & Swanson, L. W. (Elsevier Science, New York), Vol. 5, pp. 125–277. [Google Scholar]
- 7.Sperry R. (1963) Proc. Natl. Acad. Sci. USA 50, 703-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou R. (1998) Pharmacol. Ther. 77, 151-181. [DOI] [PubMed] [Google Scholar]
- 9.Cheng H. J., Nakamoto, M., Bergemann, A. D. & Flanagan, J. G. (1995) Cell 82, 371-381. [DOI] [PubMed] [Google Scholar]
- 10.Connor R. J., Menzel, P. & Pasquale, E. B. (1998) Dev. Biol. 193, 21-35. [DOI] [PubMed] [Google Scholar]
- 11.Drescher U., Kremoser, C., Handwerker, C., Loschinger, J., Noda, M. & Bonhoeffer, F. (1995) Cell 82, 359-370. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J. H., Cerretti, D. P., Yu, T., Flanagan, J. G. & Zhou, R. (1996) J. Neurosci. 16, 7182-7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monschau B., Kremoser, C., Ohta, K., Tanaka, H., Kaneko, T., Yamada, T., Handwerker, C., Hornberger, M. R., Loschinger, J., Pasquale, E. B., et al. (1997) EMBO J. 16, 1258-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamoto M., Cheng, H. J., Friedman, G. C., McLaughlin, T., Hansen, M. J., Yoon, C. H., O'Leary, D. D. & Flanagan, J. G. (1996) Cell 86, 755-766. [DOI] [PubMed] [Google Scholar]
- 15.Frisen J. & Barbacid, M. (1997) Cell Tissue Res. 290, 209-215. [DOI] [PubMed] [Google Scholar]
- 16.Feldheim D. A., Vanderhaeghen, P., Hansen, M. J., Frisen, J., Lu, Q., Barbacid, M. & Flanagan, J. G. (1998) Neuron 21, 1303-1313. [DOI] [PubMed] [Google Scholar]
- 17.Dutting D., Handwerker, C. & Drescher, U. (1999) Dev. Biol. 216, 297-311. [DOI] [PubMed] [Google Scholar]
- 18.Hornberger M. R., Dutting, D., Ciossek, T., Yamada, T., Handwerker, C., Lang, S., Weth, F., Huf, J., Wessel, R., Logan, C., et al. (1999) Neuron 22, 731-742. [DOI] [PubMed] [Google Scholar]
- 19.Brown A., Yates, P. A., Burrola, P., Ortuno, D., Vaidya, A., Jessell, T. M., Pfaff, S. L., O'Leary, D. D. & Lemke, G. (2000) Cell 102, 77-88. [DOI] [PubMed] [Google Scholar]
- 20.Gao P. P., Zhang, J. H., Yokoyama, M., Racey, B., Dreyfus, C. F., Black, I. B. & Zhou, R. (1996) Proc. Natl. Acad. Sci. USA 93, 11161-11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flanagan J. G. & Vanderhaeghen, P. (1998) Annu. Rev. Neurosci. 21, 309-345. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J. H., Pimenta, A. F., Levitt, P. & Zhou, R. (1997) Brain Res. Mol. Brain Res. 47, 202-214. [DOI] [PubMed] [Google Scholar]
- 23.Yue Y., Su, J., Cerretti, D. P., Fox, G. M., Jing, S. & Zhou, R. (1999) J. Neurosci. 19, 10026-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou R., Copeland, T. D., Kromer, L. F. & Schulz, N. T. (1994) J. Neurosci. Res. 37, 129-143. [DOI] [PubMed] [Google Scholar]
- 25.Kozak M. (1986) Cell 44, 283-292. [DOI] [PubMed] [Google Scholar]
- 26.Kozak M. (1987) Nucleic Acids Res. 15, 8125-8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gloster A., Wu, W., Speelman, A., Weiss, S., Causing, C., Pozniak, C., Reynolds, B., Chang, E., Toma, J. G. & Miller, F. D. (1994) J. Neurosci. 14, 7319-7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osborn L., Rosenberg, M. P., Keller, S. A. & Meisler, M. H. (1987) Mol. Cell. Biol. 7, 326-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franklin K. B. J. & Paxinos, G., (1997) The Mouse Brain in Stereotaxic Coordinates (Academic, San Diego).
- 30.Linke R., Pabst, T. & Frotscher, M. (1995) J. Comp. Neurol. 351, 602-616. [DOI] [PubMed] [Google Scholar]
- 31.Flanagan J. G. & Leder, P. (1990) Cell 63, 185-194. [DOI] [PubMed] [Google Scholar]
- 32.Ueno H., Colbert, H., Escobedo, J. A. & Williams, L. T. (1991) Science 252, 844-848. [DOI] [PubMed] [Google Scholar]
- 33.Kashles O., Yarden, Y., Fischer, R., Ullrich, A. & Schlessinger, J. (1991) Mol. Cell. Biol. 11, 1454-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Werner S., Smola, H., Liao, X., Longaker, M. T., Krieg, T., Hofschneider, P. H. & Williams, L. T. (1994) Science 266, 819-822. [DOI] [PubMed] [Google Scholar]
- 35.Campochiaro P. A., Chang, M., Ohsato, M., Vinores, S. A., Nie, Z., Hjelmeland, L., Mansukhani, A., Basilico, C. & Zack, D. J. (1996) J. Neurosci. 16, 1679-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Celli G., LaRochelle, W. J., Mackem, S., Sharp, R. & Merlino, G. (1998) EMBO J. 17, 1642-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pasquale E. B. (1997) Curr. Opin. Cell Biol. 9, 608-615. [DOI] [PubMed] [Google Scholar]
- 38.Wilkinson D. G. (2000) Int. Rev. Cytol. 196, 177-244. [DOI] [PubMed] [Google Scholar]
- 39.O'Leary D. D., Yates, P. A. & McLaughlin, T. (1999) Cell 96, 255-269. [DOI] [PubMed] [Google Scholar]
- 40.Gale N. W., Holland, S. J., Valenzuela, D. M., Flenniken, A., Pan, L., Ryan, T. E., Henkemeyer, M., Strebhardt, K., Hirai, H., Wilkinson, D. G., et al. (1996) Neuron 17, 9-19. [DOI] [PubMed] [Google Scholar]
- 41.Honda H. (1998) J. Theor. Biol. 192, 235-246. [DOI] [PubMed] [Google Scholar]
- 42.Gao P. P., Yue, Y., Cerretti, D. P., Dreyfus, C. & Zhou, R. (1999) Proc. Natl. Acad. Sci. USA 96, 4073-4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castellani V., Yue, Y., Gao, P. P., Zhou, R. & Bolz, J. (1998) J. Neurosci. 18, 4663-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao P. P., Sun, C. H., Zhou, X. F., DiCicco-Bloom, E. & Zhou, R. (2000) J. Neurosci. Res. 60, 427-436. [DOI] [PubMed] [Google Scholar]
- 45.Magal E., Holash, J. A., Toso, R. J., Chang, D., Lindberg, R. A. & Pasquale, E. B. (1996) J. Neurosci. Res. 43, 735-744. [DOI] [PubMed] [Google Scholar]
- 46.Freywald A., Sharfe, N. & Roifman, C. M. (2002) J. Biol. Chem. 277, 3823-3828. [DOI] [PubMed] [Google Scholar]
- 47.Holmberg J., Clarke, D. L. & Frisen, J. (2000) Nature (London) 408, 203-206. [DOI] [PubMed] [Google Scholar]
- 48.Ichijo H. & Bonhoeffer, F. (1998) J. Neurosci. 18, 5008-5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou X., Suh, J., Cerretti, D. P., Zhou, R. & DiCicco-Bloom, E. (2001) J. Neurosci. Res. 66, 1054-1063. [DOI] [PubMed] [Google Scholar]
- 50.Brownlee H., Gao, P. P., Frisen, J., Dreyfus, C., Zhou, R. & Black, I. B. (2000) J. Comp. Neurol. 425, 315-322. [DOI] [PubMed] [Google Scholar]