Abstract
Parkinson's disease (PD) is characterized by the progressive loss of substantia nigra dopaminergic neurons and the presence of cytoplasmic inclusions named Lewy bodies. Two missense mutations of the α-synuclein (α-syn; A30P and A53T) have been described in several families with an autosomal dominant form of PD. α-Syn also constitutes one of the main components of Lewy bodies in sporadic cases of PD. To develop an animal model of PD, lentiviral vectors expressing different human or rat forms of α-syn were injected into the substantia nigra of rats. In contrast to transgenic mice models, a selective loss of nigral dopaminergic neurons associated with a dopaminergic denervation of the striatum was observed in animals expressing either wild-type or mutant forms of human α-syn. This neuronal degeneration correlates with the appearance of abundant α-syn-positive inclusions and extensive neuritic pathology detected with both α-syn and silver staining. Lentiviral-mediated expression of wild-type or mutated forms of human α-syn recapitulates the essential neuropathological features of PD. Rat α-syn similarly leads to protein aggregation but without cell loss, suggesting that inclusions are not the primary cause of cell degeneration in PD. Viral-mediated genetic models may contribute to elucidate the mechanism of α-syn-induced cell death and allow the screening of candidate therapeutic molecules.
Parkinson's disease (PD) is a neurodegenerative disorder characterized by the progressive loss of dopaminergic neurons in the substantia nigra and the appearance of intracellular inclusions, named Lewy bodies and Lewy neurites in the remaining nigral neurons. Although occurring most commonly as a sporadic form, some rare familial cases of PD have been described. Two missense mutations in the α-synuclein (α-syn) gene (A53T and A30P) were identified in several families with autosomal dominant parkinsonism (1, 2). Moreover, α-syn is one of the major components of Lewy bodies and Lewy neurites in sporadic PD (3, 4). α-Syn is a presynaptic nerve terminal protein of 140 aa, which appears to be natively unfolded and represents ≈1% of the cytosolic protein in the brain (5). The generation of α-syn knock-out mice supports a role for α-syn of a presynaptic activity-dependent negative regulator of dopamine release (6). These findings support an important role of α-syn in the nigrostriatal dopamine pathway and consequently in the pathogenesis of PD and provide the rationale for creating rodent models based on the overexpression of mutated α-syn.
Genetic animal models of PD that recapitulate both pathological and cellular neurodegenerative aspects of the human disease may provide mechanistic insights into the pathogenesis of this neurological disorder. Recently, transgenic mouse and fly models were generated through the overexpression of either wild-type or mutant human forms of α-syn (7–10). The transgenic flies show adult-onset loss of dopaminergic neurons and the formation of Lewy body-like inclusions (10). In contrast, the transgenic mouse models show only mild neuropathology with occasional inclusions but no loss of dopamine nigral neurons (7–9, 11, 12). The level of human α-syn expression may explain the differences between both models. To overcome this absence of cellular degeneration in rodents, we investigated an alternative approach to classical transgenesis for developing genetic models of PD. We locally injected HIV-1-derived lentiviral vectors expressing normal or mutated α-syn in the brain region affected in the human disorder. The direct intranigral injections of viral vectors expressing wild-type or mutant forms of α-syn holds various advantages: (i) Models can be established in mammalian species other than the mice, opening the potential to establish a genetic nonhuman primate model of this neurodegenerative disorder; (ii) specific brain regions can be targeted without worrying about effects of α-syn expression in other central nervous system structures; (iii) higher gene expression is expected with the currently available lentiviral vectors than transgenesis techniques complemented recently with inducible forms allowing gene dosing experiments; (iv) ease of vector manipulation may speed the creation of multiple genetic models.
The HIV-1-derived lentiviral vector is one of the most promising gene transfer vehicles for central nervous system applications because of its strong tropism for neuronal cells (13, 14). In the striatum of rats and mice, >90% of the transduced cells are neurons (14–17) and in primates, 80–88% are NeuN-positive (18). Between 40% and 50% of dopaminergic neurons identified by immunohistochemistry for tyrosine hydroxylase are transduced in the substantia nigra of mice and rats injected with lentiviral vectors (16, 17). As recombinant lentiviral vectors efficiently infect neurons and allow high expression levels of proteins, we explored whether lentiviral-mediated overexpression of wild-type (HWT) or mutant (A53T and A30P) forms of human α-syn led to sufficient neuronal accumulation of α-syn to induce degeneration of nigral dopaminergic neurons in rats. We also investigated the specificity of the α-syn-induced toxicity by expressing the rat wild-type α-syn form.
Methods
Lentiviral Vector Production and Western Blot Analysis.
The cDNA coding for cytoplasmic β-galactosidase (LacZ), rat α-syn (nucleotides 27–496, GenBank accession no. NM_019169), and wild-type (HWT) and mutant (A53T, A30P) human α-syn (nucleotides 46–520, GenBank accession no. NM_000345) were cloned in the SIN-W-PGK lentiviral transfer vector. The packaging construct and vesicular stomatitis virus G protein (VSV-G) envelope used in this study was the pCMVDR-8.92, pRSV-Rev and the pMD.G plasmids described previously (16, 19). The viral particles were produced by transient transfection of 293T cells with these four plasmids (13). High-titer stocks were obtained by ultracentrifugation. The viral titers were determined by p24 antigen measurements. For the in vivo experiments, the different viral stocks matched for viral particle content were used at 200,000 ng of p24 per ml.
For Western blot, the SH-SY5Y human dopaminergic neuroblastoma cells were infected with the lentiviral vectors matched for particle content (800 ng of p24 antigen per well). Because of a strong and progressive toxicity of α-syn in this dopaminergic cell line, cellular lysates were harvested 2 d after infection in lysis buffer (125 mM Tris⋅HCl/0.5% SDS/1% Nonidet P-40) containing protease inhibitors (Roche Pharma, Basel, Switzerland). Protein concentration for the cytoplasmic fraction was determined by the BCA protein assay kit (Pierce). Equal amounts of protein (25 mg) were loaded onto 15% SDS-polyacrylamide gel followed by the transfer of proteins onto a nitrocellulose membrane (Bio-Rad). A polyclonal Ab generated against the 101–124 aa sequence of human α-syn was used to detect α-syn protein (1:1,000). This Ab was generated and affinity purified with the immunogen by Research Genetics (Huntsville, AL). Rat brain lysate (Transduction Laboratories, Lexington, KY) was used as a positive control. The amount of protein loaded was checked by reprobing the same membrane with an α-tubulin Ab (1:2,000; Sigma). Detections were performed by using a chemiluminescence detection kit (Amersham Pharmacia).
Stereotaxic Injection and Immunohistology.
Lentiviral vectors were injected in the right substantia nigra of rats as described (16). To target the substantia nigra, stereotaxic injection was performed in two sites (2.5 μl per site with 200,000 ng of p24 per ml) with the following anterior, lateral, and ventral coordinates (4.8, 2, 7.7, and 5.5, 1.7, 7.7). Five animals per group were injected and their brains processed as described (16). In brief, animals were transcardially perfused with PBS and their brains fixed with 4% paraformaldehyde and transferred into 25% sucrose. Coronal sections (25-μm thick) from both the substantia nigra and striatum of rat brains were harvested and processed for immunohistological labeling. Detection of α-syn inclusions was performed with an affinity-purified rabbit Ab generated against the 101- to 124-aa sequence of human α-syn (1:1,000) and revealed with an avidin-biotin-peroxidase (ABC) detection kit (Vector, Burlingame, CA).
For immunofluorescence labeling, the following primary Abs were used: tyrosine hydroxylase (TH) sheep Ab (1:500, Pel-Freez Biologicals), a dopamine transporter (DAT) rabbit Ab (1:500, Chemicon), an α-syn polyclonal rabbit Ab (1:400), an α-syn-1 mAb (1:400, Transduction Laboratories) to detect rat α-syn overexpression, a human α-syn specific mAb LB509 (1:500, Zymed), and a glutamate decarboxylase (GAD) polyclonal Ab (1:400, Chemicon). Nissl staining was performed according to classical methods.
Cell Counting and Density of Nerve Terminals.
The percentage of TH-immunoreactive (TH-IR) neurons relative to the contralateral side was determined by fluorescence microscopy in a blind manner as described (16, 17). For quantification of γ-aminobutyric acid (GABA) neurons, GAD-positive neurons were counted in 12 coronal sections throughout the substantia nigra of rats expressing the A30P mutant.
To quantify TH-IR terminals in the striatum, at least 15 striatal sections were stained for TH with ABC kit, and the optical density was evaluated with the NIH IMAGE 1.4 software. The TH staining background detected in each section was evaluated by measuring the optical density in the cortex. Finally, the density of TH-IR terminals was calculated as the percentage of optical density of the contralateral side.
For statistical analyses, the data were evaluated for significance by using analysis of variance (ANOVA) followed by a Scheffé's probable least-squares difference post hoc test (jmp 3.0, SAS Institute, Cary, NC). The significance level was set at P < 0.05.
Silver Staining and Fluorojade Labeling.
Silver staining was used to detect both protein aggregation and degenerating neurons on paraformaldehyde-fixed sections (20). The FD NeuroSilver kit was used according to the manufacturer's protocol (FD NeuroTechnologies, Baltimore, MD). For colocalization studies, selected nigral and striatal sections were stained for α-syn and TH, respectively, with ABC kit and subsequently submitted to silver staining.
Degenerating neurons also were stained with the anionic fluorescein derivative FluoroJade B (21). Sections were first washed in water, mounted on sylanized glass slides, and stained according to the supplier's manual (HistoChem, Jefferson, AR).
Transmission Electron Microscopy.
Animals were perfused with 0.1 M sodium cacodylate buffer and fixed in 2% paraformaldehyde with 1.5% glutaraldehyde. Vibratome sections from the substantia nigra of lenti-A30P- and lenti-LacZ-injected animals were processed for classical electron microscopy. Selected sections stained for α-syn by using diaminobenzidine as chromophore were postfixed in 1% osmium and flat-embedded in araldite. Ultrathin sections were collected and observed with a Philips CM12 transmission electron microscope.
Results and Discussion
Overexpression of α-Syn in the Rat Substantia Nigra.
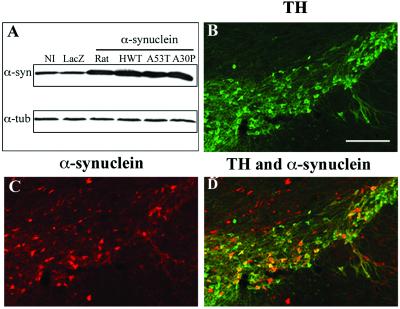
We first evaluated the functionality and the expression level of each vector on Western blots from lentiviral infected SH-SY5Y human neuroblastoma cells. To induce a high level of protein expression, the various α-syn forms were inserted between an upstream PGK promoter and a downstream posttranscriptional regulatory element of the woodchuck hepatitis virus (WPRE) in the HIV-1-derived lentiviral vector (16, 18, 22). Each lentiviral vector led to equivalent overexpression level of the different α-syn forms (Fig. 1A). We then investigated whether the injection of lentiviral vectors encoding for α-syn in rat substantia nigra led to significant transgene expression level. Tissue expression was revealed by using a polyclonal rabbit Ab generated against the 101–124 peptide of human α-syn. This Ab recognizes both rat and human forms on Western blot (Fig. 1A) but only detects human α-syn in vivo. Two weeks after intra-nigral injection of the HWT, a high proportion of TH-IR neurons expressed human α-syn (Fig. 1 B–D). The virus mainly diffused over the entire substantia nigra region (pars compacta, reticulata, and lateral substantia nigra).
Fig 1.
Lentiviral-mediated overexpression of α-syn. (A) Western blot analysis shows the overexpression of normal and mutated (A53T and A30P) human and rat α-syn in the SH-SY5Y human neuroblastoma cell line. All α-syn forms are expressed at similar levels for the same amount of viral particles. Protein (25 μg per lane) were loaded for the noninfected cells (NI) and cells transduced with lentiviral vectors encoding for cytoplasmic LacZ, rat α-syn, wild-type (HWT), and mutated forms of human α-syn. The 19-kDa α-syn bands (α-syn) were detected with a polyclonal rabbit Ab generated against the 101- to 124-aa sequence of human α-syn. This Ab recognizes both human and rat α-syn on Western blot. The amount of protein loaded was checked by reprobing the same membrane with an α-tubulin Ab (α-tub). (B–D) Lentiviral vectors encoding for wild-type and mutated human α-syn were stereotactically injected in the substantia nigra of rats. The nigral dopaminergic neurons were specifically labeled with a TH Ab (B). Detection with an α-syn polyclonal Ab revealed a significant overexpression of A30P α-syn (C) in the injected hemisphere. No α-syn staining was observed on the contralateral side. Double staining (D, yellow-orange color) shows a large proportion of TH-IR neurons overexpressing α-syn. (Scale bars = 200 μm.)
Analysis of the Neuropathology in the Rodent Brains After Lentiviral-Mediated Expression of α-Syn.
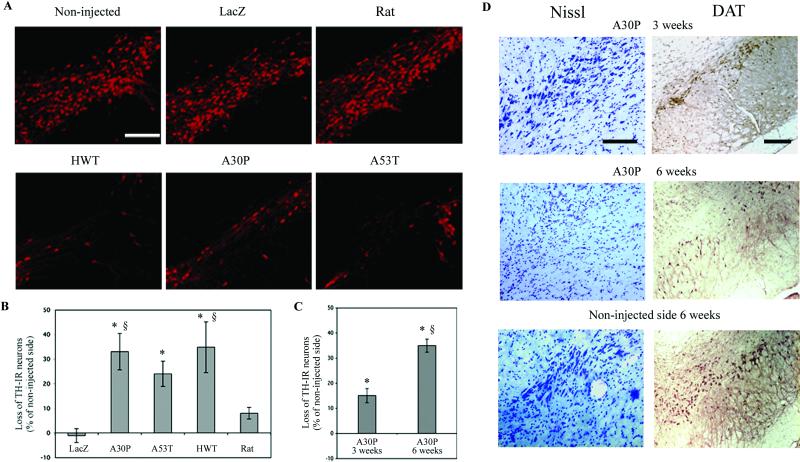
To determine whether lentiviral-mediated overexpression of α-syn induces neuronal degeneration, nigral TH-positive neurons were quantified in rats 5 mo after viral injection. Injection of recombinant lentiviruses encoding for wild-type, A30P, and A53T human α-syn induced an average of 35%, 33%, and 24%, loss of TH expression, respectively (Fig. 2 A and B). In all injected animals, this expression loss was restricted to the substantia nigra region. In contrast, no significant decrease of TH-IR neurons was observed in rats overexpressing rat α-syn. The β-galactosidase carrying lentivirus had no effect on TH expression of nigral dopaminergic neurons.
Fig 2.
Dopaminergic-specific marker (TH, DAT) expression in the substantia nigra of rats injected with lentiviral vectors encoding for wild-type (HWT) or mutated (A30P, A53T) human and wild-type rat α-syn. (A) Overexpression of normal or mutant human α-syn significantly decreases the number of TH-IR neurons. No significant loss of TH expression was observed with the lentivirus encoding for rat α-syn or the reporter protein β-galactosidase (LacZ). (B) Histograms representing the loss of TH-IR nigral neurons at 5 mo relative to the contralateral side in rats unilaterally injected with the different lentiviral constructs. (C) The loss of dopaminergic neurons was quantified at 3 and 6 weeks for the A30P mutant and compared to Lenti-LacZ- or Lenti-rat α-syn-injected animals at 5 mo. The A30P lentiviral-mediated lesion represents a time-dependant process occurring within 6 weeks. (D) Nissl and DAT staining for A30P expressing animals at 3 and 6 weeks confirming the loss of dopaminergic cells over time. Values refer to means ± SEM; n = five animals per group; *, P < 0.005; §, P < 0.05; *, compared to lenti-lacZ-injected animals; §, compared to lenti-rat α-syn-injected animals at 5 mo. (Scale bars = 200 μm.)
To follow the temporal progression of the cellular degeneration, the dopamine neuronal population of animals expressing either the A30P mutant or rat α-syn also was quantified at 3 and 6 weeks after lentiviral injection (Fig. 2C). Similar to the 5-mo data, no decrease of TH expression was detected at both 3 and 6 weeks in rats overexpressing rat α-syn. For the rats injected with lentiviral vectors expressing the A30P mutant, there was a progressive loss of TH expression starting at 3 weeks with a 15% decrease and reaching 35% at 6 weeks (Fig. 2C). Both DAT, a dopaminergic-specific marker and Nissl staining confirm the reduction of dopaminergic neurons in the substantia nigra of animals expressing the A30P mutant (Fig. 2D). This relatively fast time-dependent TH cell reduction may be explained by the abundant α-syn accumulation reached with lentiviral vectors. These data reveal that the human α-syn lentiviral-mediated lesion represents a rather rapid time-dependent process occurring within 6 weeks.
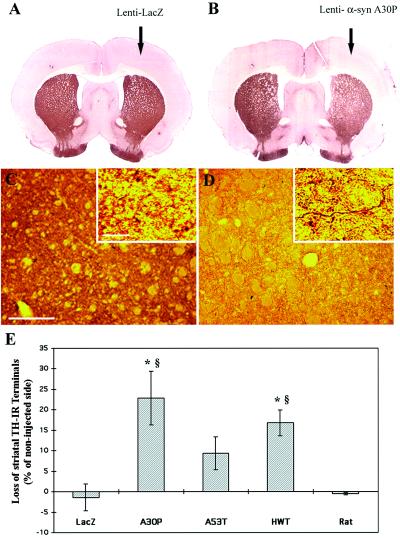
The degenerative process observed in the human α-syn-expressing animals is also associated with a considerable reduction of the TH expression in the striatum, the brain structure receiving dopaminergic projections from nigral neurons (Fig. 3 A and B). Striatal sections stained for TH and subsequently submitted to silver staining reveal that spared dopaminergic fibers contained silver deposits underlying nerve terminal degeneration (Fig. 3 C and D). Intra-nigral injections with the lenti-A53T, on the contrary to lenti-HWT and lenti-A30P, do not lead to a significant loss in TH-IR terminals (Fig. 3E). The endogenous presence of rat α-syn, which naturally shares with the human A53T mutant, a threonine at the position 53, may attenuate the A53T α-syn-induced degeneration.
Fig 3.
Dopaminergic innervation in the striatum of rats expressing the different α-syn forms after 5 mo after lentiviral injection. (A and B) Striatal sections from rats that received intra-nigral injection of either lenti-LacZ (A) or lenti-A30P (B) in the right side of the substantia nigra. Lenti-A30P-injected animals show a significant reduction of TH staining on the ipsilateral side of the striatum whereas lenti-LacZ has no effect. (C and D) Double staining for TH and silver in the striatum of an animal injected with lenti-LacZ (C) or lenti-A30P (D). Higher magnification reveals that the reduction for TH staining in A30P-injected animals is associated with the presence of degenerating fibers containing silver deposits. (E) Quantification of the density of TH-IR terminals in the striatum of rats injected with the different α-syn forms. The histogram shows a significant reduction in TH-positive terminal density for the wild-type and A30P mutant α-syn forms. Values refer to means ± SEM; n = 5 animals per groups; *, P < 0.05; §, P < 0.01; *, compared to lenti-LacZ-injected animals; §, compared to lenti-rat α-syn-injected animals. [Scale bars = 200 μm (C and D) and 10 μm for higher magnification of (C and D).]
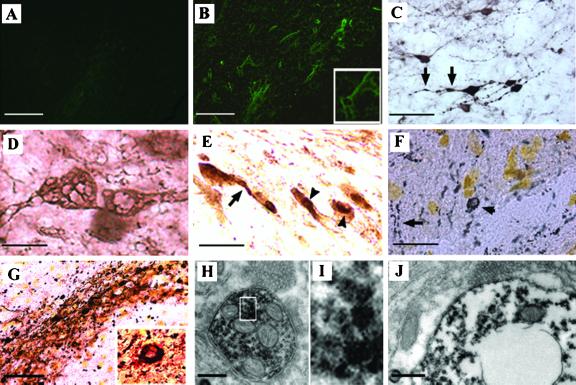
To further evaluate the degenerative process, FluoroJade, a fluorescent dye that selectively stains the cell body and neurites of degenerating neurons was used (21). No fluoroJade-labeled neurons were detected in both Lenti-LacZ-injected animals and noninjected side of animals expressing human α-syn (Fig. 4A). Scattered neurons in the substantia nigra of animals expressing the A30P mutant show FluoroJade labeling in the perikaria, axon, and dendrites (Fig. 4B). The degeneration process was confirmed by the presence of dystrophic α-syn neurites (Fig. 4C) and abnormally swollen structures with axonal and perikaria distribution (Fig. 4D).
Fig 4.
Neuropathology induced by lentiviral-mediated expression of α-syn. (A) No fluoroJade B labeled neurons were detected in both Lenti-LacZ-injected animals and noninjected side of animals expressing human α-syn. (B) Scattered FluoroJade B-positive neurons detected in the substantia nigra of rats expressing A30P α-syn. Higher magnification shows a degenerating neuron with FluoroJade B labeling in their perikaria and neurites. (C and D) α-Syn staining revealed that transduced neurons develop dystrophic neurites (C, arrows) and abnormal α-syn immunopositive swollen structures (D) with axonal and cell body distribution. (E) Nigral neurons abnormally accumulate cytoplasmic α-syn-immunopositive aggregates in cell bodies and neurites in rats overexpressing A30P α-syn. To confirm the presence of inclusions rather than a particular cellular sublocalization of α-syn, a silver staining (dark deposits) was performed alone (F) or in combination with α-syn staining (red-brown color) (G). Cytoplasmic inclusions (arrowheads) and extensive neuritic pathology (arrows) are detected with both methods. Higher magnification of a double-stained aggregate reveals that the inclusions contain abundant α-syn. (H–J) Ultrastructural analysis of A30P α-syn expressing nigral neurons. Abundant α-syn immunoreactive aggregation was detected in both the axon (H and I) and cell body (J) of nigral neurons expressing human α-syn. These cytoplasmic structures are mainly present as scattered granular microaggregates, which are occasionally associated with mitochondria or synaptic vesicles (H) and nuclear or cytoplasmic membrane (J). A degenerating cell with clustered aggregates in its perikaria reveals an important loss of organelles in its cytoplasm and a disorganized nuclear membrane (J). The size of the cell body and the presence of a varicosity full of synaptic vesicules observed (on the top) in close apposition with α-syn-positive structures support that this degenerating cell is a neuron. [Scale bars = 50 μm (A, B, and G); 25 μm (C, E, and F); 8 μm (D); and 0.3 μm (H and J).]
The surviving nigral neurons expressing, either human or rat α-syn, accumulate cytoplasmic α-syn in both neurites and cell bodies (Fig. 4E). To confirm the presence of inclusions rather than a particular cellular sublocalization of α-syn, a silver staining was performed alone (Fig. 4F) or in combination with α-syn immunostaining (Fig. 4G), knowing that Lewy body and Lewy neurites are efficiently detected by silver-staining in the human disease (20). Clear granular silver deposits were observed in neurites and larger inclusions in the cell body of the substantia nigra neurons of rats injected with lentivirus encoding for human α-syn (Fig. 4 F and G). These neuropathological features closely resemble Lewy body and Lewy neurites pathology observed in patients with PD (4). There was no silver and α-syn staining in the control animals expressing the cytoplasmic β-galactosidase. Almost all silver deposits were restricted to the viral-targeted region and colocalized with α-syn staining (Fig. 4G). No observable differences in the localization and abundance of aggregates were observed at 5 mo between the different human forms or the rat α-syn (data not shown). Similarly to the α-syn transgenic fly model (10), no significant ubiquitination was observed in rats expressing human α-syn or rat α-syn (data not shown). Interestingly, up to 10% of inclusions were found to be negative for ubiquitin in PD brains (4), suggesting that ubiquitination of α-syn containing inclusions may represent a late process in the human pathology. Recently, α-syn was also shown to be degraded by the 20S proteasome in an ubiquitin-independent manner (23). These findings may explain the absence of ubiquinated aggregates in this viral-based model.
Ultrastructural Analysis of α-Syn Positive Inclusions.
To elucidate the ultrastructural features of these inclusions, transmission electron microscopy was performed 5 mo after lentiviral injection from the substantia nigra of animals expressing wild-type or mutant human α-syn. Abundant α-syn immunoreactive aggregation was detected in both the axon (Fig. 4 H and I) and cell body (Fig. 4J) of nigral neurons. These cytoplasmic structures were mainly present as scattered granular microaggregates, which were occasionally associated with mitochondria, synaptic vesicles (Fig. 4H), and nuclear or cytoplasmic membrane (Fig. 4J). No α-syn aggregation was observed in the nucleus of neurons overexpressing α-syn. These microaggregates showed a dense core and a more diffuse surrounding staining (Fig. 4I). The presence of granular structures is also a feature of the human Lewy body (24), α-syn transgenic fly (10), and pesticide-induced model of PD (25). However, these inclusions lack the classical fibrillar elements found in human Lewy bodies. The simultaneous presence of human and endogenous rat α-syn may explain the absence of such fibrillar structures. This hypothesis is supported by the appearance of both granular and fibrillar inclusions in the α-syn transgenic fly, in which no α-syn homolog has been identified.
Selective Loss of Dopaminergic Neurons.
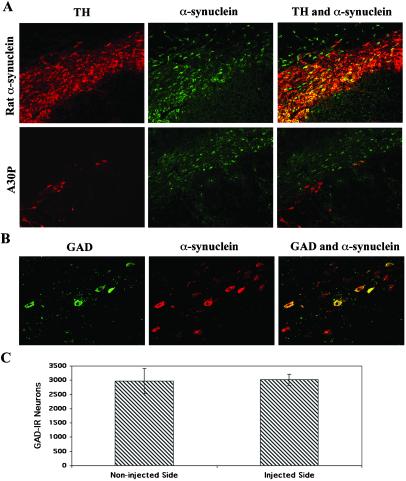
At 5 mo after lentiviral injection, some TH-IR nigral neurons still overexpressed rat α-syn (Fig. 5A). Although a significant loss of TH-IR neurons was observed in animals overexpressing the A30P human mutant, numerous nondopaminergic neurons overexpressing human α-syn survived inside the lesioned area of the substantia nigra (Fig. 5A). Based on these observations, the selectivity of the α-syn-induced lesion was assessed by quantifying the GABAergic nigral neuronal population. Lentiviral transduction of this nondopaminergic nigral population was confirmed with a double staining for GAD and α-syn (Fig. 5B). The number of GAD-positive neurons was assessed in animals injected with lentivirus encoding the A30P mutant (Fig. 5C). No difference of GAD-positive neurons was observed between the lenti-A30P injected and noninjected side of the substantia nigra, indicating a specificity of the toxicity for nigral dopaminergic neurons.
Fig 5.
Specificity of the lesion induced by human A30P α-syn expression. (A) Double staining for TH (red) and α-syn (green) in rat α-syn or A30P expressing animals at 5 mo after lentiviral injection. TH-IR nigral neurons still overexpress rat α-syn (yellow-orange) whereas a significant loss of TH-IR neurons is observed in animals overexpressing the A30P human mutant. However, nondopaminergic neurons overexpressing human α-syn (green) survived inside the lesioned area of the substantia nigra. (B) GABAergic neurons are preserved in rats expressing A30P mutant α-syn. Double staining showing spared GAD-positive neurons (green) transduced and expressing high level of A30P α-syn (red). GABAergic neurons expressing human α-syn appear yellow-orange. (C) The number of GAD-positive neurons in the substantia nigra was determined for the noninjected and lenti-A30P-injected side. Overexpression of A30P α-syn does not induce a significant loss of GABAergic neurons. Values refer to means ± SEM. [Scale bars = 200 μm (A) and 50 μm (B).]
This lentiviral-based rodent model of α-syn reproduces the most salient hallmarks of PD: (i) an accumulation of α-syn in cytoplasmic structures similar to Lewy body and Lewy neurites and (ii) a selective degeneration of dopaminergic nigral neurons. Both normal and mutant human α-syn induce neuronal degeneration, as reported in α-syn transgenic fly (10). Although the two α-syn mutants account for only rare cases of familial PD (26), patients suffering from sporadic PD often show abnormal accumulation of wild-type and not mutant human α-syn (27).
Considering that a maximum of 50% of TH-IR neurons are transduced in the rat substantia nigra infected with a lentiviral vector encoding for nuclear LacZ (16), more than one-half of potentially transduced neurons were lost in rats expressing the human α-syn forms. Some transduced dopaminergic neurons therefore still survive after 5 mo of human α-syn accumulation. Cells expressing α-syn below a toxic threshold may resist to α-syn damages. The variability of pathological phenotypes is encountered in animal models as a function of protein expression level (28). As observed in PD patients (29), differences in vulnerability of dopaminergic neuron subpopulations may also occur in lentiviral-mediated overexpression of α-syn.
Expression of rat α-syn in rat leads to inclusion formation (data not shown) without loss of TH-IR cell, suggesting that the sole formation of α-syn aggregates is not sufficient to induce cell death. This latter observation supports a specific toxic property for human α-syn. Human α-syn accumulation may result in the formation of toxic oligomers, on the contrary to rat α-syn. Our results are consistent with some in vitro findings suggesting that the inclusion formation itself may not be the pathogenic cause of cell death in PD but rather a protective event of neurons to more toxic, prefibrillar intermediates (30, 31). Although both rodent or human α-syn forms amyloid fibrils in vitro, only the mixture of human and rodent α-syn leads to the accumulation of nonfibrillar, potentially toxic oligomer (32). These prefibrillar α-syn intermediates effectively induce the destruction of vesicular membranes (33). Similarly, the toxic role of inclusions in the neurodegenerative process also is presently debated in other human disorders such as polyglutamine expansion-related diseases (34).
The present work demonstrates that the overexpression of wild-type or mutated human α-syn leads to dopamine neuronal cell death in rodents. The use of lentiviral vectors to mimic neurodegenerative disorders opens new perspectives to generate a genetic nonhuman primate model of PD and to dissect the signaling pathway leading to selective degeneration of dopaminergic neurons by injecting lentivirus coding for human α-syn in transgenic animals expressing anti-apoptotic or protective proteins. Consequently, this virally induced model of PD may help understanding the underlying pathogenic process related to α-syn and allows for the development of more efficient therapies for PD.
Acknowledgments
We thank Dr. Jack Tseng for helping with stereotaxic injections. We thank Christel Sadeghi, Katya Auderset, Fabienne Pidoux, Maria Rey, Laurence Winkel, and Stephanie Julien for excellent technical assistance; Dr. Michel Goedert for the human α-syn cDNA (HWT, A53T, and A30P); and Drs. Robert E. Burke and Nikolai Kholodilov for the rat α-syn cDNA. This work was supported by the Swiss National Science Foundation.
Abbreviations
PD, Parkinson's disease
α-syn, α-synuclein
TH-IR, tyrosine hydroxylase-immunoreactive
GABA, γ-aminobutyric acid
GAD, glutamate decarboxylase
HWT, human wild type
References
- 1.Polymeropoulos M. H., Lavedan, C., Leroy, E., Ide, S. E., Dehejia, A., Dutra, A., Pike, B., Root, H., Rubenstein, J., Boyer, R., et al. (1997) Science 276, 2045-2047. [DOI] [PubMed] [Google Scholar]
- 2.Kruger R., Kuhn, W., Muller, T., Woitalla, D., Graeber, M., Kosel, S., Przuntek, H., Epplen, J. T., Schols, L. & Riess, O. (1998) Nat. Genet. 18, 106-108. [DOI] [PubMed] [Google Scholar]
- 3.Spillantini M. G., Schmidt, M. L., Lee, V. M., Trojanowski, J. Q., Jakes, R. & Goedert, M. (1997) Nature (London) 388, 839-840. [DOI] [PubMed] [Google Scholar]
- 4.Spillantini M. G., Crowther, R. A., Jakes, R., Hasegawa, M. & Goedert, M. (1998) Proc. Natl. Acad. Sci. USA 95, 6469-6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwai A., Masliah, E., Yoshimoto, M., Ge, N., Flanagan, L., de Silva, H. A., Kittel, A. & Saitoh, T. (1995) Neuron 14, 467-475. [DOI] [PubMed] [Google Scholar]
- 6.Abeliovich A., Schmitz, Y., Farinas, I., Choi-Lundberg, D., Ho, W. H., Castillo, P. E., Shinsky, N., Verdugo, J. M., Armanini, M., Ryan, A., et al. (2000) Neuron 25, 239-252. [DOI] [PubMed] [Google Scholar]
- 7.Masliah E., Rockenstein, E., Veinbergs, I., Mallory, M., Hashimoto, M., Takeda, A., Sagara, Y., Sisk, A. & Mucke, L. (2000) Science 287, 1265-1269. [DOI] [PubMed] [Google Scholar]
- 8.van der Putten H., Wiederhold, K. H., Probst, A., Barbieri, S., Mistl, C., Danner, S., Kauffmann, S., Hofele, K., Spooren, W. P., Ruegg, M. A., et al. (2000) J. Neurosci. 20, 6021-6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahle P. J., Neumann, M., Ozmen, L., Muller, V., Jacobsen, H., Schindzielorz, A., Okochi, M., Leimer, U., van Der Putten, H., Probst, A., et al. (2000) J. Neurosci. 20, 6365-6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feany M. B. & Bender, W. W. (2000) Nature (London) 404, 394-398. [DOI] [PubMed] [Google Scholar]
- 11.Dawson V. L. (2000) Science 288, 631-632. [DOI] [PubMed] [Google Scholar]
- 12.Beal M. F. (2001) Nat. Rev. Neurosci. 2, 325-334. [DOI] [PubMed] [Google Scholar]
- 13.Naldini L., Blomer, U., Gallay, P., Ory, D., Mulligan, R., Gage, F. H., Verma, I. M. & Trono, D. (1996) Science 272, 263-267. [DOI] [PubMed] [Google Scholar]
- 14.Blomer U., Naldini, L., Kafri, T., Trono, D., Verma, I. M. & Gage, F. H. (1997) J. Virol. 71, 6641-6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blomer U., Kafri, T., Randolph-Moore, L., Verma, I. M. & Gage, F. H. (1998) Proc. Natl. Acad. Sci. USA 95, 2603-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Déglon N., Tseng, J. L., Bensadoun, J. C., Zurn, A. D., Arsenijevic, Y., Pereira de Almeida, L., Zufferey, R., Trono, D. & Aebischer, P. (2000) Hum. Gene Ther. 11, 179-190. [DOI] [PubMed] [Google Scholar]
- 17.Bensadoun J. C., Déglon, N., Tseng, J. L., Ridet, J-L., Zurn, A. D. & Aebischer, P. (2000) Exp. Neurol. 164, 15-24. [DOI] [PubMed] [Google Scholar]
- 18.Kordower J. H., Bloch, J., Ma, S. Y., Chu, Y., Palfi, S., Roitberg, B. Z., Emborg, M., Hantraye, P., Déglon, N. & Aebischer, P. (1999) Exp. Neurol. 160, 1-16. [DOI] [PubMed] [Google Scholar]
- 19.Hottinger A. F., Azzouz, M., Déglon, N., Aebischer, P. & Zurn, A. D. (2000) J. Neurosci. 20, 5587-5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandmann-Keil D., Braak, H., Okochi, M., Haass, C. & Braak, E. (1999) Acta Neuropathol. 98, 461-464. [DOI] [PubMed] [Google Scholar]
- 21.Schmued L. C. & Hopkins, K. J. (2000) Toxicol. Pathol. 28, 91-99. [DOI] [PubMed] [Google Scholar]
- 22.Kordower J. H., Emborg, M. E., Bloch, J., Ma, S. Y., Chu, Y., Leventhal, L., McBride, J., Chen, E. Y., Palfi, S., Roitberg, B. Z., et al. (2000) Science 290, 767-773. [DOI] [PubMed] [Google Scholar]
- 23.Tofaris G. K., Layfield, R. & Spillantini, M. G. (2001) FEBS Lett. 509, 22-26. [DOI] [PubMed] [Google Scholar]
- 24.Pollanen M. S., Dickson, D. W. & Bergeron, C. (1993) J. Neuropathol. Exp. Neurol. 52, 183-191. [DOI] [PubMed] [Google Scholar]
- 25.Betarbet R., Sherer, T. B., MacKenzie, G., Garcia-Osuna, M., Panov, A. V. & Greenamyre, J. T. (2000) Nat. Neurosci. 3, 1301-1306. [DOI] [PubMed] [Google Scholar]
- 26.Vaughan J., Durr, A., Tassin, J., Bereznai, B., Gasser, T., Bonifati, V., De Michele, G., Fabrizio, E., Volpe, G., Bandmann, O., et al. (1998) Ann. Neurol. 44, 270-273. [DOI] [PubMed] [Google Scholar]
- 27.Wakabayashi K., Matsumoto, K., Takayama, K., Yoshimoto, M. & Takahashi, H. (1997) Neurosci. Lett. 239, 45-48. [DOI] [PubMed] [Google Scholar]
- 28.Link C. D. (2001) Mech. Ageing Dev. 122, 1639-1649. [DOI] [PubMed] [Google Scholar]
- 29.Gibb W. R. & Lees, A. J. (1991) J. Neurol. Neurosurg. Psychiatry 54, 388-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conway K. A., Lee, S. J., Rochet, J. C., Ding, T. T., Williamson, R. E. & Lansbury, P. T. J. (2000) Proc. Natl. Acad. Sci. USA 97, 571-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldberg M. S. & Lansbury, P. T. J. (2000) Nat. Cell Biol. 2, E115-E119. [DOI] [PubMed] [Google Scholar]
- 32.Rochet J. C., Conway, K. A. & Lansbury, P. T. J. (2000) Biochemistry 39, 10619-10626. [DOI] [PubMed] [Google Scholar]
- 33.Volles M. J., Lee, S. J., Rochet, J. C., Shtilerman, M. D., Ding, T. T., Kessler, J. C. & Lansbury, P. T. J. (2001) Biochemistry 40, 7812-7819. [DOI] [PubMed] [Google Scholar]
- 34.Saudou F., Finkbeiner, S., Devys, D. & Greenberg, M. E. (1998) Cell 95, 55-66. [DOI] [PubMed] [Google Scholar]