Abstract
Psychological studies have found better memory in women than men for emotional events, but the neural basis for this difference is unknown. We used event-related functional MRI to assess whether sex differences in memory for emotional stimuli is associated with activation of different neural systems in men and women. Brain activation in 12 men and 12 women was recorded while they rated their experience of emotional arousal in response to neutral and emotionally negative pictures. In a recognition memory test 3 weeks after scanning, highly emotional pictures were remembered best, and remembered better by women than by men. Men and women activated different neural circuits to encode stimuli effectively into memory even when the analysis was restricted to pictures rated equally arousing by both groups. Men activated significantly more structures than women in a network that included the right amygdala, whereas women activated significantly fewer structures in a network that included the left amygdala. Women had significantly more brain regions where activation correlated with both ongoing evaluation of emotional experience and with subsequent memory for the most emotionally arousing pictures. Greater overlap in brain regions sensitive to current emotion and contributing to subsequent memory may be a neural mechanism for emotions to enhance memory more powerfully in women than in men.
Emotionally arousing experiences are more memorable than neutral experiences. There is superior memory for traumatic relative to mundane events (1) and for emotionally provocative relative to neutral words (2) and pictures (3). Memory for emotional stimuli and experiences differs between the sexes (4, 5). Women recall more emotional autobiographical events than men in timed tests, produce memories more quickly or with greater emotional intensity in response to cues, and report more vivid memories than their spouses for events related to their first date, last vacation, and a recent argument (4, 6–8).
Two explanations for the difference in memory performance have been proposed. The “affect-intensity” hypothesis posits that women have better memory because they experience life events more intensely than men and thus may better encode such events into memory (4). Controlling for affect intensity at encoding should therefore eliminate women's superior memory performance. The “cognitive-style” hypothesis posits that women may differ from men in how they encode, rehearse, or think about their affective experiences or in how they generate responses in a memory test (5). According to this view, controlling for affect intensity at encoding should not remove sex-based differences in memory performance.
The goal of this study was to examine the neural bases, as measured by functional MRI, of putative sex differences in the evaluation and memory encoding of negative emotional experiences, specifically of negative scenes (e.g., pictures of mutilated bodies; ref. 9). The affect-intensity and cognitive-style hypotheses lead to different predictions about brain activation patterns. The affect-intensity view predicts that women will have the same pattern of activation as men but to a greater degree. Further, there would be no differences in activation if emotional intensity is equated for men and women. The cognitive-style view suggests that men and women would have different patterns of activation even when emotional intensity is equated for men and women.
Materials and Methods
Subjects.
Twelve men and twelve women (all right-handed native speakers) participated in this study. Informed consent was obtained from all subjects. Data from 10 women in the amygdala only were reported previously (10).
Task Procedure.
During functional scanning, subjects rated each of 96 neutral to highly negative pictures selected from the International Affective Picture Series (11). Stimuli were presented for a period of 2.88 s with an intertrial interval of 12.96 s, during which a fixation cross was presented. Subjects rated their emotional experience of each picture on a scale from 0 (“not emotionally intense at all”) to 3 (“extremely emotionally intense”). Three weeks after the scan, subjects participated in an unexpected recognition memory test, in which they viewed all of the previously seen pictures (targets) and 48 new pictures (foils), and judged whether they had seen each picture previously during scanning. For pictures that were judged as seen previously, subjects indicated whether their decision was based on a distinct, vivid recollection of having seen the picture (condition “remember”) or rather based on a less certain feeling of familiarity (condition “familiar”). Thus, each picture presented to a subject during scanning was associated with one of three memory outcomes: forgotten (assigned a value of “0”), familiar (“1”), or remembered (“2”).
Stimuli Characteristics.
Normative ratings for all stimuli were available on a 9-point scale for the dimensions of emotional valence (1, most unpleasant, and 9, most pleasant) and arousal (1, calm/dull, and 9, most arousing or intense). Normative valence for target pictures ranged from 1.17 (highly negative) to 5.44 (neutral), and normative arousal ranged from 1.97 (tranquil) to 7.63 (highly arousing). Arousal and valence ratings were highly correlated (r = 0.89). Foils were selected to match target pictures; normative valence ratings ranged from 1.31 (highly negative) to 5.78 (neutral), and normative arousal ratings ranged from 2.74 (tranquil) to 7.22 (highly arousing). There was no significant difference between foils and target pictures for normative ratings of valence [t(142) = 0.16, P = 0.88] or arousal [t(142) = 0.98, P = 0.33].
Imaging Procedure.
Images were acquired on a 1.5 T General Electric Signa whole-body scanner with a whole-head elliptical bird-cage coil. For structural images, eight 7-mm-thick slices perpendicular to the axial plane of the hippocampus were obtained with a standard T1-weighted pulse sequence, with the first slice positioned 10 mm anterior to the amygdala. Functional images were obtained by using a gradient echo T2*-weighted spiral-scan sequence with two interleaves (repetition time per slice = 90 ms, echo time = 40 ms, flip angle = 80°, field of view = 20 cm, acquisition time = 1.44 s per frame, number of frames = 264). Data were not smoothed and thus retained the original spatial resolution of 2.4 mm. Head movement was minimized by means of a bite-bar formed with each subject's dental impression, and motion artifact was examined and corrected automatically for all scans with AIR 3.0 (12).
Data Analysis.
Each individual trial was captured in 11 time frames of 2.88 s each. Individual frames in each trial were assigned to the baseline period (frames 1, 2, 10, and 11) or to the activation period (frames 5–8) based on a lag in peak hemodynamic response of ≈4 s after the presentation of the stimulus (13). For each trial, the average value from baseline scans (frames 1, 2, 10, and 11) was subtracted from the average value from activation scans (frames 5–8); thus each trial served as its own control.
Correlation maps were constructed to identify areas in which greater activation during encoding was correlated positively with ratings of greater emotional arousal or recognition memory accuracy. Correlation maps were based on a random-effects model calculated by a Kendall rank-order correlation for each voxel between event-related activation and behavioral measures of emotional arousal or recognition memory, with correlation coefficients transformed into z scores.
Composite correlation maps were based on structural MRI scans for all subjects that were normalized with respect to a template brain from one subject. The template then was used for subsequent estimation of Talairach coordinates (14). Composite correlation maps were subjected to a cluster analysis procedure (15) to correct for multiple statistical comparisons by using a spatial extent threshold of P < 0.01 significance level (one-tailed) over the entire image. A secondary conjunction analysis then was performed to identify clusters that correlated significantly with both measures of emotional arousal and recognition memory accuracy (one-tailed spatial extent, P < 0.025). For all composite maps, significant clusters were colored according to their level of significance and overlaid on an averaged structural image.
Results
Behavioral Data.
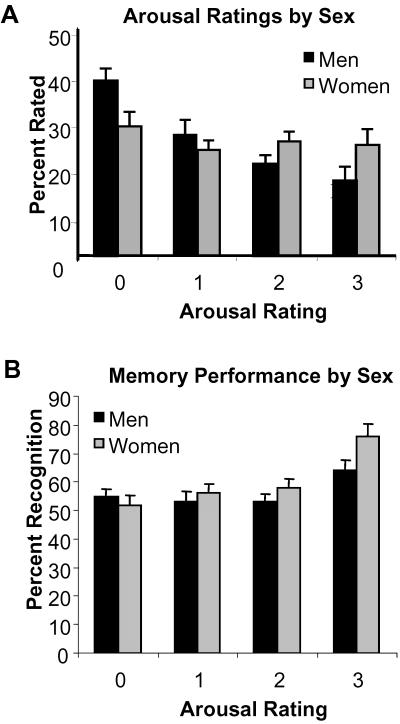
There were sex differences in reported emotional experience and in memory for emotionally provocative pictures (Fig. 1 A and B). ANOVA was performed with factors of “sex” (male and female), “arousal rating” (ratings 0–3), and “memory accuracy” (0, forgotten; 1, familiar; 2, remembered). Pictures that provoked high emotional arousal were more likely to be remembered than those that yielded little or no sense of arousal when collapsed across sex (arousal rating × memory accuracy, F(6,120) = 7.40, P < 0.0001). There was a significant interaction between sex and emotional arousal (sex × arousal rating, F(3,66) = 3.37, P < 0.025). Women rated significantly more pictures as highly arousing (rated 3) than did men [t(22) = 2.41, P < 0.025]. Women had better memory for emotional pictures than men (Fig. 1D); pictures rated as most highly arousing were recognized significantly more often by women than by men as familiar [t(20) = 2.40, P < 0.05] or remembered [t(20) = 2.38, P < 0.05]. There were no significant sex differences in memory for pictures rated less intense (0–2) or in false-positive rates (12 and 10% for women and men, respectively). Thus, women had superior memory for only the most intensely negative pictures even when subjective ratings of arousal were equated.
Fig 1.
Emotional ratings and memory in women and men. (A) Proportion of pictures rated neutral (0) to emotionally very arousing (3) by men and women. (B) Proportion of pictures rated neutral (0) to emotionally very arousing (3) that were recognized (summed percentage of familiar and remembered pictures) by men and women. The error bars represent SD.
Functional MRI Data.
Correlation with ratings of emotional arousal.
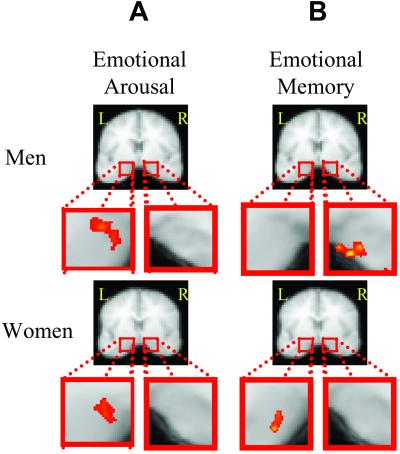
Brain activation correlated with higher ratings of emotional arousal in a large network of structures in both sexes (see Table 2, which is published as supporting information on the PNAS web site, www.pnas.org). Of particular interest was the amygdala that had been associated previously with ratings of emotional arousal (10); activation in the left but not right amygdala was correlated with emotional arousal ratings for both sexes (Fig. 2A).
Fig 2.
Amygdala correlation with arousal ratings and recognition memory for highly emotional pictures. (A) Clusters of significant correlation between brain activation and subjects' arousal ratings. Left amygdala activation correlates with emotional arousal in both men (−27, −8, −16) and women (−25, −8, −14). (Top) Oblique slice prescriptions in red and slice containing region of interest in green. L, left; R, right. (B) Clusters of significant correlation between amygdala activation and subjects' subsequent memory for scenes rated as emotionally highly intense (rated 3). Correlation clusters in the right amygdala for men (Tailarach coordinates, +16, −8, −17) and left amygdala for women (−25, −8, −17) are shown. (Top) Slice prescriptions in red and slice containing region of interest in green. The red squares in coronal slices identify region of interest.
In addition to the left amygdala, men and women had other common (although not necessarily overlapping) areas in which activation correlated significantly with reported emotional experience. These areas included the bilateral superior frontal gyrus [Brodmann area (BA) 6], right middle (BA 46), and bilateral inferior frontal (BA 44, 45) gyri, left-lateralized anterior cingulate (BA 32), right precentral gyrus (BA 4), left thalamus, and left insula. This pattern of correlation loci suggests that both sexes share an extensive network of structures associated with attention, language, and motor control that are associated with emotional arousal
Women but not men exhibited correlations in the postcentral gyrus and hippocampus. Men but not women exhibited a significant correlation in the putamen. Correlations in BA 37 of the fusiform gyrus were lateralized by gender: right-lateralized for men and left-lateralized for women. There was evidence for different patterns of hemispheric asymmetry between the sexes. Women had significantly more clusters in the left than in the right hemisphere (χ2 = 5.90, P < 0.05), whereas men showed no hemispheric asymmetry in the number of clusters in either hemisphere (χ2 = 0.12, P = not significant).
Correlation with recognition memory for emotional pictures.
Sex differences in emotional memory were evident only for the most emotionally arousing pictures. Further, in this and a prior study (10) only pictures rated as most intense yielded any correlation between amygdala activation and later memory. Therefore, trials with maximal arousal ratings of 3 were used to identify brain regions in which greater activation during encoding was correlated positively with superior recognition accuracy. This analysis revealed that recognition accuracy for maximally arousing (but not for less arousing) pictures was correlated with greater left amygdala activation in women and right amygdala activation in men (Fig. 2B), consistent with a prior report (16). Both correlations were so robust that they were present even with correction for multiple comparisons across the brain and without selecting the amygdala as a region of interest (i.e., no small volume correction).
We tested the interaction between memory for maximally emotional pictures and sex by calculating for each subject the laterality index between the left and right amygdala region of interest (anatomically defined on the basis of the averaged, normalized brain volumes for women and men, respectively). The laterality index was based on each subject's maximal z score within the boundaries of the left and right amygdala region of interest. These z scores did not represent amygdala activation but rather the significance level of the correlation (based on an r-to-z-conversion) between amygdala activation to pictures rated 3 and subsequent memory (forgotten, 0; familiar, 1; remembered, 2) for these pictures. Thus, the index quantified the degree to which the memory correlation was lateralized toward the left or right amygdala. A one-way ANOVA tested for the effects of sex on laterality index and revealed a significant group difference [F(1,19) = 4.7, P < 0.05], confirming a significant laterality difference between the sexes.
The pattern of laterality between amygdala activation and memory correlation, although statistically reliable, is not absolute. For example, there were individual differences in the laterality index in both groups. The reported left-lateralized amygdala memory correlations were seen in 72% of the women and reported right-lateralized correlations in 50% of the men, with the remaining subjects exhibiting very minor to modest lateralization in the opposite direction. Mean z scores (indicating the strength of the memory correlation, see above) were larger in the left for women (mean + SD: L = 1.7 + 0.37, R = 1.3 + 0.42) and larger in the right for men (mean + SD: L = 1.8 + 0.74, R = 1.9 + 0.46) but were significantly larger than zero in all cases (t values 7.9–15.4, all P < 0.0001). Thus, amygdala activation correlates with better memory for highly emotional pictures at greater-than-chance levels in both hemispheres but is more robust in the left hemisphere for women and the right hemisphere for men when using standard statistical threshold techniques (as seen in Fig. 2B).
The analysis identified additional loci at which activation was correlated with recognition accuracy for the most emotionally arousing pictures (see Table 3, which is published as supporting information on the PNAS web site). A χ2 test comparing the number of significant clusters between men and women revealed that the number of loci was significantly greater for men than for women (χ2 = 9.0, P < 0.01). There were no significant differences in the number of left- or right-lateralized clusters in either sex. In addition to the amygdala, both sexes exhibited correlation clusters in the left anterior cingulate gyrus (BA 24) and the left precentral gyrus (BA 4). Both groups also had a cluster of significant correlation located within the right fusiform gyrus, although the loci were separated by 16 mm along the anterior–posterior axis. Women but not men exhibited correlations in the posterior region of the insula. Men but not women exhibited correlations in the basal ganglia (caudate and putamen). Men also exhibited extensive correlations across all frontal gyri, primarily in the left hemisphere, with both superior and inferior temporal gyri, and along the medial and ventral temporal lobe (hippocampus, parahippocampal gyrus, and fusiform gyrus).
Conjunction of emotional memory and experience.
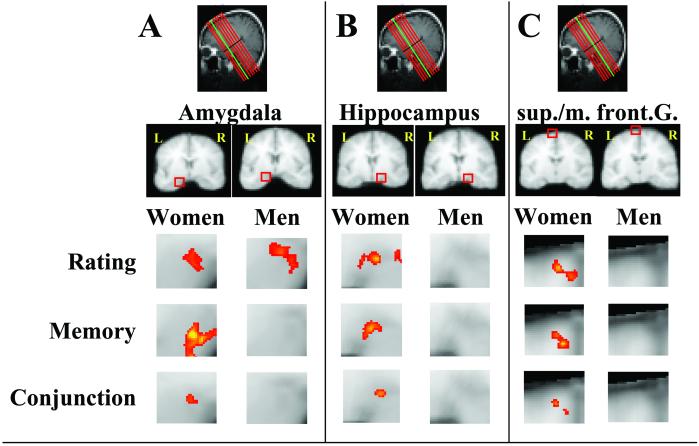
A conjunction analysis was performed to identify regions in which greater activation correlated with both concurrent emotional intensity and subsequent memory accuracy (collapsed across all trials, Fig. 3). Such conjunctions of activation were found for women in nine regions (Table 1) including loci associated with memory or emotional experience such as the left amygdala, bilateral hippocampus, and left anterior cingulate. In contrast, men exhibited such conjunctions in only two frontal regions and none of the medial temporal regions. A χ2 test showed that women had significantly more conjunctions than men (χ2 = 4.23, P < 0.05).
Fig 3.
Colocalization of brain regions associated with emotional-intensity ratings and recognition memory. Areas of activation for women and men that correlated with greater emotional-intensity ratings (Rating), with better subsequent memory (Memory), and as a result of a conjunction analysis of these two conditions (Conjunction) are shown. Also shown are three locations in the left amygdala (−22, −8, −14), right hippocampus (+20, −16, −11), and left superior frontal gyrus (−12, 12, 60) and medial frontal gyrus (−3, 12, 55) in which brain activation correlated with both rating and memory in women but not men in overlapping or adjacent brain regions. (A–C) Oblique slice prescriptions in red and slice containing region of interest in green. R, right; L, left.
Table 1.
Brain regions in which activation was correlated positively with both greater emotional arousal ratings and better subsequent memory for pictures (collapsed across all trials)
| Structure
|
Talairach coordinates | Cluster size, mm3
|
Z
|
||
|---|---|---|---|---|---|
| x | y | z | |||
| Men | |||||
| R inferior frontal gyrus | 39 | 16 | 31 | 104 | 2.58 |
| L anterior cingulate gyrus, BA 24 | −7 | −8 | 40 | 224 | 2.19 |
| Women | |||||
| Frontal lobe (gyrus) | |||||
| L superior, BA 6 | −12 | 12 | 60 | 104 | 2.56 |
| L middle, BA 6 | −39 | 0 | 47 | 38 | 2.28 |
| L medial, BA 6 | −6 | −4 | 53 | 156 | 3.51 |
| −3 | 12 | 55 | 120 | 2.78 | |
| R superior temporal gyrus/insula | 37 | −8 | −10 | 246 | 2.93 |
| L anterior cingulate gyrus, BA 23 | −3 | −12 | 31 | 180 | 2.63 |
| L amygdala | −22 | −8 | −14 | 140 | 2.49 |
| R hippocampus | 20 | −16 | −11 | 107 | 2.86 |
| L hippocampus | −23 | −20 | −9 | 117 | 3.17 |
Z score indicates maximal Z score within the cluster that was obtained for a correlation between brain activation and either ratings or memory performance based on an r-to-z transformation. R, right; L, left.
Discussion
This study used functional MRI to discover whether there are differences between women and men in the neural bases of the evaluation and memory encoding of negative pictures. Behaviorally, women rated more pictures as highly negative and remembered better pictures rated as highly negative by both men and women. These psychological differences were accompanied by a complex set of activations, both common and distinct across the sexes, that correlated with measures of emotional evaluation and/or memory encoding. There was also a marked sex difference in the relations between those neural systems. Women had significantly more areas than men that were activated both by subjective emotional experience and by successful encoding of that experience into long-term memory. This “colocalization” of evaluative and encoding processes may be the mechanism by which women may attain better emotional memory than men.
Ratings of Emotional Arousal.
There were sex differences in emotional experience and the neural substrate associated with ratings of emotional experience. Women, compared with men, rated a greater proportion of pictures as maximally arousing and exhibited a lateralized pattern of correlation clusters. For women, activation loci were left-lateralized, whereas for men there was no significant difference between the numbers of loci in either hemisphere. The pattern of activations that correlated with evaluation of emotional arousal included both common and distinct regions across the sexes.
Common regions included the left amygdala, insula, and thalamus. Activation of the amygdala as a function of emotional arousal is consistent with its role in the encoding of affective experiences (see below). The correlational clusters associated with the insula were located in the posterior area, which is concerned with somatosensory processing (17). It therefore is possible that activation of this region represents visceral responses to highly emotionally arousing pictures. Activation in the thalamus (most likely the dorsomedial nucleus) suggests the interaction of cortical and subcortical regions and may reflect the integration of visceral and evaluative processes. What is striking about all three structures is the fact that they were left-lateralized in both men and women. Traditional accounts of hemispheric laterality would ascribe affective function to the right rather than left hemisphere (the “right-hemisphere” hypothesis, see ref. 18 for a review). Davidson's valence hypothesis, on the other hand, would ascribe approach-related emotion to the left and withdrawal-related emotion to the right hemisphere (19). The left-hemispheric laterality seen in these subcortical regions may indicate descending unilateral influences from language regions engaged in the evaluation required to rate emotional intensity. Indeed, both men and women exhibited activation in anterior speech areas of BA 44 and 45 that correlated with greater self-reported emotional arousal.
Distinct regions included the postcentral gyrus and the hippocampus for women and the putamen for men. Of these, the involvement of the hippocampus during emotional evaluation and experience is perhaps least surprising, because it receives afferent input from the amygdala (20). The involvement of the postcentral gyrus, a sensory area, and putamen, a region in the basal ganglia that may have inhibitory control over motor activities, is less interpretable. However, these regions have been identified in other imaging studies of emotion (21–23), and their specific roles in affective processes remains to be examined.
Encoding of Emotional Memories.
Consistent with prior findings (10, 24–26), better memory for highly emotional pictures correlated with greater amygdala activation in both sexes. There was a noticeable sex difference in the laterality pattern. At standard threshold levels to determine statistical significance, amygdala correlations were left-lateralized for women and right-lateralized for men, consistent with a prior report showing results from positron-emission tomography (16). Laterality differences between the sexes in amygdala encoding of emotional memories may reflect different encoding strategies. Participants were not warned before scanning that they would receive a later memory test, thus this sort of strategy reflects spontaneous or incidental differences between men and women. For example, women but not men may have used a language-based, left-lateralized encoding strategy. This hypothesis is consistent with evidence that the left but not right amygdala responds to verbally communicated threat (27). A related explanation is that women but not men encoded emotional stimuli by consciously accessible means (e.g., introspection or internal verbalization), which is consistent with a demonstration that the left and right amygdala are associated with conscious and unconscious learning, respectively (28). Men, in contrast, may have used a more visual-spatial strategy associated with right-hemisphere processes. Future research will need to test these hypotheses by explicitly manipulating subjects' cognitive encoding strategies.
In addition to the amygdala, this study shows that a number of other areas exhibit significant correlations between activation and better emotional memory. Some of these areas were common in men and women, such as the left anterior cingulate, fusiform, and precentral gyri. Activation of the anterior cingulate may represent subjects' attention to their own emotional responses (29, 30) rather than memory encoding per se. Evidence from a variant of the emotion Stroop task further indicates that this region is involved in emotional attention (31). Consistent with its presumed role in emotional attention, activation in this structure also correlated with self-reported emotional arousal in both sexes. The significant correlation of fusiform activation with better memory may represent an interaction between emotional arousal during encoding and modulation of early visual processing centers. A similar explanation has been proposed by Critchley et al. (32) when they observed a significant correlation between subjects' galvanic skin response and activation in the fusiform gyrus. Interestingly, the same study also reported negative correlations with skin conductance response in the supplemental motor area (BA 6) and premotor cortex (BA 4), which in the current study correlated with better emotional memory for men and women, respectively (see Table 2).
Relation Between Current Emotional Experience and Future Memories.
Women had significantly more brain regions than men in which greater activation correlated with both emotional-intensity ratings and better recognition memory for the most emotionally intense pictures. Such areas may be termed as being “concordant” in processing current emotional intensity and driving encoding processes that determine what experiences are remembered later. Areas of concordant processing included a number of structures previously identified in imaging studies as associated with memory encoding such as amygdala (16, 24–26), insula (27, 33), and hippocampus (34, 35) or with conscious emotional experience, emotion regulation, or attention to emotional stimuli such as the anterior cingulate (36–38). The greater number of concordant relations in women than men cannot be explained simply by the number of activations for each group, because men had significantly more memory-related activations than women.
It is possible that such concordant processing in the brain provides a direct and powerful neural mechanism between ongoing emotional experience and later memory for that experience. Conversely, separation of these two processes, at both psychological and neural levels of analysis, likely would weaken their relation. In that case, other nonemotional processes would play a greater role in determining what is to be remembered. It is noteworthy that the structure best known to mediate emotional memory enhancement, the amygdala, shows concordant activation in women. In contrast, emotional evaluation and emotional memory were “discordant” in men, because the evaluation and memory encoding activated different amygdalae. Those physically distinct activations suggest that, for men, different processes were engaged for either emotional evaluation (left amygdala) or emotional memory encoding (right amygdala). Therefore, these functional MRI findings raise the possibility that women's superior memory for emotional pictures is because of better integration of brain processes associated with emotional experience and encoding of that experience into memory.
Because sex differences in memory were found only for maximally arousing pictures, some critical analyses were based on maximal arousal ratings made by men and women. This approach had the advantage that only pictures rated as maximally arousing by each participant were analyzed for activations predicting later memory, which avoided a confound between these analyses and the fact that women rated more pictures as maximally arousing than did men. However, it is difficult to know whether equal ratings reported by both sexes corresponded to equal subjective emotional experiences. Use of a four-point scale may have introduced a ceiling effect. Future research can use alternative self-report measures (39, 40) that may have greater sensitivity of detecting group differences (41–43) or physiological measures of emotional arousal. However, men and women differ in physiological reactivity to emotional stimuli even when they evoke comparable subjective emotional experiences (11). Emotional arousal is likely to be a complex process, and no single measure is likely to quantify inner emotional experience.
The data presented here demonstrate that men and women differ in the neural networks engaged during emotional experience and memory encoding. The brain differences in activation do not indicate the etiology of those differences, because both genetic and environmental factors mold brain function. The different patterns of activation, however, are more consistent with the cognitive-style hypothesis (5), which predicts activation in different neural systems for emotional experience of comparable intensity, than the affect-intensity hypothesis (4), which predicts that women will have the same pattern of activation as men but to a greater degree. It may be possible to manipulate the cognitive style of subjects through instruction or different types of stimuli and thus shift the observable laterality pattern as a function of task rather than sex.
Supplementary Material
Abbreviations
BA, Brodmann area
References
- 1.Christianson S.-A. & Loftus, E. F. (1987) Appl. Cogn. Psychol. 1, 225-239. [Google Scholar]
- 2.LaBar K. S. & Phelps, E. A. (1998) Psychol. Sci. 9, 490-493. [Google Scholar]
- 3.Bradley M. M., Greenwald, M. K., Petry, M. C. & Lang, P. J. (1992) J. Exp. Psychol. Learn. Mem. Cognit. 18, 379-390. [DOI] [PubMed] [Google Scholar]
- 4.Fujita F., Diener, E. & Sandvik, E. (1991) J. Pers. Soc. Pychol. 61, 427-434. [DOI] [PubMed] [Google Scholar]
- 5.Seidlitz L. & Diener, E. (1998) J. Pers. Soc. Pychol. 74, 262-271. [DOI] [PubMed] [Google Scholar]
- 6.Herz R. S. & Cupchick, G. C. (1992) Chem. Senses 17, 519-528. [Google Scholar]
- 7.Robinson J. A. (1976) Cognit. Psychol. 8, 578-595. [Google Scholar]
- 8.Ross M. & Holmberg, D. (1990) in Self-Inference Processes: The Ontario Symposium, eds. Olson, J. M. & Zanna, M. P. (Earlbaum, Hillsdale, NJ), Vol. 6, pp. 135–152. [Google Scholar]
- 9.Lang P. J. & Greenwald, M. K., (1993) International Affective Picture System Standardization Procedure and Results for Affective Judgments: Technical Reports 1A–1C (Univ. of Florida Center for Research in Psychophysiology, Gainesville, FL).
- 10.Canli T., Zhao, Z., Brewer, J., Gabrieli, J. D. E. & Cahill, L. (2000) J. Neurosci. 20, RC99., 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lang P. J., Greenwald, M. K., Bradley, M. M. & Hamm, A. O. (1993) Psychophysiology 30, 261-273. [DOI] [PubMed] [Google Scholar]
- 12.Woods R. P., Cherry, S. R. & Mazziotta, J. C. (1992) J. Comput. Assist. Tomogr. 16, 620-633. [DOI] [PubMed] [Google Scholar]
- 13.Malonek D. & Grinwald, A. (1996) Science 272, 551-554. [DOI] [PubMed] [Google Scholar]
- 14.Talairach J. & Tournoux, P., (1988) Co-planar Stereotaxic Atlas of the Human Brain (Thieme, New York).
- 15.Xiong J., Gao, J.-H., Lancaster, J. L. & Fox, P. T. (1995) Hum. Brain Mapp. 3, 287-301. [DOI] [PubMed] [Google Scholar]
- 16.Cahill L., Haier, R. J., White, N. S., Fallon, J., Kilpatrick, L., Lawrence, C., Potkin, S. G. & Alkire, M. T. (2001) Neurobiol. Learn. Mem. 75, 1-9. [DOI] [PubMed] [Google Scholar]
- 17.Schneider R. J., Friedman, D. P. & Mishkin, M. (1993) Brain Res. 621, 116-120. [DOI] [PubMed] [Google Scholar]
- 18.Canli T. (1999) Neuroscientist 5, 201-207. [Google Scholar]
- 19.Davidson R. J. (1992) Psychol. Sci. 3, 39-43. [Google Scholar]
- 20.Tamraz J. C. & Comair, Y. G., (2000) Atlas of Regional Anatomy of the Brain Using MRI (Springer, New York).
- 21.Lane R. D., Chua, P. M. & Dolan, R. J. (1999) Neuropsychologia 37, 989-997. [DOI] [PubMed] [Google Scholar]
- 22.Damasio A. R., Grabowski, T. J., Bechara, A., Damasio, H., Ponto, L. L. B., Parvizi, J. & Hichwa, R. D. (2000) Nat. Neurosci. 3, 1049-1056. [DOI] [PubMed] [Google Scholar]
- 23.George M. S., Ketter, T. A., Parekh, P. I., Herscovitch, P. & Post, R. M. (1996) Biol. Psychiatry 40, 859-871. [DOI] [PubMed] [Google Scholar]
- 24.Canli T., Zhao, Z., Desmond, J. E., Glover, G. & Gabrieli, J. D. E. (1999) Psychobiology 27, 441-452. [Google Scholar]
- 25.Cahill L., Haier, R. J., Fallon, J., Alkire, M. T., Tang, C., Keator, D., Wu, J. & McGaugh, J. L. (1996) Proc. Natl. Acad. Sci. USA 93, 8016-8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamann S. B., Ely, T. D., Grafton, S. T. & Kilts, C. D. (1999) Nat. Neurosci. 2, 289-293. [DOI] [PubMed] [Google Scholar]
- 27.Phelps E. A., O'Connor, K. J., Gatenby, J. C., Gore, J. C., Grillon, C. & Davis, M. (2001) Nat. Neurosci. 4, 437-441. [DOI] [PubMed] [Google Scholar]
- 28.Morris J. S., Ohman, A. & Dolan, R. J. (1998) Nature (London) 393, 467-470. [DOI] [PubMed] [Google Scholar]
- 29.Lane R. D., Fink, G. R., Chau, P. M. & Dolan, R. J. (1997) NeuroReport 8, 3969-3972. [DOI] [PubMed] [Google Scholar]
- 30.Lane R. D., Reiman, E. M., Axelrod, B., Yun, L. S., Holmes, A. & Schwartz, G. E. (1998) J. Cogn. Neurosci. 10, 525-535. [DOI] [PubMed] [Google Scholar]
- 31.Whalen P. J., Bush, G., McNally, R. J., Wilhelm, S., McInerney, S. C., Jenike, M. A. & Rauch, S. L. (1998) Biol. Psychiatry 44, 1219-1228. [DOI] [PubMed] [Google Scholar]
- 32.Critchley H. D., Elliott, R., Mathias, C. J. & Dolan, R. J. (2000) J. Neurosci. 20, 3033-3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ploghaus A., Tracey, I., Gati, J. S., Clare, S., Menon, R. S., Matthews, P. M. & Rawlins, J. N. (1999) Science 284, 1979-1981. [DOI] [PubMed] [Google Scholar]
- 34.Gabrieli J. D. E., Brewer, J. B., Desmond, J. E. & Glover, G. H. (1997) Science 276, 264-266. [DOI] [PubMed] [Google Scholar]
- 35.Wagner A. D., Schacter, D. L., Rotte, M., Koutstaal, W., Maril, A., Dale, A. M., Rosen, B. R. & Buckner, R. L. (1998) Science 281, 1188-1191. [DOI] [PubMed] [Google Scholar]
- 36.Lane R. D., Reiman, E. M., Axelrod, B., Yun, L.-S., Holmes, A. & Schwartz, G. E. (1998) J. Cogn. Neurosci. 10, 525-535. [DOI] [PubMed] [Google Scholar]
- 37.Beauregard M., Levesque, J. & Bourgouin, P. (2001) J. Neurosci. 21, RC165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elliott R., Rubinsztein, J. S., Sahakian, B. J. & Dolan, R. J. (2000) NeuroReport 11, 1739-1744. [DOI] [PubMed] [Google Scholar]
- 39.Green B. G., Dalton, P., Cowart, B., Rankin, K. & Higgins, J. (1996) Chem. Senses 21, 323-334. [DOI] [PubMed] [Google Scholar]
- 40.Green B. G., Shaffer, G. S. & Gilmore, M. M. (1993) Chem. Senses 18, 683-702. [Google Scholar]
- 41.Bartoshuk L. M., Cunningham, K. E., Dabrila, G. M., Duffy, V. B., Etter, L., Fast, K. R., Lucchina, L. A., Prutkin, J. M. & Snyder, D. J. (1999) in Tastes and Aromas: The Chemical Senses in Science and Industry, eds. Bell, G. & Watson, A. J. (Univ. New South Wales Press, Sydney), pp. 12–22.
- 42.Bartoshuk L. M. (2000) Chem. Senses 25, 447-460. [DOI] [PubMed] [Google Scholar]
- 43.Lucchina L. A., Curtis, O. F., Putnam, P., Drewnowski, A., Prutkin, J. M. & Bartoshuk, L. M. (1998) Ann. N.Y. Acad. Sci. 855, 816-819. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.