Abstract
Circadian organization changes with age, but we do not know the extent to which age-related changes are the result of alterations in the central pacemakers, the peripheral oscillators, or the coupling mechanisms that hold the system together. By using transgenic rats with a luciferase (luc) reporter, we assessed the effects of aging on the rhythm of expression of the Period 1 (Per1) gene in the suprachiasmatic nucleus (SCN) and in peripheral tissues. Young (2 months) and aged (24–26 months) Per1-luc transgenic rats, entrained to light–dark cycles, were killed, and tissues were removed and cultured. Per1-luc expression was measured from 10 tissues. In the SCN, the central mammalian pacemaker, Per1-luc expression was robustly rhythmic for more than 7 weeks in culture. The only difference between SCN rhythmicity in young and old rats was a small but significant age-related shortening of the free-running period. Circadian rhythmicity in some peripheral tissues was unaffected by aging, whereas rhythmicity in other tissues was either phase advanced relative to the light cycle or absent. Those tissues that were arrhythmic could be induced to oscillate by application of forskolin, suggesting that they retained the capacity to oscillate but were not being appropriately driven in vivo. Overall, the results provide new insights into the effects of aging on the mammalian circadian system. Aging seems to affect rhythms in some but not in all tissues and may act primarily on interactions among circadian oscillators, perhaps attenuating the ability of the SCN to drive damped oscillators in the periphery.
The SCN is a circadian pacemaker structure that drives myriad behavioral and physiological rhythms in mammals (1). Surgical destruction of the SCN abolishes most circadian rhythms (2–5); however, some rhythms persist including mitosis in cornea and gut and disk shedding in the retina (6, 7). Circadian rhythms in wheel-running behavior, absent following complete SCN lesions, reappear when methamphetamine is provided chronically to SCN-lesioned rats (8). Furthermore, SCN-lesioned rats express a rhythm in food-anticipatory activity when exposed to restricted feeding and later fasted (9). Collectively, these observations reveal the existence of extra-SCN circadian oscillators. More recent results suggest that they may be present in most mammalian cells and tissues (10, 11).
Rapid progress in the cloning and identification of clock genes has revealed that these genes are expressed not only in the SCN but in many peripheral tissues as well (12). The recent development of a transgenic rat model allows for the continuous monitoring of Per1 transcription through use of a luciferase reporter (13). By using this methodology, it was discovered that several peripheral structures, as well as extra-SCN brain areas, exhibit circadian oscillations in vitro (13–15). Consequently, there is now direct evidence that the mammalian circadian system is composed of multiple circadian oscillators, a central pacemaker in the SCN, and damped oscillators in other regions of the brain and peripheral tissues. Such organization is similar to the circadian systems of Drosophila and zebra fish (16, 17).
Age-related changes in the mammalian circadian timing system have been studied in rodents and in humans. In several species of mammals, aging affects the amplitude and phase of circadian rhythms (18–20). The period of the circadian locomotor activity rhythm is shortened in aged rats (21–24) and in aged hamsters by most reports (20, 25–28); however, two studies of hamsters failed to reveal age-related changes in period (29, 30). The effect of aging on the free-running period in mice has not been consistent (18, 20). Similarly, in humans there have been conflicting results. An early study reported that the free-running period changed with age (19); however, a recent, more comprehensive study reveals that the period of the sleep/wake rhythm is essentially identical in young and elderly subjects (31).
A central unresolved issue is to what extent age-related changes in circadian behavior are the result of effects on the central SCN pacemaker, on peripheral oscillators, or on the mechanisms providing synchronization among contributing oscillators. There is evidence suggesting that some of the effects of aging are because of changes within the SCN. Histological changes in the SCN have been reported in aged rats (refs. 32 and 33); electrical activity rhythms in SCN slice cultures and dispersed SCN neurons taken from aged animals have lower amplitudes and are less precise than in SCN cultures prepared from young animals (34–37). Fetal SCN transplants restore or improve the circadian behavioral rhythms in aged animals (38, 39). These results suggest that the age-related changes in period and amplitude of overt rhythms may be because of age-related changes within the SCN.
Because it has been shown that electrical activity and neuropeptide content in the SCN are altered by aging, it is important to determine whether molecular oscillations in the SCN are likewise altered. The recent discovery of mammalian clock genes that are thought to comprise the central molecular oscillatory mechanism (12) provides the opportunity to measure directly age effects on a component of the clock mechanism. This opportunity has recently been exploited in a study in which several “clock” genes were measured from SCN, pineal, and paraventricular nucleus of the hypothalamus (PVN; ref. 40). Although the measurements were made during only a single cycle in vivo, the results suggest that the cycling of important clock genes [Per1, Per2, Cryptochrome1(Cry1)] appears largely unaffected by aging (the sole exception is an attenuation of light induction of the Per1 and Per2 genes).
By using transgenic rats with a luciferase reporter, we have been able to measure circadian rhythms of Per1 expression for multiple cycles in cultured tissues; consequently, we have been able to assess the effects of aging on the Per1 circadian rhythm in the SCN and nine other tissues. The results reveal several unanticipated effects of aging on molecular rhythms that may provide insights into the general effects of aging on the mammalian circadian system.
Materials and Methods
Animals.
A Per1-luciferase transgenic rat line [W(per1)1] maintained at the University of Virginia (13–15) was used in this study. All rats were raised in a 12-h light/12-h dark cycle (lights on at 0500, lights off at 1700) and kept in the same light/dark cycles until they were killed. One homozygous male, two homozygous females, and three heterozygous males comprised both the young (2 months old) and old (24–26 months old) experimental groups. As previously described, no differences were found between males and females (13–15). Although light signals from homozygous tissue exhibited higher photon counts than those from heterozygous tissues, no other differences were observed. All animal studies were conducted in accordance with the regulations of the Committee on Animal Care and Use at the University of Virginia.
Culture.
Within the hour before lights-off (1700), rats were anesthetized with CO2 and killed by decapitation. Brain, eyes, pineal, pituitary, liver, lung, and kidney were removed quickly and chilled in Hanks' buffered salt solution (GIBCO). Coronal sections of the brain (300 μm) were made by a Vibroslicer. The SCN, retrochiasmatic area (RCA), PVN, and arcuate nucleus were dissected and cultured separately on Millicell culture plate inserts (Millipore). Pineals were cut halfway through, flattened, and placed on the plate inserts. Pituitaries were hand-sliced, reduced to a small piece (≈1 mm3), and placed on the plate inserts. Whole corneas were dissected from eyes; hand-sliced liver, lung, and kidney were dissected into small pieces. They were cultured without the culture plate inserts. Each tissue was cultured in a 35-mm Petri dish with 1.2 ml of culture medium (serum-free, low sodium bicarbonate, no phenol red), DMEM (GIBCO) supplemented with 10 mM Hepes (pH 7.2), B27 (2%, GIBCO), and 0.1 mM luciferin (beetle luciferin, potassium salt, Promega) and antibiotics (25 units/ml penicillin, 25 μg/ml streptomycin). Bioluminescence was measured with photomultiplier tube (PMT) detector assemblies (MOD Hamamatsu), modified from HC135–01. PMTs (R3550) were specially selected with dark counts below 20 counts per sec at room temperature, and the prescale factor was reduced to 2. The modules and cultures were maintained in a light-tight, water-jacketed incubator at 36°C and interfaced to IBM PC-type computers for continuous data acquisition. The PMT was positioned about 2 cm above the culture, and photon counts were done through the glass coverslip and integrated over 1-min intervals. Dark counts (nonspecific counts) from the PMTs were about 20–40 per sec at 36°C. Light emission from cultured tissues was measured immediately upon placement in culture and continued without interruption for 7 days.
To determine whether forskolin could reinitiate oscillation in tissues with damped rhythms or induce oscillation in arrhythmic tissue, tissues (except SCN) taken from aged rats were stimulated by adding forskolin (final concentration 10 μM). After 1 h, the forskolin-containing medium was replaced with fresh medium.
We have previously shown that SCN explants from young rats exhibit sustained circadian oscillations for more than 1 month (13). To test whether the SCN taken from aged rats also sustains rhythmicity for long durations, we maintained four SCN cultures from aged rats for 40 days [5% of CO2, CO2 DMEM (GIBCO) with 10% new born calf serum; medium was changed once a week]. After 40 days these cultures were transferred into the recording incubator, and light signals were measured.
Data Analysis.
Phase and period measurements were calculated as in previous studies (14, 15). Briefly, the original data (1-min bins) were smoothed by an adjacent-averaging method with 2-h running means. The peak was calculated as the highest point of smoothed data. Because SCN, pituitary, and cornea exhibited robust oscillations usually sustained for more than 6 cycles, we were able to calculate periods from these tissues. From the data sets, 3-h and 24-h running means were calculated, and crossings of these two estimated profiles provided the rising and falling phase markers for each cycle. The free-running period was computed as the mean between rising markers in each cycle (15). Statistically significant differences of mean periods between young and old animals are based on t tests (P < 0.05).
Results
Prior research with rodents revealed age-related changes in the amplitude and regularity of rhythm expression as well as changes in the free-running period and phase of the behavioral rhythm. Consequently, in evaluating molecular rhythmicity in different tissues, we focused our attention on the “robustness” (i.e., amplitude, sustainability, cycle-to-cycle regularity) of the rhythm, its free-running period, and the phase relationship between the rhythm, measured in vitro, and the prior light cycle. Our measurements of Per1-luc activity conform to and extend earlier in vivo observations in aged animals (40), indicating that our in vitro reporter gene measurements reflect faithfully the in vivo Per1 rhythm.
Robustness of Circadian Rhythm Expression.
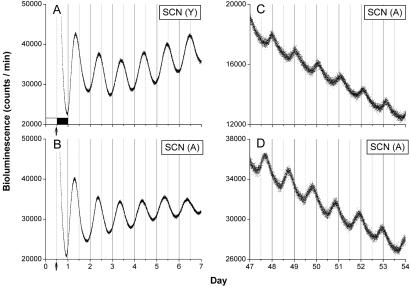
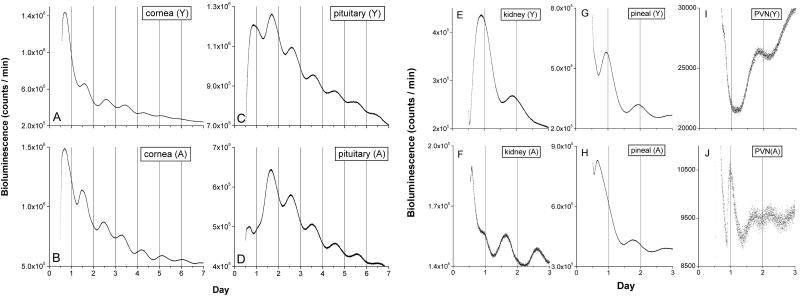
Aging had little effect on the robustness of the circadian rhythm of Per1 expression in several tissues, including the SCN. All SCN cultures taken from both young and old rats exhibited prominent circadian rhythms during 7 days of recording (Fig. 1 A and B). Four SCN from aged rats were maintained in vitro, and all oscillations were sustained and could be recorded after 50 days in vitro (Fig. 1 C and D). In addition to the SCN, aging also appeared to have little effect on the strength of rhythmic expression of several other tissues including cornea, pituitary, kidney, pineal, PVN (Fig. 2), liver, and arcuate nucleus (data not shown).
Fig 1.
SCN from aged rats display robust circadian oscillations in vitro. Representative circadian rhythms of Per1-luc luminescence from cultured SCN explanted from young (A) and aged (B) rats. The tissue was explanted just before lights-off (arrows). Light output (in counts/min) is plotted against previous light onset (hour 0). Four SCN from aged rats were maintained in vitro for 40 days before recording (two examples are shown in C and D). Circadian rhythms persisted in all four explants, and as expected, the explants were out of phase with one another after 40 days in culture.
Fig 2.
Circadian rhythms of cultured cornea, pituitary, kidney, pineal, and PVN. Data were plotted as in Fig. 1 (A and B). Aging did not alter the robustness of circadian oscillations in cornea and pituitary. Shown are representative recordings from cornea of young (A) and aged (B) rats and pituitary from young (C) and aged (D) rats. Aging did alter the phase of kidney, pineal, and PVN. Shown are examples of circadian rhythms from kidney of young (E) and old (F), pineal from young (G) and old (H), and PVN from young (I) and old (J) animals. There was no difference in the robustness of these oscillations between young and old animals. Peak Per-luc activity in kidney and pineal cultures from aged rats were ≈4-h phase advanced compared with tissue from young rats. A sharp peak at the beginning of subjective day (J) was observed in most PVN from aged rats but not from young rats (see Results and Fig. 5 for details).
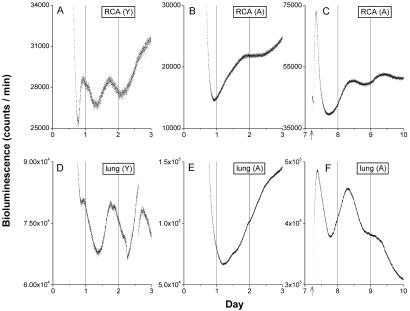
In other two-tissue explants from the RCA and lung, the expression of rhythmicity was affected by aging. Whereas most RCA and lung tissue from young rats displayed robust circadian rhythms (Fig. 3 A and D), in only half of the aged rats did the RCA and lung display circadian rhythms similar to those from young animals. The RCA and lung tissues of the remaining aged rats were arrhythmic in vitro (Fig. 3 B and E). Circadian rhythms could be initiated in these arrhythmic tissues by forskolin stimulation (Fig. 3 C and F), indicating that the aged tissues retained the capacity to oscillate.
Fig 3.
Aging disrupted the circadian rhythms in RCA and lung. Luminescence of cultured RCA from young (A) and old (B) and from lung of young (D) and old (E) animals are plotted against previous light onset (hour 0). RCA and lung from young rats showed clear circadian oscillations, but RCA and lung from old rats showed weak or no circadian oscillation. Circadian oscillation in old tissue could be induced by forskolin stimulation (arrows) in previously arrhythmic RCA (C) and lung (F).
Free-Running Period of Per1 Rhythm.
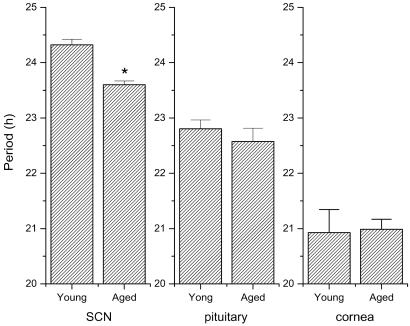
We were able to obtain reliable free-running period estimates from the SCN, pituitary, and cornea of young and aged animals (Fig. 4). We found that the free-running periods of the cornea and pituitary were not affected by aging. The free-running period of the pituitary was approximately 23 h and the cornea was about 21 h in both young and old rats. In contrast to these tissues, the free-running period of the SCN rhythm calculated from the first 7 days in culture in aged rats was shortened significantly compared with younger animals (0.72 h). All four aged SCN cultures maintained over 50 days exhibited a lengthening of the free-running period when compared with the initial 7 days of recording (Fig. 1 C and D). A similar lengthening of the free-running period after extended durations in culture was observed in young and neonate SCN tissue, suggesting that the lengthening in free-running period during maintenance in culture is not related to the age of the tissue (S.Y., M.M., and G.D.B., unpublished results).
Fig 4.
Aging shortened the circadian free-running period of the SCN but not of the pituitary or cornea. Mean period (± SEM) of SCN, pituitary, and cornea are shown. *, Statistically significant (P < 0.001, t test). These periods were based on the first 6–7 days of culture. The sample size is shown in Fig. 5.
Effects of Aging on the Entrained Phase of Tissue Rhythms.
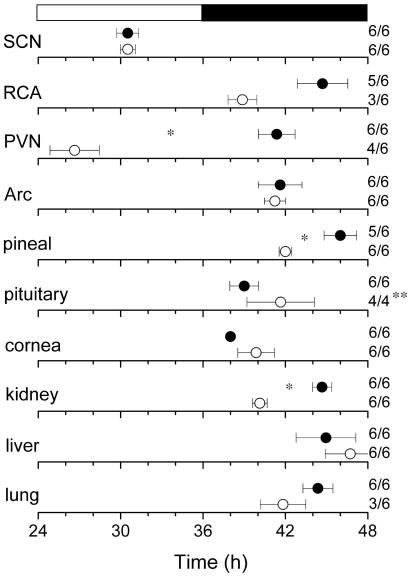
Prior studies revealed that different brain and peripheral tissues have different phase relationships to the entraining light cycles or feeding schedule (13–15). Whereas the SCN, when entrained by light–dark = 12:12 light cycles, exhibits peak activity during the middle of the subjective day, most other tissues, both neural and nonneural, exhibit peak Per1-luc activity during the subjective night. In the present study, most aged tissues, including the SCN, exhibited an unchanged phase relationship with the light–dark cycle (Fig. 5). There were, however, at least three exceptions. Both the pineal and the kidney were phase advanced by 4.0 h and 4.5 h, respectively, in aged animals. The RCA also appeared to be phase advanced by ≈6 h; however, perhaps because of the small sample size from aged animals, statistical analysis did not reveal significant differences. Finally, the PVN showed a very large change in entrained phase angle. However, three of four PVNs in aged animals exhibited an unusual rhythm with a sharp peak near dawn, followed by a low amplitude oscillation making it difficult to calculate phase with certainty (Fig. 2J).
Fig 5.
Phase map for central and peripheral circadian oscillators. The peak of the circadian oscillation was determined during the interval between 12 h and 36 h in culture. The average times (± SEM) of peaks were plotted against the time of last lights-on. •, data from young rats; ○, data from aged rats. The light–dark cycle to which the animals were exposed before killing (black and white bar) is shown at Top. (Right) The sample size is shown (number of rhythmic tissues/number of tissues tested). *, Statistically significant (P < 0.01, t test); **, the pituitary in two aged rats had tumors and consequently were excluded from the analysis. Arc, arcuate nucleus.
Discussion
Persistence and Loss of Rhythmicity in Aged Tissues.
Asai and coinvestigators observed that the in vivo expression profiles of rPer1, rPer2, and rCry1 mRNA were very similar in the SCN, the PVN, and the pineal of young and aged (22–26 months old) rats (40). This conclusion was based on sampling mRNA levels by in situ hybridization at five or six different time points following one complete cycle of darkness. Our in vitro results, employing dynamic imaging of the luciferase reporter of Per1 activity in those three tissues, are in general agreement with their data. Furthermore, we were able to measure free-running circadian rhythms in five other tissues in isolation with similar results. It should be born in mind that there are substantial differences in the capacity of different tissues to generate sustained circadian rhythms of gene expression (13–15), with the SCN producing by far the most robust, sustained circadian periodicity. The fact that many tissues damp rapidly in vitro permits only a qualitative comparison between rhythmicity of the tissue level in young and old animals, making it impossible to detect small age-related changes in rhythm expression. The current study departs from earlier in situ hybridization studies in measuring the ability of young and aged tissues to sustain circadian rhythmicity in vitro. Our results demonstrate that intrinsic rhythmicity persists in many tissues of aged animals. There were two tissues from aged rats that were not always rhythmic in vitro; RCA and lung were rhythmic in only half of the aged rats. However, these tissues did not lose their capacity to oscillate because rhythmicity could be initiated by application of forskolin, an adenylate cyclase activator. The fact that these tissues required stimulation to express rhythmicity suggests that they may not have been oscillating in vivo. Indeed, it is possible that the aged SCN has lost some of its effectiveness during damped peripheral oscillations.
We recognize that we must be cautious in interpreting changes in the waveform and “sustainability” of rhythmic tissues. First, we cannot exclude the possibility that rhythmicity in some tissues is either stopped or initiated at the time of tissue preparation. Although we find no evidence of initial phase being linked to dissection time, this possibility cannot be fully excluded. We must also be cautious in our interpretation of the circadian waveform. By using in situ hybridization methods, we and other investigators have confirmed that the phase of the Per 1 mRNA rhythm matches Per1-luc rhythmicity; however, the amplitude of the bioluminescent signal may not accurately reflect the amplitude of the Per 1 mRNA (41).
There is some evidence to suggest that age-related changes in the SCN could affect its ability to entrain peripheral oscillators. The amplitude of the electrical activity rhythm from SCN slices is reduced in aged rats (34–36), and although the total number of SCN neurons remains the same (32, 42), the number of neurons expressing arginine vasopressin (AVP) and vasoactive intestinal polypeptide (VIP) is decreased (32, 33). Krajnak et al. (43) reported that VIP mRNA levels in the SCN exhibited a daily rhythm in young female rats but that the rhythm was absent by the time animals were middle-aged. A decrease in neurotransmitter production of the SCN and a low amplitude of electrical activity might weaken the ability of the SCN to entrain peripheral oscillators.
Another possible explanation for the presence of arrhythmic tissues in aged rats is that weakened behavioral and physiological rhythms may provide less effective signals to the periphery. Such signals (e.g., from feeding rhythms) are known to be important for entraining peripheral oscillators (14). Aged rats have lower amplitude locomotor activity rhythms and are less active overall (28, 44). If locomotor activity feeds back to the lung (e.g., the lung is more active when animals are exercising), the lungs of aged, inactive animals may fail to receive sufficient periodic stimulation to continue oscillating. It has been reported that the amplitude of the feeding and drinking rhythm is reduced in aged rats, although the age-related decline of the amplitude of drinking rhythm is less prominent than that of feeding behavior (45). However, we did not observe any clear age-related difference in robustness of the oscillation in either the liver or kidney. Unfortunately, little is presently known about the coupling signals that stimulate peripheral oscillators to remain rhythmic in vivo. The identification of these signals, either released directly by the SCN or secondarily generated by SCN control of periodic behaviors and physiology, will enable a more systematic study of the effects of aging on the network properties of the circadian system.
Age-Related Changes in the Free-Running Period.
Age-dependent changes in the free-running period of locomotor activity in several mammals have been reported, although the effects vary among species. However, in rats, aging consistently shortens the free-running period (21–24).
In the present study, we observed age-related shortening of the free-running period of the Per1-luc rhythm in the SCN, but not in rhythms of the pituitary or cornea. These results suggest that the shortening observed in the behavioral rhythm in rats is caused by the shortening of the free-running period of molecular events within the SCN. However, although the period shortening of the molecular rhythm may be responsible for the altered period in rhythmic locomotor behavior, other age-related changes in locomotor behavior do not appear to be reflected in the Per1 molecular rhythm. For example, whereas the Per1-luc rhythms within the SCN of young and aged rats display similar amplitude and phase, behavioral rhythms of old rats exhibit reduced amplitude and an advanced phase angle of entrainment. This suggests that some age-related alterations are because of circadian system changes outside of the SCN or, alternatively, because of changes within the SCN other than the Per1 transcript that we have measured. Very recently, Weinert et al. (46) reported that the amplitude of day–night expression of mPer2 but not of mPer1 in the SCN was decreased in aged mice. However, this change in mPer2 amplitude may be species-specific. No change in amplitude was observed in rPer2 in the SCN of aged rats measured by in situ hybridization (40).
Advanced Phase Angle for Entrainment.
Many rhythms in behavior and physiology in old animals are phase advanced during steady-state entrainment. This phenomenon has been observed in the human sleep–wake rhythm (47, 48), temperature rhythms (48), and hormonal rhythms (49). Mice and hamsters initiate locomotor activity earlier when they get old (50, 51). Body temperature and activity rhythms in rats and plasma insulin and corticosterone rhythms in mice are also advanced by aging (20, 44, 52).
Despite the age-related change in the period of the SCN Per1-luc rhythmicity, there was no change in the entrained phase angle of SCN (Fig. 5). However, the kidney and pineal of aged rats showed significantly advanced phase relative to the light cycle. These results suggest that the change in phase relationships of behavioral and physiological rhythms is not because of age-related changes in the entrained phase of the SCN itself but rather is because of age-related alterations in other rhythmic components of the circadian system. The observation that the pineal and kidney are phase advanced reveals that some but not all circadian oscillators exhibit age-related changes in entrained phase. Once again, an important caveat is that the absence of a phase advance in the Per1-luc molecular rhythm does not rule out the possibility that the SCN as a whole is phase advanced in aged animals. It will be important to look at the phase of the SCN electrical rhythm in both brain slice and in vivo to determine whether the SCN electrical output signals of aged animals exhibit a normal or advanced phase.
The Effects of Aging on Circadian Organization.
Our current understanding of the multioscillatory nature of the mammalian circadian system provides many potential targets on which the aging process could exert its effects (13–15). Our results reveal that aging acts differentially at several different loci within the circadian system. The observation that some tissues, such as lung and RCA, are not rhythmic when removed from the animal but can be induced to rhythmicity suggests a failure of periodic stimulation in vivo. This may reflect a deterioration of coupling mechanisms between the robust self-sustained and damped oscillators. In addition, aging appears to act on some but not all tissues exhibiting circadian oscillations by altering the phase of the entrained rhythm (e.g., kidney and pineal) or shortening the free-running period (as in the SCN).
The lack of age-related impact on molecular cycling within the SCN is surprising. With the exception of a shortening of the free-running period, the Per1-luc molecular rhythm appears unaffected by aging as is also the case for rPer2 and rCry1 (40). In contrast, there is evidence in hamster, mouse, and rat that SCN rhythms of neuronal electrical activity, both in brain slices (34–36) and in dispersed neurons (37), is affected significantly by aging. Brain slices from aged animals exhibit lower amplitude and more irregular rhythms than those from young animals (34–36). Pronounced deterioration in electrical rhythmicity also has been reported from dispersed neurons from middle-aged mice (37).
There are several possible explanations for the differential effects of aging on the robustness of neuronal and molecular rhythms of the SCN. Age-related changes in electrical activity may be because of alterations in the amplitude and regularity of molecular components of the clock, other than Per1. Other molecular components of the biological clock of SCN may be more profoundly affected by aging, and their behavior may contribute to the changes in the electrical rhythm. However, this explanation is weakened by the failure to observe changes in the amplitude of RNA expression in three central clock genes (40). Another possibility is that aging acts “downstream” of the molecular feedback loop, either on clock-regulated gene expression or directly on the biochemical and biophysical events involved in the production and expression of the electrical impulse rhythm. Another possibility that must be considered is that the recording process itself, which is much more invasive for electrical than for optical measurements, may introduce stress on aged tissues that affects the expression of the rhythm. Thus, the more pronounced effects on electrical rhythmicity may reflect an inability of aged tissue to tolerate in vitro experimental procedures rather than represent bona fide age-related change in the clock mechanism. These issues can be experimentally resolved by recording electrical and molecular rhythmicity simultaneously in both SCN tissue and dispersed neurons. In any event, it is not clear how either the molecular or the electrical events within the SCN are coupled to behavioral or physiological outputs. Indeed, several studies suggest, perhaps counterintuitively, that the SCN output signal controlling locomotor behavior is chemical rather than electrical (53, 54).
The current study has provided evidence that the aging process does not impact all oscillatory tissues in a uniform manner; there are system-level alterations as well as changes within individual oscillatory tissues. This suggests that the effects of aging on behavioral and physiological rhythmicity are likely to be complex—the results of changes at several levels of circadian organization. To fully understand the impact of aging on biological timing, we will have to look beyond the SCN to rhythmic and quasi-rhythmic tissues both within and outside of the nervous system. In addition, we will have to identify and understand the mechanisms that maintain normal temporal order within the multioscillator ensemble that is the mammalian circadian system.
Acknowledgments
We thank Tom Breeden for computer programming assistance; Crystal D. Wilson and Catharine R. Cowan for technical assistance; and Edward M. Blumenthal, Alec Davidson, and Tomoko Yoshikawa for comment on this manuscript. This work was supported in part by the National Science Foundation Center for Biological Timing and National Institutes of Health Grants MH62517 (to G.D.B.) and MH56647 (to M.M.).
Abbreviations
SCN, suprachiasmatic nucleus
RCA, retrochiasmatic area
PVN, paraventricular nucleus of the hypothalamus
PMT, photomultiplier tube
References
- 1.Klein D. C., Moore, R. Y. & Reppert, S. M., (1991) The Mind's Clock (Oxford Univ. Press, New York).
- 2.Moore R. Y. & Eichler, V. B. (1972) Brain Res. 42, 201-206. [DOI] [PubMed] [Google Scholar]
- 3.Stephan F. K. & Zucker, I. (1972) Proc. Natl. Acad. Sci. USA 69, 1583-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rusak B. & Zucker, I. (1979) Physiol. Rev. 59, 449-526. [DOI] [PubMed] [Google Scholar]
- 5.Meyer-Bernstein E. L., Jetton, A. E., Matsumoto, S. I., Markuns, J. F., Lehman, M. N. & Bittman, E. L. (1999) Endocrinology 140, 207-218. [DOI] [PubMed] [Google Scholar]
- 6.Scheving L. E., Tsai, T. H., Powell, E. W., Pasley, J. N., Halberg, F. & Dunn, J. (1983) Anat. Rec. 205, 239-249. [DOI] [PubMed] [Google Scholar]
- 7.Terman J. S., Reme, C. E. & Terman, M. (1993) Brain Res. 605, 256-264. [DOI] [PubMed] [Google Scholar]
- 8.Honma K., Honma, S. & Hiroshige, T. (1987) Physiol. Behav. 40, 767-774. [DOI] [PubMed] [Google Scholar]
- 9.Mistlberger R. E. (1994) Neurosci. Biobehav. Rev. 18, 171-195. [DOI] [PubMed] [Google Scholar]
- 10.Tosini G. & Menaker, M. (1996) Science 272, 419-421. [DOI] [PubMed] [Google Scholar]
- 11.Balsalobre A., Damiola, F. & Schibler, U. (1998) Cell 93, 929-937. [DOI] [PubMed] [Google Scholar]
- 12.Reppert S. M. & Weaver, D. R. (2000) J. Biol. Rhythms 15, 357-364. [DOI] [PubMed] [Google Scholar]
- 13.Yamazaki S., Numano, R., Abe, M., Hida, A., Takahashi, R., Ueda, M., Block, G. D., Sakaki, Y., Menaker, M. & Tei, H. (2001) Science 288, 682-685. [DOI] [PubMed] [Google Scholar]
- 14.Stokkan K.-A., Yamazaki, S., Tei, H., Sakaki, Y. & Menaker, M. (2001) Science 291, 490-493. [DOI] [PubMed] [Google Scholar]
- 15.Abe M., Herzog, E. D., Yamazaki, S., Straume, M., Tei, H., Sakaki, Y., Menaker, M. & Block, G. D. (2002) J. Neurosci. 22, 350-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plautz J. D., Kaneko, M., Hall, J. C. & Kay, S. A. (1997) Science 278, 1632-1635. [DOI] [PubMed] [Google Scholar]
- 17.Whitmore D., Foulkes, N. S. & Sassone-Corsi, P. (2000) Nature (London) 404, 27-28. [DOI] [PubMed] [Google Scholar]
- 18.Valentinuzzi V. S., Scarbrough, K., Takahashi, J. S. & Turek, F. W. (1997) Am. J. Physiol. 273, R1957-R1964. [DOI] [PubMed] [Google Scholar]
- 19.Wever R. A., (1979) The Circadian System of Man (Springer, Berlin).
- 20.Weinert D. (2001) Chronobiol. Int. 17, 261-283. [DOI] [PubMed] [Google Scholar]
- 21.Richter C. P. (1967) in Comparative Psychopathology, eds. Zubin, J. & Hunt, H. F. (Grune & Stratton, London), pp. 205–227.
- 22.van Gool W. A., Witting, W. & Mirmiran, M. (1987) Brain Res. 413, 384-387. [DOI] [PubMed] [Google Scholar]
- 23.Rietveld W. J., Buijs, P. & Ruis, J. (1988) J. Interdiscipl. Cycle Res. 19, 289-295. [Google Scholar]
- 24.Witting W., Mirmiran, M., Bos, N. P. & Swaab, D. F. (1994) Chronobiol. Int. 2, 103-112. [DOI] [PubMed] [Google Scholar]
- 25.Pittendrigh C. S. & Daan, S. (1974) Science 186, 548-550. [DOI] [PubMed] [Google Scholar]
- 26.Morin L. P. (1988) J. Biol. Rhythms 3, 237-248. [Google Scholar]
- 27.Rosenberg R. S., Zee, P. C. & Turek, F. W. (1991) Am. J. Physiol. 261, R491-R495. [DOI] [PubMed] [Google Scholar]
- 28.Aschoff J. (1994) in Evolution of Circadian Clock, eds. Hiroshige, T. & Honma, K. (Hokkaido Univ. Press, Sapporo, Japan), pp. 23–44.
- 29.Davis F. C. & Viswanathan, N. (1998) Am. J. Physiol. 275, R960-R968. [DOI] [PubMed] [Google Scholar]
- 30.Duffy J. F., Viswanathan, N. & Davis, F. C. (1999) Neurosci. Lett. 271, 77-80. [DOI] [PubMed] [Google Scholar]
- 31.Czeisler C. A., Duffy, J. F., Shanahan, T. L., Brown, E. N., Mitchell, J. F., Rimmer, D. W., Ronda, J. M., Silva, E. J., Allan, J. S., Emens, J. S., et al. (1999) Science 284, 2177-2181. [DOI] [PubMed] [Google Scholar]
- 32.Roozendaal B., van Gool, W. A., Swaab, D. F., Hoogendijk, J. E. & Mirmiran, M. (1987) Brain Res. 409, 259-264. [DOI] [PubMed] [Google Scholar]
- 33.Chee C. A., Roozendaal, B., Swaab, D. F., Goudsmit, E. & Mirmiran, M. (1988) Neurobiol. Aging 9, 307-312. [DOI] [PubMed] [Google Scholar]
- 34.Satinoff E., Li, H., Tcheng, T. K., Liu, C., McArthur, A. J., Medanic, M. & Gillette, M. U. (1993) Am. J. Physiol. 265, R1216-R1222. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe A., Shibata, S. & Watanabe, S. (1995) Brain Res. 695, 237-239. [DOI] [PubMed] [Google Scholar]
- 36.Ruby N. F., Edgar, D. M., Heller, H. C. & Miller, J. D. (1998) Brain Res. 779, 338-341. [DOI] [PubMed] [Google Scholar]
- 37.Aujard F., Herzog, E. D. & Block, G. D. (2001) Neuroscience 106, 255-261. [DOI] [PubMed] [Google Scholar]
- 38.Van Reeth O., Zhang, Y., Zee, P. C. & Turek, F. W. (1994) Brain Res. 643, 338-342. [DOI] [PubMed] [Google Scholar]
- 39.Li H. & Satinoff, E. (1998) Am. J. Physiol. 275, R1735-R1744. [DOI] [PubMed] [Google Scholar]
- 40.Asai M., Yoshinobu, Y., Kaneko, S., Mori, A., Nikaido, T., Moriya, T., Akiyama, M. & Shibata, S. (2001) J. Neurosci. Res. 66, 1133-1139. [DOI] [PubMed] [Google Scholar]
- 41.Wilsbacher L. D., Yamazaki, S., Herzog, E. D., Song, E. J., Radcliffe, L. A., Abe, M., Block, G., Spitznagel, E., Menaker, M. & Takahashi, J. S. (2002) Proc. Natl. Acad. Sci. USA 99, 489-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madeira M. D., Sousa, N., Santer, R. M., Paula-Barbosa, M. M. & Gundersen, H. J. (1995) J. Comp. Neurol. 361, 585-601. [DOI] [PubMed] [Google Scholar]
- 43.Krajnak K., Kashon, M. L., Rosewell, K. L & Wise, P. M. (1998) J. Nerurosci. 18, 4767-4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benstaali C., Bogdan, A. & Touitou, Y. (2002) Pflügers Arch. 444, 73-79. [DOI] [PubMed] [Google Scholar]
- 45.Peng M. T., Chen, Y. T., Hung, S. H. & Yaung, C. L. (1990) Proc. Natl. Sci. Council Repub. China B 14, 98-104. [PubMed] [Google Scholar]
- 46.Weinert H., Weinert, D., Schurov, I., Maywood, E. S. & Hastings, M. H. (2001) Chronobiol. Int. 18, 559-565. [DOI] [PubMed] [Google Scholar]
- 47.Reilly T., Waterhouse, J. & Atkinson, G. (1997) Occup. Environ. Med. 54, 812-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duffy J. F., Dijk, D. J., Klerman, E. B. & Czeisler, C. A. (1998) Am. J. Physiol. 275, R1478-R1487. [DOI] [PubMed] [Google Scholar]
- 49.Van Cauter E., Leproult, R. & Kupfer, D. J. (1996) J. Clin. Endocrinol. Metab. 81, 2468-2473. [DOI] [PubMed] [Google Scholar]
- 50.Zee P. C., Rosenberg, R. S. & Turek, F. W. (1992) Am. J. Physiol. 263, R1099-R1103. [DOI] [PubMed] [Google Scholar]
- 51.Weinert H. & Weinert, D. (1998) Biol. Rhythm Res. 29, 159-178. [Google Scholar]
- 52.Yunis E. J., Fernandes, G., Nelson, W. & Halberg, F. (1974) in Chronobiology, eds. Scheving, L. E., Halberg, F. & Pauly, J. E. (Georg Thieme, Stuttgart), pp. 358–363.
- 53.Silver R., LeSauter, J., Tresco, P. A. & Lehman, M. N. (1996) Nature (London) 382, 810-813. [DOI] [PubMed] [Google Scholar]
- 54.Kramer A., Yang, F. C., Snodgrass, P., Li, X., Scammell, T. E., Davis, F. C. & Weitz, C. J. (2001) Science 294, 2511-2515. [DOI] [PubMed] [Google Scholar]