Abstract
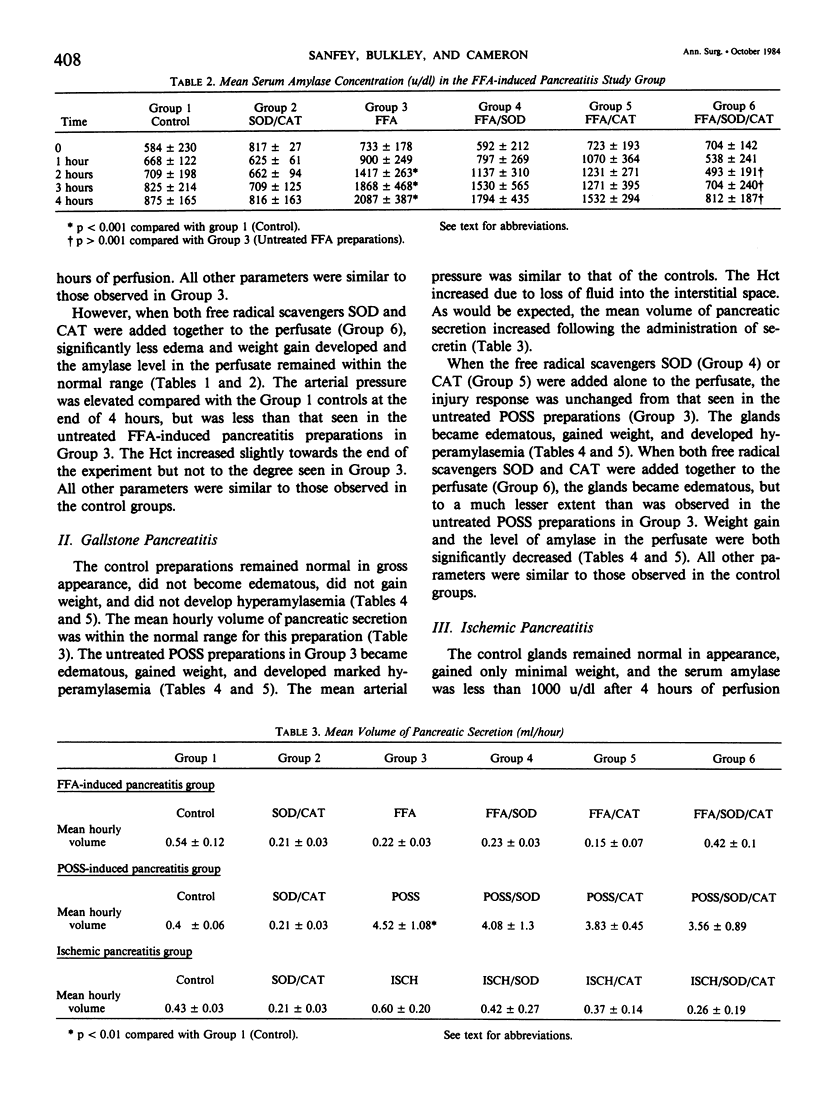
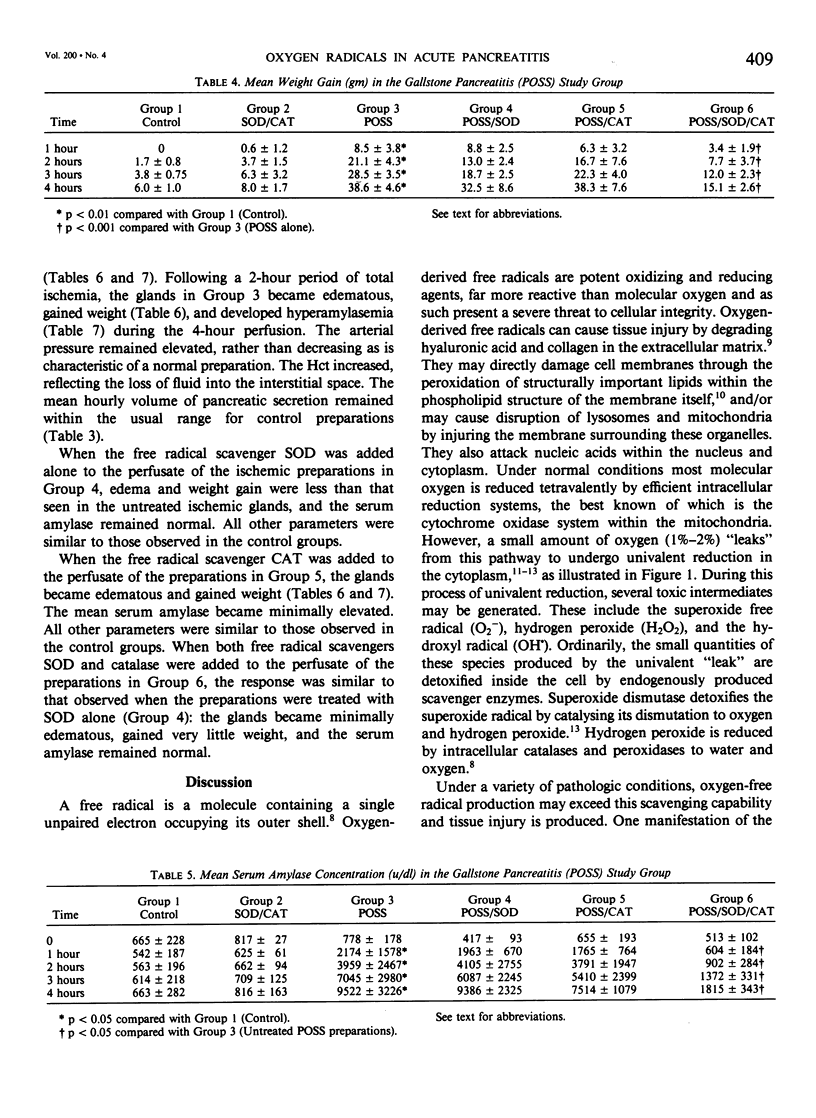
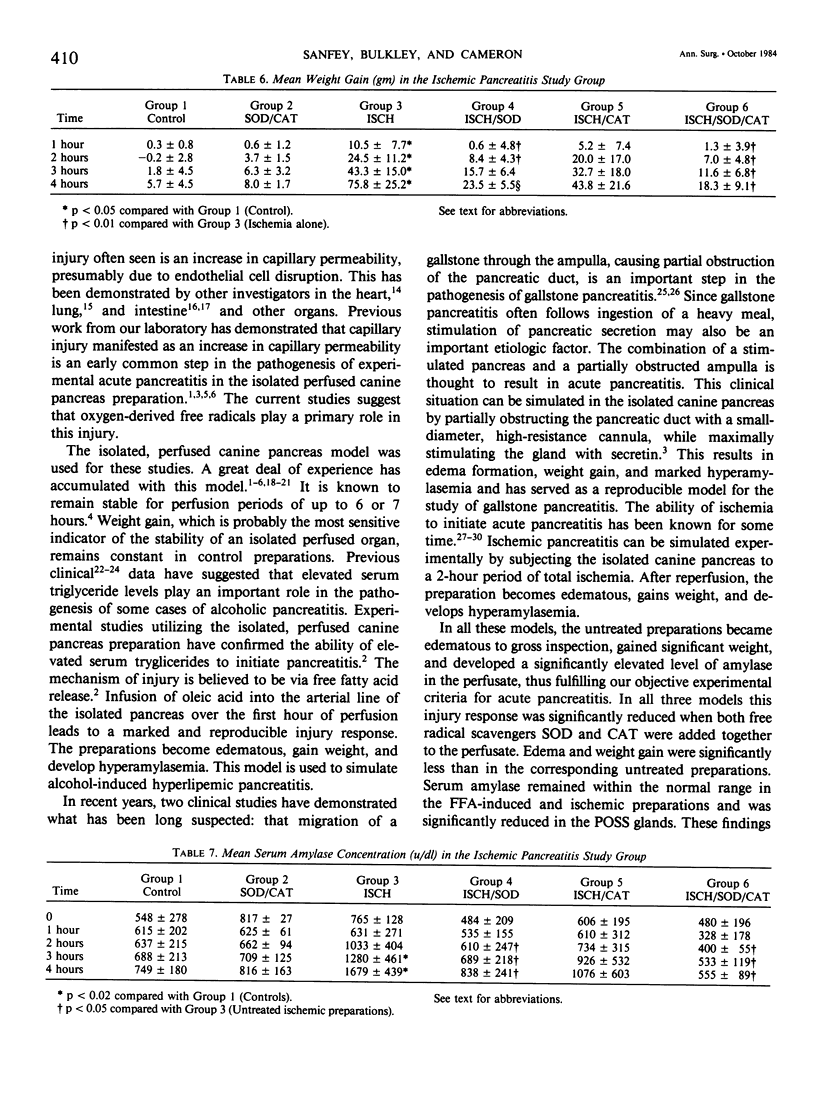
Acute pancreatitis may be initiated in the ex vivo, perfused canine pancreas preparation by a variety of stimuli. These include oleic acid infusion (FFA), partial duct obstruction with secretin stimulation (POSS), and a 2-hour period of ischemia (ISCH). In each model, pancreatitis is characterized by weight gain, edema, and hyperamylasemia. Oxygen-derived free radicals such as superoxide, hydrogen peroxide, and the hydroxyl radical are highly reactive toxic substances that are normally produced in small amounts during oxidative metabolism. Ordinarily, these substances are detoxified by endogenous intracellular enzymes called free radical scavengers (FRS), such as superoxide dismutase (SOD) and catalase (CAT). These studies were undertaken to evaluate the possible role of oxygen-derived free radicals in the initiation of acute pancreatitis in the isolated canine model. All preparations were perfused for 4 hours with autologous blood. Controls (N = 6): these glands remained normal in appearance, gained minimal weight (6 +/- 1 g), and serum amylase remained normal (less than 1000 u/dl). FFA pancreatitis, FFA alone (N = 6): these glands became edematous, gained weight (113.5 +/- 27.0 g), and developed hyperamylasemia (2087 +/- 387 u/dl). FFA + FRS (N = 6), SOD (50 mg) and CAT (50 mg) were added to the perfusate at time zero: these glands became only minimally edematous, gained less weight (31.8 +/- 10.1 g, p less than 0.05), and amylase remained normal (p less than 0.05). POSS pancreatitis, POSS alone (N = 8): these glands became edematous, gained weight (38.6 +/- 4.6 g), and developed marked hyperamylasemia (9522 +/- 3226 u/dl). POSS + FRS (N = 6): these glands did not develop edema, gained less weight (15.1 +/- 2.6 g, p less than 0.05), and serum amylase only increased to 1815 +/- 343 u/dl, (p less than 0.05). ISCH pancreatitis, ISCH alone (N = 6): these glands became edematous, gained weight (75.8 +/- 25 g), and developed hyperamylasemia (1679 +/- 439 u/dl). ISCH + FRS (N = 6): these glands did not develop edema, gained only 18.3 +/- 9.0 g (p less than 0.005), and serum amylase remained normal (p less than 0.05). These studies demonstrate that, in this canine preparation, acute pancreatitis is significantly ameliorated by oxygen-free radical scavengers. Since this was true whether the pancreatitis was produced by FFA infusion, POSS, or ischemia, it suggests that oxygen-derived free radicals may mediate a common essential step in the pathogenesis of all forms of pancreatitis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acosta J. M., Ledesma C. L. Gallstone migration as a cause of acute pancreatitis. N Engl J Med. 1974 Feb 28;290(9):484–487. doi: 10.1056/NEJM197402282900904. [DOI] [PubMed] [Google Scholar]
- Brawn K., Fridovich I. Superoxide radical and superoxide dismutases: threat and defense. Acta Physiol Scand Suppl. 1980;492:9–18. [PubMed] [Google Scholar]
- Broe P. J., Cameron J. L. Azathioprine and acute pancreatitis: studies with an isolated perfused canine pancreas. J Surg Res. 1983 Feb;34(2):159–163. doi: 10.1016/0022-4804(83)90055-0. [DOI] [PubMed] [Google Scholar]
- Broe P. J., Cameron J. L. Experimental gallstone pancreatitis. Pathogenesis and response to different treatment modalities. Ann Surg. 1982 May;195(5):566–573. doi: 10.1097/00000658-198205000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broe P. J., Zuidema G. D., Cameron J. L. The role of ischemia in acute pancreatitis: studies with an isolated perfused canine pancreas. Surgery. 1982 Apr;91(4):377–382. [PubMed] [Google Scholar]
- Bulkley G. B. The role of oxygen free radicals in human disease processes. Surgery. 1983 Sep;94(3):407–411. [PubMed] [Google Scholar]
- Cameron J. L., Capuzzi D. M., Zuidema G. D., Margolis S. Acute pancreatitis with hyperlipemia. Evidence for a persistent defect in lipid metabolism. Am J Med. 1974 Apr;56(4):482–487. doi: 10.1016/0002-9343(74)90480-x. [DOI] [PubMed] [Google Scholar]
- Cameron J. L., Capuzzi D. M., Zuidema G. D., Margolis S. Acute pancreatitis with hyperlipemia: the incidence of lipid abnormalities in acute pancreatitis. Ann Surg. 1973 Apr;177(4):483–489. doi: 10.1097/00000658-197304000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron J. L., Crisler C., Margolis S., DeMeester T. R., Zuidema G. D. Acute pancreatitis with hyperlipemia. Surgery. 1971 Jul;70(1):53–61. [PubMed] [Google Scholar]
- Del Maestro R. F. An approach to free radicals in medicine and biology. Acta Physiol Scand Suppl. 1980;492:153–168. [PubMed] [Google Scholar]
- Feiner H. Pancreatitis after cardiac surgery; a morphologic study. Am J Surg. 1976 Jun;131(6):684–688. doi: 10.1016/0002-9610(76)90178-1. [DOI] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Gardner T. J., Stewart J. R., Casale A. S., Downey J. M., Chambers D. E. Reduction of myocardial ischemic injury with oxygen-derived free radical scavengers. Surgery. 1983 Sep;94(3):423–427. [PubMed] [Google Scholar]
- Hermon-Taylor J. A technique for perfusion of the isolated canine pancreas. Responses to secretin and gastrin. Gastroenterology. 1968 Oct;55(4):488–501. [PubMed] [Google Scholar]
- Kelly T. R. Gallstone pancreatitis: pathophysiology. Surgery. 1976 Oct;80(4):488–492. [PubMed] [Google Scholar]
- Kimura T., Zuidema G. D., Cameron J. L. Acute pancreatitis. Experimental evaluation of steroid, albumin and trasylol therapy. Am J Surg. 1980 Sep;140(3):403–408. doi: 10.1016/0002-9610(80)90178-6. [DOI] [PubMed] [Google Scholar]
- Kimura T., Zuidema G. D., Cameron J. L. Experimental pancreatitis: influence of secretory state of the pancreas. Surgery. 1980 Nov;88(5):661–666. [PubMed] [Google Scholar]
- Kimura T., Zuidema G. D., Cameron J. L. Steroid administration and acute pancreatitis: studies with an isolated, perfused canine pancreas. Surgery. 1979 May;85(5):520–524. [PubMed] [Google Scholar]
- McCord J. M. Free radicals and inflammation: protection of synovial fluid by superoxide dismutase. Science. 1974 Aug 9;185(4150):529–531. doi: 10.1126/science.185.4150.529. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McCord J. M. The superoxide free radical: its biochemistry and pathophysiology. Surgery. 1983 Sep;94(3):412–414. [PubMed] [Google Scholar]
- Parks D. A., Bulkley G. B., Granger D. N. Role of oxygen-derived free radicals in digestive tract diseases. Surgery. 1983 Sep;94(3):415–422. [PubMed] [Google Scholar]
- Saharia P., Margolis S., Zuidema G. D., Cameron J. L. Acute pancreatitis with hyperlipemia: studies with an isolated perfused canine pancreas. Surgery. 1977 Jul;82(1):60–67. [PubMed] [Google Scholar]
- Taylor A. E., Martin D., Parker J. C. The effects of oxygen radicals on pulmonary edema formation. Surgery. 1983 Sep;94(3):433–438. [PubMed] [Google Scholar]
- Warshaw A. L., O'Hara P. J. Susceptibility of the pancreas to ischemic injury in shock. Ann Surg. 1978 Aug;188(2):197–201. doi: 10.1097/00000658-197808000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]



