Abstract
The hippocampus has a critical role in several fundamental memory operations, including the conditioning of fear to contextual information. We show that the hippocampus is necessary also for unconditioned fear, and that the involved circuitry is at the ventral pole of the hippocampus. Rats with selective hippocampal lesions failed to avoid open arms in an elevated plus-maze and had decreased neuroendocrine stress responses during confinement to a brightly lit chamber. These effects were reproduced by lesions of the ventral half of the hippocampus, but not by damage to the dorsal three-quarters of the hippocampus or the amygdala. Ventral lesions failed to impair contextual fear conditioning or spatial navigation, suggesting that the ventral hippocampus may specifically influence some types of defensive fear-related behavior.
Keywords: defensive, rat, plus-maze, water maze, corticosterone
The experience of anxiety and fear is controlled by a modular neural system including regions in the brainstem, hypothalamus, and deeper parts of the temporal lobe (1–4). The amygdala plays a pivotal role in this system. It controls a broad range of fear reactions, and exhibits neural plasticity that may permit fear responses to be conditioned to new types of experience (refs. 1–5; but see ref. 6). Conditioned fear depends strongly on the basolateral complex of the amygdala (the lateral, basolateral, and basomedial nuclei), whereas the central nucleus and the bed nucleus of the stria terminalis are thought to be the principal output structures, mediating fear-related signals to behavioral, autonomic, and endocrine response systems in the hypothalamus and brainstem (1–5).
Conditioning of fear to multimodal stimuli such as context and spatial location also requires the integrity of the hippocampus (7–9). The hippocampus is strongly involved in the encoding of spatial and episodic memories (10–12), and contextual fear conditioning is thought to require many of the associative algorithms responsible for these types of memory (13–15). Although the hippocampal influence on fear reactions may be a necessary consequence of its mnemonic operations, it is also possible that the hippocampus controls fear and anxiety independently of learning (16). This view is based particularly on the similar effects that anxiolytic drugs and septal-hippocampal lesions have on behavior in aversively motivated tasks. However, except for early studies showing that rats with large nonselective hippocampal lesions exhibit reduced food neophobia (17–19) and reduced freezing in the presence of a predator (20), direct evidence is absent. We now show (i) that the hippocampus controls defensive fear responses during exposure to a potentially threatening environment, (ii) that the relevant circuitry is located at the ventral pole of the hippocampus, and (iii) that this circuit may be dissociable from the associative circuits involved in contextual fear conditioning.
Methods
Subjects.
A total of 187 male Long Evans rats (250–450 g) were housed individually in transparent polycarbonate cages (40 × 15 × 26 cm) in a temperature- and humidity-controlled room and were kept on a 12-h light/dark schedule (lights on at 8 a.m.). Behavior was tested between 8 a.m. and 8 p.m., and corticosterone was sampled between 10 a.m. and 3 p.m.
Lesions.
The rats were anesthetized with Equithesin (1.0 ml/250 g, i.p.). Ibotenic acid (Sigma; 10 mg/ml, pH 7.4) was injected with a 1-μl Hamilton syringe mounted to the stereotaxic frame (21, 22). Injections of 0.05–0.12 μl were made for 10–20 s at each of 28 hippocampal sites (complete bilateral lesion; n = 37) or at a subset of these (dorsal lesion: 20 injections, n = 44; ventral lesion: 16 injections, n = 35). The syringe was retracted 2 min after the injection. In sham-operated rats (n = 50), the syringe was lowered through the neocortex. Lesions of the basolateral amygdala (n = 5) were made by infusion of 0.10 μl ibotenic acid bilaterally 3.3 mm posterior to bregma, 5.0 mm lateral to bregma, and 7.3–8.0 mm ventral to dura. The rats recovered for 7 days.
Elevated Plus Maze.
The maze consisted of four equally illuminated white steel arms (12 × 50 cm) radiating at square angles from a central platform (12 × 12 cm) 50 cm above the floor (23). The maze was placed in a silent and dimly lit room (3 × 4 m; background noise: <55 dB at 50 kHz; light intensity on open arms: 30 lux), except in the initial lesion study (1,000 lux).
The rats were handled 5 min/day for 5 days before the two test trials (10 min each). In trial 1, two opposite arms were enclosed by 40-cm-high walls of white steel. The other two arms were open but had transparent plastic ledges (0.3 cm) to prevent the rats from falling. In trial 2 (24 h later), walls were mounted around all four arms. In both trials, the rat started from the central platform, with the face pointing toward an enclosed arm. After each trial, the maze was cleaned with wet tissue paper. Tissue paper was never reused.
An observer watching the animal's behavior on a monitor behind a curtain counted entries into open and closed arms. An entry was scored when the rat moved into the arm with all four paws. It had to leave the arm entirely before another entry was scored. The observer was blind to the treatment of the rat. Position was tracked at 10 Hz (VP 200, HVS IMAGE, Hampton, U.K.; WATERMAZE, Watermaze Software, Edinburgh, U.K.; ref. 22), and path lengths and time spent in each arm and in the center were calculated.
To validate the interpretation of the avoidance of open arms as an index of fear expression, we gave the anxiolytic drug midazolam (Alpharma, Oslo) to eight naïve rats 30 min before exposing them to the elevated maze (0.5 mg/kg in 0.9% saline injected s.c.). Eight control rats received saline.
Corticosterone Measurement.
Rats were confined for 10 min to a white Perspex box (50 × 50 × 50 cm) with a grid floor (21 bars; 8-mm diameter, 2.2 cm apart center to center) twice daily for 5 days. The box was located in the center of a brightly lit empty water maze (1,000 lux). The rat's behavior was video recorded and tracked as above, and feces left in the box were counted. The box was cleaned with wet tissue paper after each trial. Plasma corticosterone was measured after 20 min of confinement on day 6. Within 3–4 min, the rats were taken to a procedure room and anesthetized with Fluothane, and 2 ml of blood was sampled by cardiac puncture from vena jugularis (heparinized 1-ml syringes, 0.8 × 40 mm hypodermic needle). Blood was collected in heparinized plastic tubes, centrifuged at 6,000 rpm for 15 min, and plasma stored at −20°C for later HPLC.
Contextual Conditioning.
Seven days later, the rats were reexposed to the Perspex box. They received three electric foot shocks (1 mA, 1 s) between alternating bars at 3, 5, and 7 min. The rats were returned to the box for 20 min 24 h later, and blood was then sampled from vena jugularis. Freezing was scored every 10 s during both trials (9).
Water Maze.
Experimentally naïve rats were trained in a white Morris water maze (diameter 198 cm, height 50 cm, water depth 40 cm, water temperature 23 ± 2°C; 22). The southwest quadrant contained a remotely controlled escape platform (11-cm diameter) that could be moved between an available level (submerged 1.5 cm) and an unavailable level (submerged 21 cm) (22). Training consisted of 10 blocks of four consecutive trials (two blocks on day 1, four blocks on days 2 and 3; four start positions varied in a predetermined and pseudorandom order; maximal trial length, 120 s; time on platform, 30 s). Probe trials (platform unavailable for 60 s) were conducted at the beginning of days 3 and 4. Time spent in a zone around the platform (20-cm radius) was compared with time in corresponding zones of the other quadrants. Data were collected at 50 Hz (Axona, Herts, U.K.).
Histology.
The rats received an overdose of Equithesin and were perfused intracardially with saline and 4% formaldehyde. The brains were stored in 4% formaldehyde, frozen sections were cut coronally (30 μm) and stained with cresyl violet, and images of every tenth section were taken into Canvas (Deneba Systems, Miami), where outlines of the remaining hippocampus (dentate gyrus, CA3–CA1) and subiculum were traced to determine their area and volume (percentage of average values of sham group; ref. 22). The subiculum was distinguished from the hippocampus by the width and density of the pyramidal cell layer (24).
Statistical Analyses.
The distributions of corticosterone concentration, defecation, and entries in the plus maze deviated from normality and were evaluated with nonparametric statistics.
Results
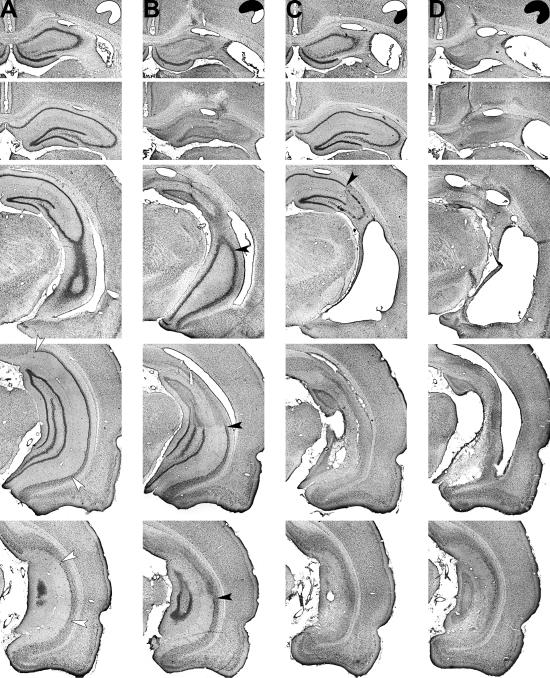
All lesions caused significant damage to the hippocampus and subiculum (Fig. 1). There was neocortical damage around the cannula track, which sometimes was accompanied by a spot of degeneration in the dorsal thalamus. Four rats with complete hippocampal lesions and one with a ventral lesion had minor damage at the anteroventral end of the entorhinal cortex. These lesions were small, and the animals were not excluded.
Fig 1.
Representative cresyl violet stains of intact neuronal cell bodies at five coronal levels through the hippocampus after sham surgery (A) or ibotenate lesions of the dorsal hippocampus (B), ventral hippocampus (C), or entire hippocampus (D). Filled arrowheads, borders between lesioned and healthy tissue; open arrowheads, borders of the subiculum.
The contribution of the hippocampus to unconditioned fear was examined in an elevated plus maze (23). During their first exposure to the maze, rats typically make few entries into open arms. Clinically effective anxiolytics reduce this avoidance (23). In the present apparatus, open-arm entries were more frequent in rats that received the anxiolytic drug Midazolam than in saline-treated rats [medians 63.4% vs. 27.3%; Kruskal–Wallis ANOVA on ranks: H(1) = 9.3, P < 0.005].
We first asked whether hippocampal lesions increased visits to open arms. The lesions removed 90.8 ± 2.4% of the hippocampus and 73.8 ± 5.5% of the subiculum (means ± SEM; n = 13). Rats with hippocampal damage visited open arms more frequently (Mann–Whitney U test: Z = 2.5, P = 0.01) and spent more time on these arms (Z = 2.7, P < 0.01) than sham-operated rats (n = 14). However, the absolute level of avoidance was high in both groups (16,7% vs. 0% of arms visited), possibly because of the light intensity in the test room (1,000 lux).
We next asked whether defensive behavior in the plus maze was maintained by a specific part of the hippocampus. Rats received either complete lesions of the hippocampus (n = 13), lesions of the dorsal three-quarters (n = 9) or the ventral half (n = 10) of the hippocampus, or sham surgery (n = 10) (Fig. 1). Only small remnants were left in the complete lesion group (hippocampal damage, 96.2 ± 1.0%, mean ± SEM). The dorsal lesions affected 75.8 ± 2.2% of the hippocampus. The ventral lesions affected 54.5 ± 3.3%, and included the tip of the hippocampus. No significant damage to adjacent structures occurred, except for the ventral subiculum (complete lesion group, 81.2 ± 4.3% of subiculum; dorsal lesion group, 54.4 ± 3.8%; ventral group, 59.5 ± 5.5%).
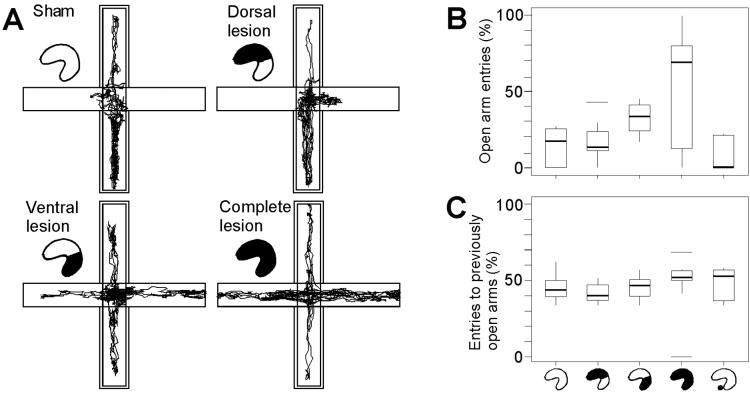
To reduce the threshold for leaving the closed arms, the rats were handled extensively and tested in a silent and dimly lit room (30 lux on the open arms). Rats with complete hippocampal lesions readily approached the open arms (Fig. 2 A and B). Similar behavior was seen after lesions that specifically targeted the ventral pole. Sham-operated rats and rats with damage to the dorsal three-quarters of the hippocampus stayed away from the exposed areas. The group difference was significant [H(3) = 10.4, P < 0.05]. Rats with ventral lesions had a significantly higher percentage of visits to open arms than sham-operated rats and rats with dorsal lesions (P < 0.01; Mann–Whitney U test with sequential Bonferroni correction). They were not different from the rats with complete lesions, nor did a difference between the dorsal group and the sham-operated group occur. Time spent on the open arms was also group-dependent [H(3) = 15.4, P = 0.001; complete lesions, median value 65.2%; ventral lesions, 28.4%; dorsal lesions, 16.7%; sham surgery, 7.4%]. When tested subsequently with four arms enclosed, all groups visited previously open arms as frequently as previously enclosed arms (Fig. 2C), implying that the group differences were caused by the exposed nature of two of the arms rather than some other aversive perceptual feature. The total number of arm entries was not affected by ventral lesions but increased after dorsal or complete lesions, both when two arms were open [ventral, 11.5; dorsal, 16; complete, 20; sham, 8.5; H(3) = 10.3, P < 0.05] and when all arms were enclosed [ventral, 15; dorsal, 31; complete, 29; sham, 12; H(3) = 13.4, P < 0.005], which suggests that the rats with ventral hippocampal lesions did not enter the open arms because of general hyperactivity.
Fig 2.
Reduced open-arm avoidance after selective lesions of the ventral hippocampus. (A) Paths of representative animals with sham operations or dorsal, ventral, or complete lesions of the hippocampus, during 10 min of exposure to an elevated plus maze with two open arms. Double contours indicate enclosed arms. (B) Box plot comparing performance in rats with dorsal, ventral, or complete hippocampal lesions, or lesions of the adjacent parts of the amygdala (hippocampal icons as in Fig. 1; amygdala group to the right). The diagram shows median percentage of visits to open arms (thick horizontal line inside box), interquartile distances (boxes), upper and lower limits [Q1 − 1.5 (Q3–Q1) and Q3 + 1.5 (Q3–Q1), where Q1 and Q3 are first and third quartiles, respectively], and outliers (horizontal lines). (C) Visits to previously open arms on a subsequent trial with all arms enclosed.
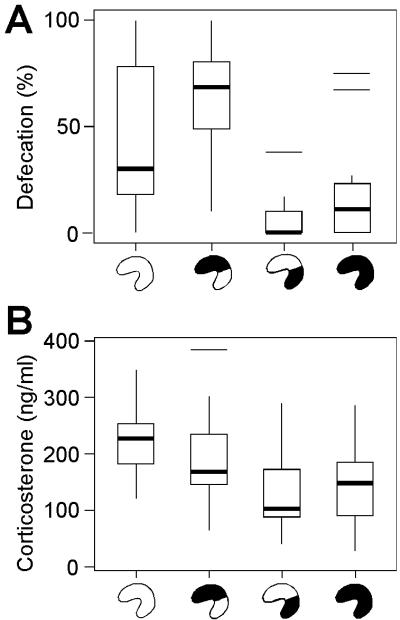
If rats with lesions in the ventral hippocampus approach open arms because they are less fearful, other indices of fear might be affected correspondingly. In a second experiment, we first confined rats to a brightly lit white chamber (1,000 lux) for 10 min twice daily for 5 days. Eleven of the rats had complete hippocampal lesions (95.7 ± 0.9% of total hippocampal tissue), 15 had ventral lesions (63.4 ± 2.3% damage), 25 had dorsal lesions (60.9 ± 1.5% damage), and 14 were sham-operated. The subiculum was damaged partly (complete, 84.5 ± 4.4%; ventral, 56.3 ± 6.0%; dorsal, 39.9 ± 3.3%). Confinement did not cause appreciable freezing (<8.3% of total time), but most animals with sham lesions or dorsal lesions left one or several feces during each session (Fig. 3A), suggesting that the event provoked moderate fear in these rats. Animals with complete or ventral lesions, in contrast, almost never defecated. The percentage of sessions during which the rats defecated was strongly group-dependent [H(3) = 29.2; P < 0.001]. We next measured corticosterone secretion after 20 min of confinement on day 6. Plasma corticosterone concentrations were lower in rats with complete or ventral lesions than in those with dorsal or sham lesions [Fig. 3B; H(3) = 15.8; P < 0.001]. On both measures (defecation and corticosterone), significant pairwise differences occurred between the ventral and dorsal groups and between the ventral and sham groups after Bonferroni correction, but no difference occurred between the dorsal group and the sham-operated group. The attenuated corticosterone response of the ventral group did not reflect a ceiling effect. When exposed to the test cage 24 h after receiving a foot shock in the cage, plasma corticosterone increased in 13 of the 15 rats in this group (median increase, 85 ng/ml; sham group, 74 ng/ml; dorsal group, 31 ng/ml; complete lesion group, 16 ng/ml). Altogether, these data show that ventral hippocampal lesions affect multiple indices of fear.
Fig 3.
Selective lesions of the ventral hippocampus reduced defecation and corticosterone secretion during exposure to a brightly lit test chamber (box plots; symbols as in Fig. 2). (A) Percentage of trials during which rats left one or several feces during confinement to a brightly lit test chamber. (B) Plasma corticosterone concentration 20 min after exposure to the test chamber.
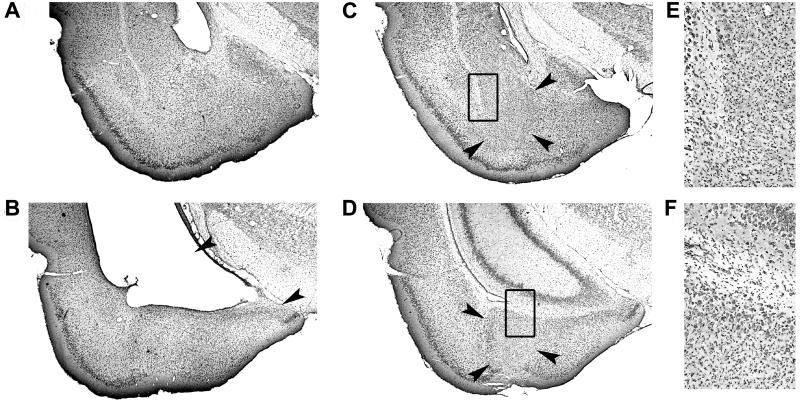
A potential concern is that the ventral hippocampal lesions might extend into nuclei of the amygdala that are essential for conditioning and expression of fear, such as the basolateral complex and the central nucleus (1–5). Cells in these nuclei seemed normal after ventral hippocampal damage (Fig. 4 A and B). To examine the question more directly, we made excitotoxic lesions in those parts of the amygdala that were closest to the hippocampus (n = 5). Most of the basolateral complex was affected, but also the central, medial, and cortical nuclei, and the amygdalo-hippocampal transition zone received partial damage (Fig. 4 C–F). Rats with lesions in the amygdala avoided the open arms of the elevated maze as strongly as the control rats (Fig. 2B; Mann–Whitney U test: Z < 1), suggesting that the effects of ventral hippocampal lesions were not due to inadvertent damage in the amygdala.
Fig 4.
Coronal sections showing representative cresyl violet stains including amygdala and ventral hippocampus after ibotenate lesions targeted either at the ventral hippocampus (A and B) or the adjacent nuclei of the amygdala (C–F). Arrowheads indicate approximate borders between lesioned and intact tissue. Transitions between intact and lesioned tissue in C and D (boxes) are shown at high magnification in E and F, respectively. The behavior of rats with lesions in the amygdala is shown in Fig. 2.
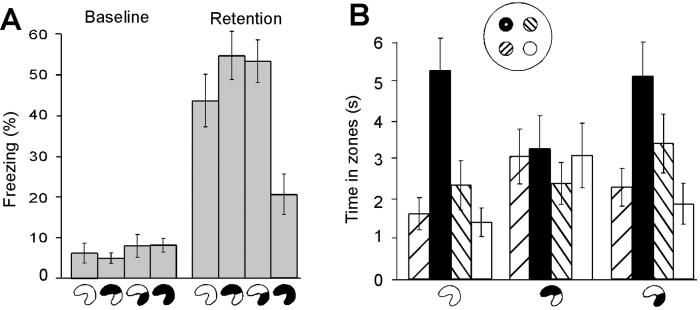
Finally, we asked whether lesions in the ventral hippocampus reduced fear because the animals were unable to process spatial or contextual information. First, rats with partial or complete damage to the hippocampus received three electric foot shocks in the white chamber with the grid floor. To examine contextual learning, we reexposed the rats to the chamber 24 h later. Most rats exhibited long periods of freezing (Fig. 5A). Freezing was attenuated only in rats with complete hippocampal ablation [F(3,51) = 6.8, P < 0.001]. Rats with ventral damage froze as much as control animals, suggesting that they did recognize the context. To examine the issue further, we trained rats with sham lesions (n = 12), dorsal lesions (n = 10; mean hippocampal damage, 40.1%), or ventral lesions (n = 10; mean damage, 49.0%) to find a hidden platform at a constant position in a Morris water maze during a 3-day period. As reported (22, 25, 26), rats with ventral lesions searched as accurately as sham-operated rats (Fig. 5B). Only dorsal lesions attenuated learning. Rats with damage to the dorsal hippocampus failed to show any bias toward the platform location on day 3 [group × zone effect: F(6,87) = 2.2, P < 0.05] but caught up by day 4 (Group × Zone effect: F < 1). Together, these results indicate that the processing of spatial and contextual information was functional in rats with ventral hippocampal damage and so cannot account for their frequent visits to the exposed arms.
Fig 5.
Intact contextual and spatial memory after lesions of the ventral hippocampus. (A) Percentage of freezing (means ± SEM) during the first 5 min of confinement to a test chamber before and 24 h after the rats received electric foot shock in this chamber. (B) Retention on a probe trial at the beginning of day 3 in the water maze. The diagram shows the time that rats spent in a circular zone around the platform position (black) and in corresponding zones of the three other quadrants (means ± SEM). Each zone covered 4.1% of the pool surface (radius, 20 cm; expected time, 2.5 s; Inset).
Discussion
The results suggest that the hippocampus is necessary for defensive and fear-related behavior, and that the involved circuitry lies at the ventral pole of the structure. Rats with lesions in the ventral hippocampus spent more time in the open arms of an elevated plus maze, as did animals receiving the clinically effective anxiolytic drug midazolam, and they left fewer feces and secreted less corticosterone when confined to a brightly lit environment. Lesions of the dorsal three-quarters of the hippocampus had no detectable effects on these measures. The deficit did not depend on prior experience, and was not caused by poor processing of spatial or contextual information. The results suggest that normal defensive behavior depends on the ventral hippocampus, or the ventral subiculum, which also was damaged. The range of defensive responses influenced by the hippocampus remains to be determined. Rats with large hippocampal lesions exhibit less food neophobia (17–19) and less predator-induced freezing (20), but do avoid predators when escape is possible (20), suggesting that not all defensive reactions depend on the ventral hippocampus.
The hippocampal circuits for unconditioned fear behavior seemed to some extent to be dissociable from those responsible for fear conditioning. Whereas the ventral tip of the structure was required for expression of unconditioned fear, fear could be conditioned to context with small circuits of hippocampal tissue at either pole. Other studies have reported that contextual conditioning is blocked specifically by ventral lesions (27, 28), but the impaired retention of contextual fear observed after damage to the dorsal hippocampus (29) implies that rats normally acquire contextual fear associations with the dorsal hippocampus as well. If so, how can animals with ventral lesions express conditioned fear without a hippocampal circuitry for expression of unconditioned fear? One possibility is that the associative networks of the remaining hippocampus can access executive regions of the brain's fear systems independently. Another is that the ventral hippocampus controls approach behavior and endocrine reactions but not the type of fear response by which contextual conditioning was measured, namely freezing.
The attenuated defensive behavior after lesions of the ventral hippocampus and subiculum is consistent with the intimate and partly bidirectional connectivity between this region and structures involved in the control of fear expression, such as the hypothalamus and amygdala (30–33). Several pathways exist through which the ventral hippocampus could influence the executive fear and defense systems of the brain. On one hand, the ventral poles of CA1, subiculum, and entorhinal cortex receive substantial input from the basolateral complex of the amygdala and the ventral hippocampus and subiculum send strong projections back to the basolateral complex and the central nucleus (30, 31), suggesting that the ventral hippocampus and the amygdala may organize defensive behavior and fear expression as a single integrated system. On the other hand, the ventral subiculum also has connections that bypass the amygdala and directly contact defensive response systems in the septum, hypothalamus, and bed nucleus of the stria terminalis (31, 32). Damage to the amygdala did not attenuate open-arm avoidance in the elevated plus maze, nor did extensive electrolytic lesions of the amygdala in previous studies (34). These results suggest that the hippocampus may influence fear expression in our tasks through direct projections to downstream neuroendocrine and behavioral control systems. However, other types of fear responses, such as food neophobia and predator-induced freezing, are apparently affected also by damage to the amygdala (35, 36). Although connections between ventral subiculum and downstream fear-response systems might be disrupted by these lesions, the findings have been replicated with neurotoxic lesions (37), suggesting that the temporal lobe may organize defensive behavior by means of several parallel systems.
Whereas the expression of unconditioned fear depended exclusively on the ventral pole of the hippocampus, spatial learning in the water maze was impaired only after damage to the dorsal hippocampus, as shown (22, 25, 26), and as expected if signals from the sensory association cortices to the entorhinal cortex primarily reach the dorsal hippocampus (33, 38, 39). The learning impairment was temporary (40), suggesting that the ventral hippocampus does support some spatial navigation, albeit less efficiently than more dorsal regions, which is in accordance with the presence of place cells in the ventral hippocampus (41, 42), although these are less numerous and less specific than in the dorsal hippocampus (41). How relevant sensory information reaches the ventral hippocampus (e.g., from amygdala or entorhinal cortex) and what computations can be performed with this information (e.g., elemental vs. configural associations) remain to be determined.
The uniform structure of the hippocampus suggests that it may perform a unitary function. Even if dorsal and ventral parts of the hippocampus receive input from different brain regions, the principal computations performed on these inputs may be similar. Could the deficit in fear expression after ventral lesions reflect the disruption of the same associative algorithms that are used by the dorsal hippocampus for successful spatial or contextual learning? The hippocampal influence on spatial and contextual learning strongly depends on associative plasticity, whereas the control of defensive behavior seemed to be independent of prior experience. It is possible, however, that the hippocampal influence on defensive behavior reflects a general nonassociative operation that is performed throughout the hippocampus, such as the comparison of multiple response alternatives and the subsequent strategic selection of an optimal active response (16).
Acknowledgments
We thank K. Barmen, R. Dahl, R. Espelid, I. Hammer, K. Haugen, J. Hoff, K. Jenssen, E. Nordeide, G. T. Storsveen, and R. Ulriksen for technical assistance. This work was supported by the Norwegian Research Council (148357/300, 139786/300, and 133958/420) and the Torstein Erbo Foundation.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Davis M., Walker, D. L. & Lee, Y. (1997) Philos. Trans. R. Soc. London B 352, 1675-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fendt M. & Fanselow, M. S. (1999) Neurosci. Biobehav. Rev. 23, 743-760. [DOI] [PubMed] [Google Scholar]
- 3.LeDoux J. E. (2000) Annu. Rev. Neurosci. 23, 155-184. [DOI] [PubMed] [Google Scholar]
- 4.Maren S. (2001) Annu. Rev. Neurosci. 24, 897-931. [DOI] [PubMed] [Google Scholar]
- 5.Aggleton J. P., (2000) The Amygdala: A Functional Analysis (Oxford Univ. Press, Oxford).
- 6.Cahill L., Weinberger, N. M., Roozendaal, B. & McGaugh, J. L. (1999) Neuron 23, 227-228. [DOI] [PubMed] [Google Scholar]
- 7.Selden N. R., Everitt, B. J., Jarrard, L. E. & Robbins, T. W. (1991) Neuroscience 42, 335-350. [DOI] [PubMed] [Google Scholar]
- 8.Kim J. J. & Fanselow, M. S. (1992) Science 256, 675-677. [DOI] [PubMed] [Google Scholar]
- 9.Phillips R. G. & LeDoux, J. E. (1992) Behav. Neurosci. 106, 274-285. [DOI] [PubMed] [Google Scholar]
- 10.O'Keefe J. & Nadel, L., (1978) The Hippocampus as a Cognitive Map (Clarendon, Oxford).
- 11.Squire L. R. (1992) Psychol. Rev. 99, 195-231. [DOI] [PubMed] [Google Scholar]
- 12.Eichenbaum H. (2000) Nat. Rev. Neurosci. 1, 41-50. [DOI] [PubMed] [Google Scholar]
- 13.Nadel L. & Willner, J. (1980) Physiol. Psychol. 8, 218-228. [Google Scholar]
- 14.Sutherland R. J. & Rudy, J. W. (1989) Psychobiology 17, 129-144. [Google Scholar]
- 15.Fanselow M. S. (2000) Behav. Brain Res. 110, 73-81. [DOI] [PubMed] [Google Scholar]
- 16.Gray J. A. & McNaughton, N., (2000) The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septo-hippocampal System (Oxford Univ. Press, Oxford).
- 17.Jarrard L. E. (1968) Physiol. Behav. 3, 65-70. [Google Scholar]
- 18.Krane R. V., Sinnamon, H. M. & Thomas, G. J. (1976) J. Comp. Physiol. Psychol. 90, 680-693. [DOI] [PubMed] [Google Scholar]
- 19.Miller J. S., Nonneman, A. J., Kelly, K. S., Neisewander, J. L. & Isaac, W. L. (1986) Behav. Neural Biol. 45, 240-253. [DOI] [PubMed] [Google Scholar]
- 20.Blanchard R. J. & Blanchard, D. C. (1972) J. Comp. Physiol. Psychol. 78, 77-82. [DOI] [PubMed] [Google Scholar]
- 21.Jarrard L. E. (1989) J. Neurosci. Methods 29, 251-259. [DOI] [PubMed] [Google Scholar]
- 22.Moser M.-B. & Moser, E. I. (1998) J. Neurosci. 18, 7535-7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pellow S., Chopin, P., File, S. E. & Briley, M. (1985) J. Neurosci. Methods 14, 149-167. [DOI] [PubMed] [Google Scholar]
- 24.Paxinos G. & Watson, C., (1986) The Rat Brain in Stereotaxic Coordinates (Academic, San Diego). [DOI] [PubMed]
- 25.Moser E. I., Moser, M.-B. & Andersen, P. (1993) J. Neurosci. 13, 3916-3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moser M.-B., Moser, E. I., Forrest, E., Andersen, P. & Morris, R. G. M. (1995) Proc. Natl. Acad. Sci. USA 92, 9697-9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richmond M. A., Yee, B. K., Pouzet, B., Veenman, L., Rawlins, J. N. P., Feldon, J. & Bannerman, D. M. (1999) Behav. Neurosci. 113, 1189-1203. [DOI] [PubMed] [Google Scholar]
- 28.Bast T., Zhang, W. N. & Feldon, J. (2001) Exp. Brain Res. 139, 39-52. [DOI] [PubMed] [Google Scholar]
- 29.Maren S., Aharonov, G. & Fanselow, M. S. (1997) Behav. Brain Res. 88, 261-274. [DOI] [PubMed] [Google Scholar]
- 30.Pitkänen A., Pikkarainen, M., Nurminen, N. & Ylinen, A. (2000) Ann. N.Y. Acad. Sci. 911, 369-391. [DOI] [PubMed] [Google Scholar]
- 31.Petrovich G. D., Canteras, N. S. & Swanson, L. W. (2001) Brain Res. Rev. 38, 247-289. [DOI] [PubMed] [Google Scholar]
- 32.Risold P. Y., Thompson, R. H. & Swanson, L. W. (1997) Brain Res. Rev. 24, 197-254. [DOI] [PubMed] [Google Scholar]
- 33.Witter M. P., Groenewegen, H. J., Lopes da Silva, F. H. & Lohman, A. H. M. (1989) Prog. Neurobiol. 33, 161-254. [DOI] [PubMed] [Google Scholar]
- 34.Treit D., Pesold, C. & Rotzinger, S. (1993) Behav. Neurosci. 107, 770-785. [DOI] [PubMed] [Google Scholar]
- 35.Blanchard D. C. & Blanchard, R. J. (1972) J. Comp. Physiol. Psychol. 81, 281-290. [DOI] [PubMed] [Google Scholar]
- 36.Nachman M. & Ashe, J. H. (1974) J. Comp. Physiol. Psychol. 87, 622-643. [DOI] [PubMed] [Google Scholar]
- 37.Dunn L. T. & Everitt, B. J. (1988) Behav. Neurosci. 102, 3-23. [DOI] [PubMed] [Google Scholar]
- 38.Dolorfo C. L. & Amaral, D. G. (1998) J. Comp. Neurol. 398, 25-48. [PubMed] [Google Scholar]
- 39.Burwell R. D. & Amaral, D. G. (1998) J. Comp. Neurol. 398, 179-205. [DOI] [PubMed] [Google Scholar]
- 40.De Hoz, L., Knox, J. & Morris, R. G. M. (2002) Hippocampus, in press.
- 41.Jung M. W., Wiener, S. I. & McNaughton, B. L. (1994) J. Neurosci. 14, 7347-7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poucet B., Thinus-Blanc, C. & Muller, R. U. (1994) NeuroReport 5, 2045-2048. [DOI] [PubMed] [Google Scholar]