Abstract
Human embryonic stem (ES) cells are pluripotent cells that may be used in transplantation medicine. These cells can be induced to differentiate into cells from the three embryonic germ layers both in vivo and in vitro. To determine whether human ES cells might be rejected after transplantation, we examined cell surface expression of the MHC proteins in these cells. Our results show very low expression levels of MHC class I (MHC-I) proteins on the surface of human ES cells that moderately increase on in vitro or in vivo differentiation. A dramatic induction of MHC-I proteins was observed when the cells were treated with IFN-γ but not with IFN-α or -β. However, all three IFNs induced expression of MHC-I proteins in differentiated human ES cells. MHC-II proteins and HLA-G were not expressed on the surface of undifferentiated or differentiated cells. Ligands for natural killer cell receptors were either absent or expressed in very low levels in human ES cells and in their differentiated derivatives. In accordance, natural killer cytotoxic assays demonstrated only limited lysis of both undifferentiated and differentiated cells. To initiate a histocompatibility databank of human ES cells, we have isotyped several of the published ES cell lines for their human leukocyte antigens. In conclusion, our results demonstrate that human ES cells can express high levels of MHC-I proteins and thus may be rejected on transplantation.
Human embryonic stem (ES) cells are undifferentiated pluripotent cells derived from the inner cell mass (ICM) of blastocyst-stage embryos (1, 2). These cells may form embryonic tumors when injected into muscles or testes of severe combined immunodeficiency mice (1, 2). Additionally, human ES cells can differentiate in vitro into embryoid bodies (EBs) comprising the three embryonic germ layers (3). This differentiation process may be somewhat directed by the addition of growth factors to the media (4, 5). Indeed, several cell types have been characterized during differentiation, including neurons (5–7), pancreatic β cells (8), cardiomyocytes (9), and hematopoietic cells (10). In addition, methods for genetically modifying human ES cells now allow labeling and sorting of cells at different stages of differentiation (11). Thus, human ES cells hold promise to serve as an unlimited cell source for therapeutic transplantation. For such purposes, it is essential to determine whether human ES cells express MHC proteins.
The classical MHC class I (MHC-I) proteins generated from the HLA-A, -B, or -C loci are composed of a heavy chain, which interacts with β2 microglobulin, and a peptide. HLA-E, a nonclassical MHC-I, is expressed on the surface of all cells expressing MHC-I proteins after interacting with the leader peptides of various MHC-I proteins (12). Recognition of MHC-I molecules by cytotoxic T lymphocytes (CTL) can stimulate the killing of the MHC-I-presenting cell. Other cells capable of killing virus-infected and tumor cells are the natural killer (NK) cells. The killing of various targets by human NK cells is achieved primarily by three NK lysis receptors, i.e., NKp30, NKp44, and NKp46 (13). The cellular ligands that are recognized by these receptors and are expressed on the surface of the NK-sensitive target cells are currently unknown. A hemagglutinin protein was recently demonstrated to be the viral ligand for the NKp44 and NKp46 receptors (14, 15). The MHC-I proteins can also serve as ligands for NK cells; however, contrary to CTL, the recognition of MHC-I proteins by NK cells leads to inhibition of NK-mediated cytotoxicity (16). The nonclassical MHC-I molecules, HLA-G and -E, were also found to inhibit killing by NK cells (16). HLA-G is selectively expressed at the fetomaternal interface on the surface of cytotrophoblasts (17) and was also found in trophectodermal cells of some early human embryos but not in ICM cells (18). HLA-G is thought to protect the fetus from maternal uterine NK cells, thus playing an important role in immunological tolerance of the fetus by the mother (19).
Although MHC-I is expressed in almost all tissues, MHC-II is expressed primarily on B lymphocytes, macrophages, and dendritic cells (antigen-presenting cells). Expression of MHC-I, MHC-II, and other genes involved in the intracellular machinery that enables peptide loading to the MHC molecules can be induced in response to cytokines, in particular IFNs.
Information regarding the expression levels of MHC-I proteins on the surface of human ICM cells, from which ES cells originate, is inconclusive (18, 20). Lack of MHC-I expression on human preimplantation embryos was reported in one paper (20), whereas in another, MHC-I expression on the ICM of two of five human preimplantation embryos was demonstrated (18). In addition, it is not yet certain whether all adult stem cells express MHC-I molecules (21, 22).
In this study, we characterize the expression profile of MHC molecules on the surface of human ES cells and in their differentiated derivatives. We demonstrate low expression levels of MHC-I molecules on the surface of human ES cells. This expression is dramatically elevated when cells are incubated with IFNs. Recognition of human ES cells by NK lysis receptors was low or absent, and only a low level of killing was achieved when human ES cells were incubated with NK cells. Thus, this report presents an initial attempt to address the graft rejection issue of cells derived from human ES cells.
Materials and Methods
Cell Culture.
Human ES cell lines H9 (1), H13 (1), and HES1 (2) were cultured as previously described (3, 4). The undifferentiated cell cultures were induced to differentiate in vitro into EBs, as described (3). Two independent human teratoma cell lines derived from the H9 line were grown on 0.1% gelatin- (Merck) coated plates with human ES cell media. HeLa cells were cultured in DMEM (GIBCO/BRL), supplemented with 10% FCS (GIBCO/BRL). The MHC-I-negative human cell line 721.221 cells (23) and the various 721.221 transfectants were cultured in RPMI-1640 medium (GIBCO/BRL), supplemented with 10% heat inactivated FCS (GIBCO/BRL). NK cells were isolated from peripheral blood by using the human NK cell isolation kit and the autoMACS instrument (Miltenyi Biotec, Auburn, CA). NK cells were grown in culture as previously described (24).
Cytokine Treatment.
Lyophilized cytokines (PeproTech, Rocky Hill, NJ) were reconstituted at a concentration of 1,000 μg/ml of IFN-α, 10 μg/ml of IFN-β, and 100 μg/ml of IFN-γ, aliquoted and stored at −20°C. To find the dose response of human ES cells to IFN-γ, 2.5–50 ng/ml of the cytokine was added to the growth media for 48 h. For time-course analysis, MHC-I expression was monitored 12–72 h after addition of 25 ng/ml of IFN-γ to the growth media. In cytokine withdrawal experiments, cultures were passaged into medium lacking IFN-γ, and protein expression was monitored 3 days after the first passage and 4 more days after the second passage.
Abs.
Primary Abs used in this work were: mAb W6/32 directed against conformed MHC-I proteins (25); BBM1, which recognizes β2-microglobulin (26); HC10, which preferentially binds free HLA-B and -C heavy chains (27); anti-HLA-E and anti-HLA-G (V. Horejsi, personal communication). Staining with various mAbs was followed by staining with a secondary FITC-conjugated goat F(ab′)2 fragment to mouse IgG (ICN). For MHC-II detection, FITC-conjugated mouse anti-human HLA-DP, DQ, DR (Dako) mAbs were used.
Ig Fusion Proteins.
The generation of NKp30-Ig, NKp44-Ig, NKp46-Ig, and CD16-Ig fusion proteins and the staining procedure were previously described (14, 15). The various Ig-fusion proteins were recognized by phycoerythrin-conjugated goat anti-human Fc (Jackson ImmunoResearch) Abs.
Staining Procedures.
All adherent cells were trypsinized and resuspended in FACS medium (PBS containing 0.5% BSA, 0.05% Azid) to reach a final concentration of 3 × 105 − 106 cells/ml. Cells were dispensed into 96-well U-bottom plates (Nunclon, Naperville, IL). Blocking was performed on ice for 1 h with 10% human serum. Cells were stained by incubation with primary followed by secondary Abs for 1 h on ice. Detection of MHC-II proteins was performed as described without addition of secondary Ab. Staining for NK ligands was performed by the addition of approximately 10 μg of Ig fusion protein to 105 cells in 100-μl volume, for 2 h on ice without blocking. After centrifugation, cells were incubated with phycoerythrin-conjugated secondary Ab for 1 h on ice. Then cells were washed and resuspended, as noted above.
FACS Analysis.
Analysis was performed on a FACSCalibur system (Becton Dickinson) according to 530-nm band pass filter for detection of FITC-conjugated Abs and according to 661-nm band pass for phycoerythrin-conjugated Abs. Analysis was performed on cellquest software (Becton Dickinson). Cells (3 × 103 − 104) were acquired for each sample. Forward and side-scatter plots were used to exclude dead cells and debris from the histogram analysis plots.
Cytotoxic Assays.
The cytotoxic activity of NK cells against the various targets was assessed in 5 h 35S release assays, as described (24). In all presented cytotoxic assays, the spontaneous release was <20% of maximal release.
HLA Typing.
HLA allele typing was performed by using LiPA HLA-DRB1, -DQB1, -A, -B, and -C kits (Innogenetics, Alpharetta, GA).
Results
Expression of MHC Proteins in Human ES Cells and in Differentiated Derivatives.
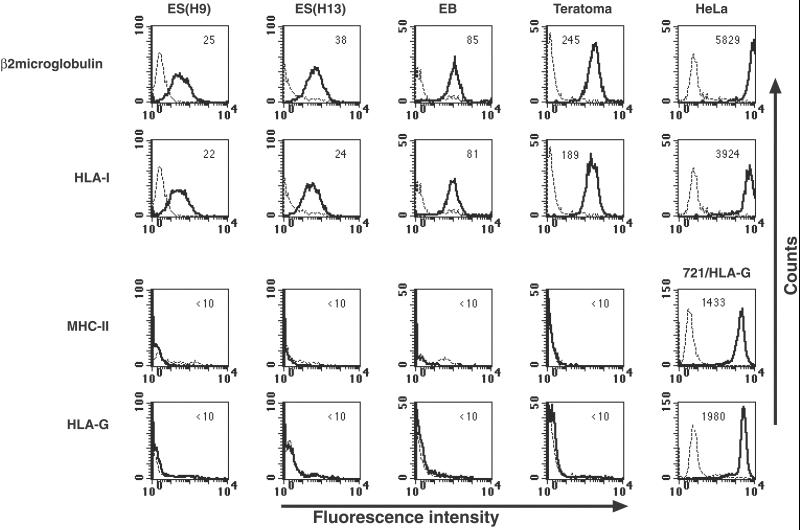
To analyze the expression of MHC-I proteins on the surface of human ES cells, we used the BBM1 mAb directed against β2 microglobulin and the W6/32 mAb directed against HLA class I (HLA-I) proteins. Staining with these Abs revealed relatively low levels of MHC-I expression in the H9 human ES cell line (Fig. 1). Low expression levels of MHC-I were also recorded in a different human ES cell line (H13) (Fig. 1). Because somatic cell lines express high levels of MHC-I molecules, we examined whether the differentiation process of human ES cells either in vitro or in vivo would cause MHC-I up-regulation. In vitro differentiation of human ES cells into EBs resulted in a slight elevation of MHC-I protein expression (2- to 4-fold increase). The median fluorescence intensity staining of β2 microglobulin was 85 in EBs as compared with 25 or 38 in undifferentiated human ES cell lines (Fig. 1). The expression level of MHC-I proteins on the surface of teratoma cells, which represent in vivo differentiation of human ES cells, was moderately elevated (approximately 8- to 10-fold) (Fig. 1). It should be noted, however, that the expression levels of MHC-I proteins on the surface of human ES cells, before or after differentiation, were one to two orders of magnitude lower than those observed in human somatic cell lines, such as HeLa cells (Fig. 1) or monocytes (data not shown). This relatively low level of MHC-I expression may reflect the embryonic and somewhat undifferentiated nature of human ES cells and their derivatives.
Figure 1.
Expression of MHC proteins in undifferentiated and differentiated human ES cells. FACS analysis of MHC molecules: expression of MHC-I was assayed by use of BBM1 and the pan-anticlass I W6/32 Abs. Expression of MHC-II was assayed by an Ab directed against HLA-DP, -DQ, -DR, and expression of the nonclassical MHC-I HLA-G was assayed by an Ab. Cell types: ES (H9) and ES (H13), two undifferentiated human ES cell lines; EB, in vitro differentiated human ES cells; Teratomas, in vivo-differentiated human ES cells; HeLa, cervix epithelial cell line used as a control for high expression level of MHC-I; 721/HLA-G, human B cell line 721.221 expressing HLA-G, used as control for high expression level of HLA-G and MHC-II. Dashed lines represent background staining with secondary Ab alone, and solid lines indicate expression of specific antigens. Median fluorescence intensity staining is indicated in the top of each box. Two to five independent experiments were performed for each analysis.
In addition to MHC-I, we analyzed human ES cells for the expression of MHC-II proteins. The expression of MHC-II proteins was analyzed by an Ab directed against HLA-DP, -DQ, and -DR (HLA-II). No staining for MHC-II was observed before or after in vitro or in vivo differentiation of human ES cells (Fig. 1). Anti-MHC-II mAb stained the human B cell line 721.221 (Fig. 1). Expression of HLA-G, a nonclassical MHC-I, was also monitored by Ab staining. The anti-HLA-G mAb specifically stained 721.221 cells transfected with HLA-G and did not stain 721.221 cells transfected with various isotypes of HLA-A, -B, or -C (data not shown). In addition, the mAb immunoprecipitates HLA-G from 721.221 cells transfected with HLA-G and from JEG3 cells. No staining of HLA-G was recorded in undifferentiated or differentiated human ES cells, although 721.221/HLA-G cells were highly stained by this Ab (Fig. 1). The expression of free HLA-B and -C proteins on the surface of human ES cells was assayed by the HC10 Ab (27). No significant binding of HC10 was observed in undifferentiated or differentiated human ES cells (data not shown). The absent binding of HC10 suggests that the association of heavy and light chain subunits of MHC-I proteins on the surface of the cells is stable.
Effects of IFNs on MHC-I Protein Expression in Undifferentiated and Differentiated Human ES Cells.
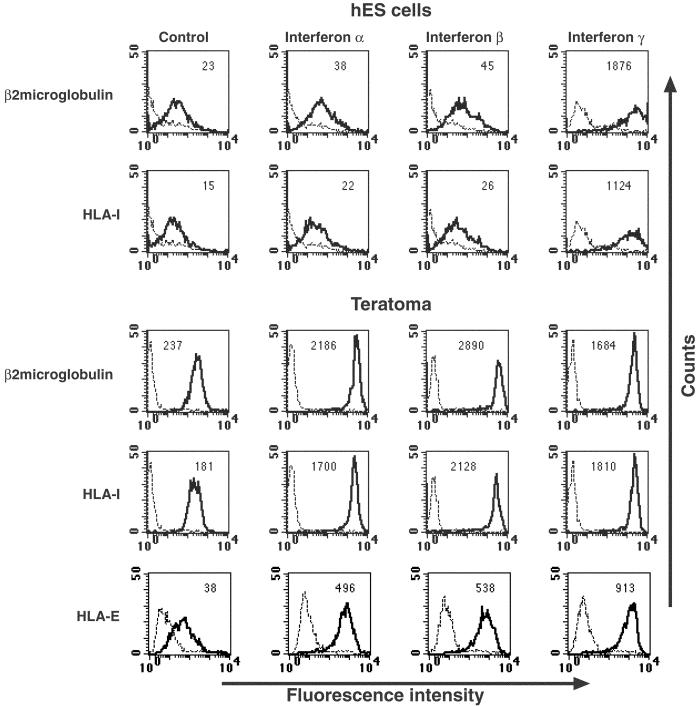
IFNs are cytokines that are released in the course of immune responses and affect many of the processes associated with effective immunity, one of which is increased expression of MHC-I and -II proteins. Because human ES cells and their differentiated derivatives express relatively low levels of MHC-I proteins, we therefore tested whether treatment of human ES cells with IFNs will result in the increased expression of MHC proteins. IFN-α, -β, or -γ was added to the growth media and MHC-I protein expression was assayed 48 h later (Fig. 2). No significant change in the level of MHC-I expression was observed after the addition of 1 ng/ml up to 100 ng/ml of IFN-α or -β to the growth media (shown in Fig. 2 are the effects of the treatment with 100 ng/ml of IFN-α or -β). In contrast, the addition of 25 ng/ml of IFN-γ resulted in a dramatic elevation in the expression of β2 microglobulin and HLA-I proteins (one to two orders of magnitude induction) (Fig. 2). Teratoma cells derived from human ES cells were also treated with IFN-α, -β, or -γ for 48 h (Fig. 2 Lower). Addition of IFN-γ (25 ng/ml) to the growth media of teratoma cells resulted in a dramatic up-regulation of MHC-I protein expression. Interestingly, IFN-α and -β, which had no effect on undifferentiated human ES or on EB cells (data not shown), caused a very high induction of MHC-I protein expression in the differentiated teratoma cells. In agreement with these results, preliminary analysis of the repertoire of genes expressed in human ES cells by using DNA microarrays demonstrates that the receptor for IFN-γ is indeed expressed in human ES cells, whereas the receptor for IFN-α and -β is absent (results not shown). The expression of the IFN-γ receptor in human ES cells was also confirmed by FACS analysis by using an Ab against IFN-γ receptor α subunit (data not shown). In contrast to MHC-I-induced expression, MHC-II proteins were absent from teratoma cells after addition of all IFNs (data not shown).
Figure 2.
Effects of IFNs on the expression of MHC-I and HLA-E in undifferentiated and differentiated human ES cells. Shown are expression levels of β2 microglobulin, HLA-I and -E proteins. IFN-α, -β, or -γ was added to the growth media of human ES cells and teratoma cells for 48 h as described in Materials and Methods. Control levels of antigen expression are shown (Left). Median fluorescence intensity staining of the antigen is indicated at the top of each box. Dashed lines represent background staining with secondary Ab alone, and solid lines indicate expression of specific antigens. Two to five independent experiments were performed for each analysis.
MHC-I-expressing cells also present HLA-E molecules on their surface. The HLA-E protein presents the leader peptides of various MHC-I proteins. As in vivo-differentiated human ES cells express low levels of MHC-I proteins, HLA-E expression was also assayed. Low levels of HLA-E expression were detected on the surface of teratoma cells, and the addition of all IFNs resulted in a significant increase of HLA-E expression (about one order of magnitude induction) (Fig. 2). Similarly, HLA-E was expressed and up-regulated in undifferentiated cells stimulated by IFN-γ (data not shown). The anti-HLA-E Ab used was specific, as it recognized 721.221 cells transfected with HLA molecule that can reconstitute surface expression of HLA-E. No staining was observed when 721.221 cells were transfected with HLA alleles that are unable to reconstitute HLA-E expression (data not shown).
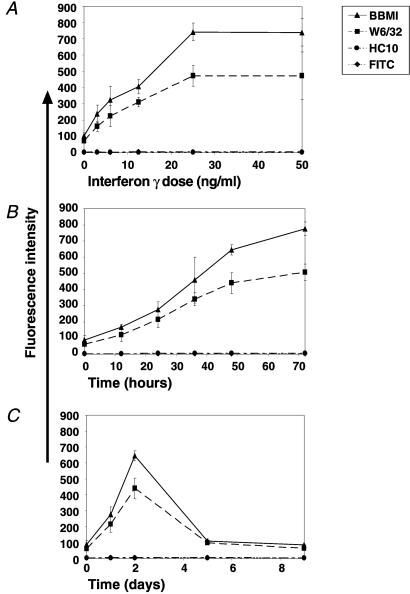
The maximal IFN-γ concentration needed for maximal induction of MHC-I expression on human ES cells was determined by the addition of various concentrations of IFN-γ to the growth media of human ES cells for 48 h (Fig. 3A). Maximal expression was observed after treatment with 25 ng/ml of IFN-γ. A time-course analysis showed that MHC-I expression was elevated as incubation periods with the cytokine were lengthened, the most dramatic increase occurring during the first 48 h (Fig. 3B). Withdrawal of IFN-γ from the growth media caused a dramatic decline in MHC-I expression to basal levels (Fig. 3C). No binding of HC10 was observed in any of the treated cells, indicating there are no MHC-I-free heavy chains on the surface of the cells. Surface expression of MHC-II was not detected before or after IFN-γ treatment (data not shown).
Figure 3.
IFN-γ induction of MHC-I in human ES cells is dose and time dependent. (A) Dose response of MHC-I expression on human ES cells on IFN-γ treatment. (B) Time dependence of MHC-I expression in human ES cells. (C) Time dependence of MHC-I expression after withdrawal of IFN-γ from the media. The FITC-conjugated secondary Ab was used as a negative control. Shown are standard error bars of three to four independent experiments.
Human ES Cells Are Not Effectively Recognized by NK Cells.
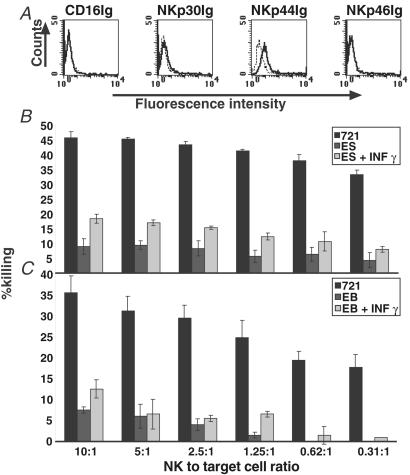
NK cells play a major role in the rejection of transplants. One of the most notable functions of NK cells is to kill cells that have lost MHC-I protein expression, a phenomenon known as the “missing self”. The killing of the class I negative target cells is mediated by a panel of NK cell lysis receptors that includes NKp30, NKp44, NKp46, and CD16 (28–32). As human ES cells express relatively low levels of MHC-I, it was important to determine whether they would be recognized by NK cells. The expression of the ligands to four newly discovered receptors mediating NK-specific recognition was assayed by using NK receptors attached to human Ig, as previously described (15). Human ES cells were found to be only slightly stained by the NKp44 receptor (29), whereas no staining was observed by NKp30 (28), NKp46 (30), and CD16 (31) receptors (Fig. 4A). We next tested whether naive and IFN-γ treated human ES cells might still be sensitive to lysis by primary NK cell lines. The NK cells efficiently killed the control NK-sensitive B cell line (721.221); however, only minimal killing was observed when human ES cells were tested (Fig. 4B). A slight increase in the killing of human ES cells by NK cells was observed when target cells were treated with IFN-γ (Fig. 4B). Similar results were obtained with cell derived from EBs (Fig. 4C). None of the NK receptors significantly stained undifferentiated or differentiated cells after IFN-γ addition (data not shown).
Figure 4.
Human ES cell recognition by NK receptors and NK-mediated lysis. (A) FACS analysis for the expression of the NK-receptor specific ligands NKp44, NKp30, NKp46, and CD16 in human ES cells. Dashed lines represent background staining with secondary Ab alone, and solid lines indicate expression of specific antigens. Two independent experiments were performed for each analysis. Histogram representation of the cytotoxic effects of NK cells on naive and IFN-γ- (IFN-γ, 25 ng/ml, 48 h) treated human ES cells (B) and human EB cells (C). (B, C). The various NK-to-target cell ratios used are indicated. Shown are standard error bars of three experiments.
HLA Typing of Human ES Cells.
To perform transplantation of foreign cells into a recipient patient without rejection by CTL and for future investigations of the role of MHC-I proteins with regard to CTL, it is necessary to determine the HLA haplotypes of the human ES cell lines. The HLA composition of three human ES cell lines, H9 (1), H13 (1), and HES1 (2), was determined by PCR analysis of their HLA A, B, C, DRB, and DQB allelic variations (Table 1). HLA typing reveals that few alleles are shared by at least two cell lines: A02, Cw04, and Cw08 alleles are shared by two cell lines, and B35 is shared by three cell lines.
Table 1.
Isotype classification of human ES cell lines H9, H13, and HES1
|
MHC-I
|
MHC-II
|
||||
|---|---|---|---|---|---|
| HLA-A | HLA-B | HLA-C | HLA-DRB | HLA-DQB | |
| H9 | A*02 | B*35 | Cw*04 | DRB1*1501 | DQB1*0602 |
| A*03 | B*44 | Cw*07 | DRB1*1601 | DQB1*0502 | |
| H13 | A*02 | B*14 | Cw*04 | DRB1*0102 | DQB*0501 |
| A*33 | B*35 | Cw*08 | DRB1*1101 | DQB1*0301 | |
| HES1 | A*11 | B*15 | Cw*08 | DRB1*14011 | DQB1*05031 |
| A*24 | B*35 | Cw*12 | DRB1*15011 | DQB1*0601 | |
HLA isotyping of three human ES cell lines was performed by PCR. The alleles examined were in MHC-I and -II loci (HLA-A, -B, -C, and -DRB, and -DQB, respectively).
Discussion
Human ES cells are derived from the ICM of blastocyst-stage embryos (1). The status of MHC-I expression in human ICM was previously examined by immunostaining with the W6/32 Ab and gave contradictory results. One report concluded that MHC-I is not expressed (20), whereas a more recent report showed a positive staining (18). Yet the expression of MHC-I and -II in human ICM was never quantified. Here we show that human ES cells express MHC-I proteins, albeit in very low levels. Although most somatic cell lines express high levels of MHC-I, the in vitro- and in vivo-differentiated derivatives of human ES cells still express low levels of MHC-I. Addition of IFNs, in particular IFN-γ, to the growth media of differentiated human ES cells resulted in high levels of MHC-I protein expression. As the expression of MHC-I molecules may be dramatically and rapidly induced by treating the cells by IFNs, it is possible that a similar phenomenon will be observed after transplantation. Thus, when transplantation of allogeneic human ES cells is performed, the cells might be rejected by CTL.
MHC-II and HLA-G were not expressed in human ES cells or in their differentiated derivatives. As HLA-G is present on extraembryonic trophectoderm cells (18), the results suggest that, like murine ES cells (33), human ES cells also do not readily differentiate into the extraembryonic trophectoderm, although they still differentiate into the extraembryonic primitive endoderm. On the other hand, HLA-E expression could be detected, and the expression of HLA-E was elevated after IFN-γ treatment. Thus, it is likely that antigen processing and presentation are intact and functional in undifferentiated and differentiated human ES cells.
NK cells recognize and kill cells that lack MHC-I expression (32). The killing is mediated primarily via lysis receptors such as the NKp44 (29), NKp30 (28), NKp46 (30), or CD16 (31). Because only a low level of NKp44 staining on human ES cells was noted, it is likely that the lack of killing of human ES cells by NK cells is not the result of MHC-I-dependent inhibition of lysis but rather due to lack of recognition. Indeed, masking of the MHC-I proteins by a specific anticlass I mAb did not result in increased killing of human ES cells (data not shown).
Human ES cells have been suggested to serve as a source of cells in transplantation medicine. We show that the expression of MHC-I in these cells is very low but can be rapidly induced. Because tissue rejection is primarily directed at nonself MHC molecules expressed by grafts, strategies to overcome rejection must be discovered. One option is to form a “histocompatibility bank,” in which all human ES cell lines will be stored after being HLA isotyped. Ideally, if large numbers of cell lines from genetically diverse populations can be maintained, adequate levels of isotype matching with patients may be achieved. Derivation of a human ES cell line specifically for each patient was also suggested as a method to overcome tissue rejection. Therapeutic cloning is highly problematic from an ethical and practical point of view; however, recently it was shown that somatic nuclear transfer to human eggs and development of the embryo past pronuclear stage are possible (34). Another option is to engineer a “universal cell” that may be suitable for all patients. As genetic manipulation of human ES cells is now feasible (11), and human ES cells were found to be insensitive to NK cell-mediated killing, such a cell line might be one that does not express MHC-I proteins.
Acknowledgments
We thank Vaclav Horejsi (Institute of Molecular Genetics ASCR, Prague, Czech Republic) for kindly providing anti-HLA-E and anti-HLA-G Abs, Arnon Tal for critical help with cell lines and fusion proteins, Dr. Varda Deutsch for the use of Abs, and the tissue-typing unit at the Hadassah Medical Center for the HLA typing. We thank ES Cell International Pte Ltd for the HES1 cell line. This research was partially supported by funds from the Herbert Cohn Chair (Hebrew University) and by a grant from the Juvenile Diabetes Fund (USA).
Abbreviations
- ES
embryonic stem
- ICM
inner cell mass
- EBs
embryoid bodies
- CTL
cytotoxic T lymphocytes
- NK
natural killer
References
- 1.Thomson J A, Itskovitz-Eldor J, Shapiro S S, Waknitz M A, Swiergiel J J, Marshall V S, Jones J M. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Reubinoff B E, Pera M F, Fong C Y, Trounson A, Bongso A. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 3.Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, Soreq H, Benvenisty N. Mol Med. 2000;6:88–95. [PMC free article] [PubMed] [Google Scholar]
- 4.Schuldiner M, Yanuka O, Itskovitz-Eldor J, Melton D A, Benvenisty N. Proc Natl Acad Sci USA. 2000;97:11307–11312. doi: 10.1073/pnas.97.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuldiner M, Eiges R, Eden A, Yanuka O, Itskovitz-Eldor J, Goldstein R S, Benvenisty N. Brain Res. 2001;913:201–205. doi: 10.1016/s0006-8993(01)02776-7. [DOI] [PubMed] [Google Scholar]
- 6.Reubinoff B E, Itsykson P, Turetsky T, Pera M F, Reinhartz E, Itzik A, Ben-Hur T. Nat Biotechnol. 2001;19:1134–1140. doi: 10.1038/nbt1201-1134. [DOI] [PubMed] [Google Scholar]
- 7.Zhang S C, Wernig M, Duncan I D, Brustle O, Thomson J A. Nat Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 8.Assady S, Maor G, Amit M, Itskovitz-Eldor J, Skorecki K L, Tzukerman M. Diabetes. 2001;50:1691–1697. doi: 10.2337/diabetes.50.8.1691. [DOI] [PubMed] [Google Scholar]
- 9.Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, Livne E, Binah O, Itskovitz-Eldor J, Gepstein L. J Clin Invest. 2001;108:407–414. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaufman D S, Hanson E T, Lewis R L, Auerbach R, Thomson J A. Proc Natl Acad Sci USA. 2001;98:10716–10721. doi: 10.1073/pnas.191362598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eiges R, Schuldiner M, Drukker M, Yanuka O, Itskovitz-Eldor J, Benvenisty N. Curr Biol. 2001;11:514–518. doi: 10.1016/s0960-9822(01)00144-0. [DOI] [PubMed] [Google Scholar]
- 12.Lee N, Goodlett D R, Ishitani A, Marquardt H, Geraghty D E. J Immunol. 1998;160:4951–4960. [PubMed] [Google Scholar]
- 13.Biassoni R, Cantoni C, Pende D, Sivori S, Parolini S, Vitale M, Bottino C, Moretta A. Immunol Rev. 2001;181:203–214. doi: 10.1034/j.1600-065x.2001.1810117.x. [DOI] [PubMed] [Google Scholar]
- 14.Arnon T I, Lev M, Katz G, Chernobrov Y, Porgador A, Mandelboim O. Eur J Immunol. 2001;31:2680–2689. doi: 10.1002/1521-4141(200109)31:9<2680::aid-immu2680>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 15.Mandelboim O, Lieberman N, Lev M, Paul L, Arnon T I, Bushkin Y, Davis D M, Strominger J L, Yewdell J W, Porgador A. Nature (London) 2001;409:1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 16.Moretta L, Bottino C, Cantoni C, Mingari M C, Moretta A. Curr Opin Pharmacol. 2001;1:387–391. doi: 10.1016/s1471-4892(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 17.Kovats S, Main E K, Librach C, Stubblebine M, Fisher S J, DeMars R. Science. 1990;248:220–223. doi: 10.1126/science.2326636. [DOI] [PubMed] [Google Scholar]
- 18.Jurisicova A, Casper R F, MacLusky N J, Mills G B, Librach C L. Proc Natl Acad Sci USA. 1996;93:161–165. doi: 10.1073/pnas.93.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rouas-Freiss N, Goncalves R M, Menier C, Dausset J, Carosella E D. Proc Natl Acad Sci USA. 1997;94:11520–11525. doi: 10.1073/pnas.94.21.11520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desoye G, Dohr G A, Motter W, Winter R, Urdl W, Pusch H, Uchanska-Ziegler B, Ziegler A. J Immunol. 1988;140:4157–4159. [PubMed] [Google Scholar]
- 21.Bailey K A, Drago J, Bartlett P F. J Neurosci Res. 1994;39:166–177. doi: 10.1002/jnr.490390207. [DOI] [PubMed] [Google Scholar]
- 22.McLaren F H, Svendsen C N, Van der Meide P, Joly E. J Neuroimmunol. 2001;112:35–46. doi: 10.1016/s0165-5728(00)00410-0. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu Y, Geraghty D E, Koller B H, Orr H T, DeMars R. Proc Natl Acad Sci USA. 1988;85:227–231. doi: 10.1073/pnas.85.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandelboim O, Reyburn H T, Vales-Gomez M, Pazmany L, Colonna M, Borsellino G, Strominger J L. J Exp Med. 1996;184:913–922. doi: 10.1084/jem.184.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnstable C J, Bodmer W F, Brown G, Galfre G, Milstein C, Williams A F, Ziegler A. Cell. 1978;14:9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- 26.Brodsky F M, Bodmer W F, Parham P. Eur J Immunol. 1979;9:536–545. doi: 10.1002/eji.1830090709. [DOI] [PubMed] [Google Scholar]
- 27.Stam N J, Spits H, Ploegh H L. J Immunol. 1986;137:2299–2306. [PubMed] [Google Scholar]
- 28.Pende D, Parolini S, Pessino A, Sivori S, Augugliaro R, Morelli L, Marcenaro E, Accame L, Malaspina A, Biassoni R, et al. J Exp Med. 1999;190:1505–1516. doi: 10.1084/jem.190.10.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cantoni C, Bottino C, Vitale M, Pessino A, Augugliaro R, Malaspina A, Parolini S, Moretta L, Moretta A, Biassoni R. J Exp Med. 1999;189:787–796. doi: 10.1084/jem.189.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pessino A, Sivori S, Bottino C, Malaspina A, Morelli L, Moretta L, Biassoni R, Moretta A. J Exp Med. 1998;188:953–960. doi: 10.1084/jem.188.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mandelboim O, Malik P, Davis D M, Jo C H, Boyson J E, Strominger J L. Proc Natl Acad Sci USA. 1999;96:5640–5644. doi: 10.1073/pnas.96.10.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ljunggren H G, Karre K. Immunol Today. 1990;11:237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 33.Beddington R S, Robertson E J. Development (Cambridge, UK) 1989;105:733–737. doi: 10.1242/dev.105.4.733. [DOI] [PubMed] [Google Scholar]
- 34.Cibelli J B, Lanza R P, West M D, Ezzell C. Sci Am. 2002;286:44–51. doi: 10.1038/scientificamerican0102-44. [DOI] [PubMed] [Google Scholar]