Abstract
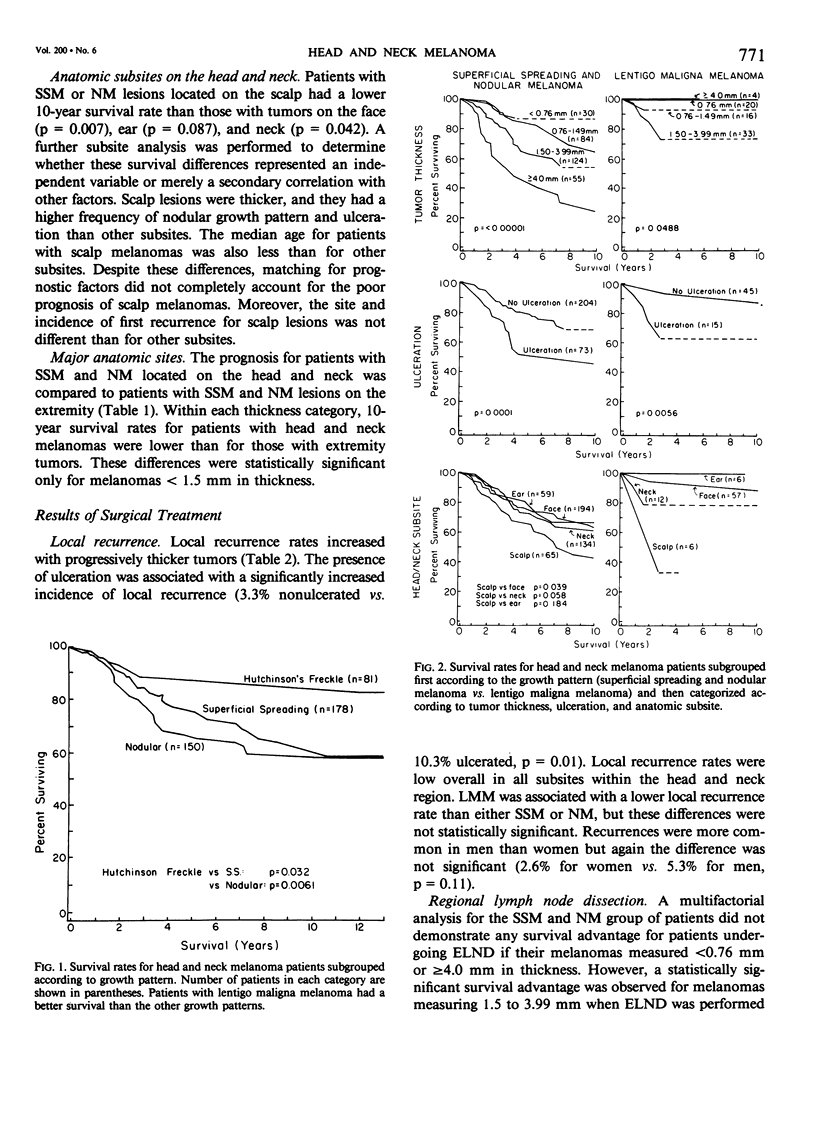
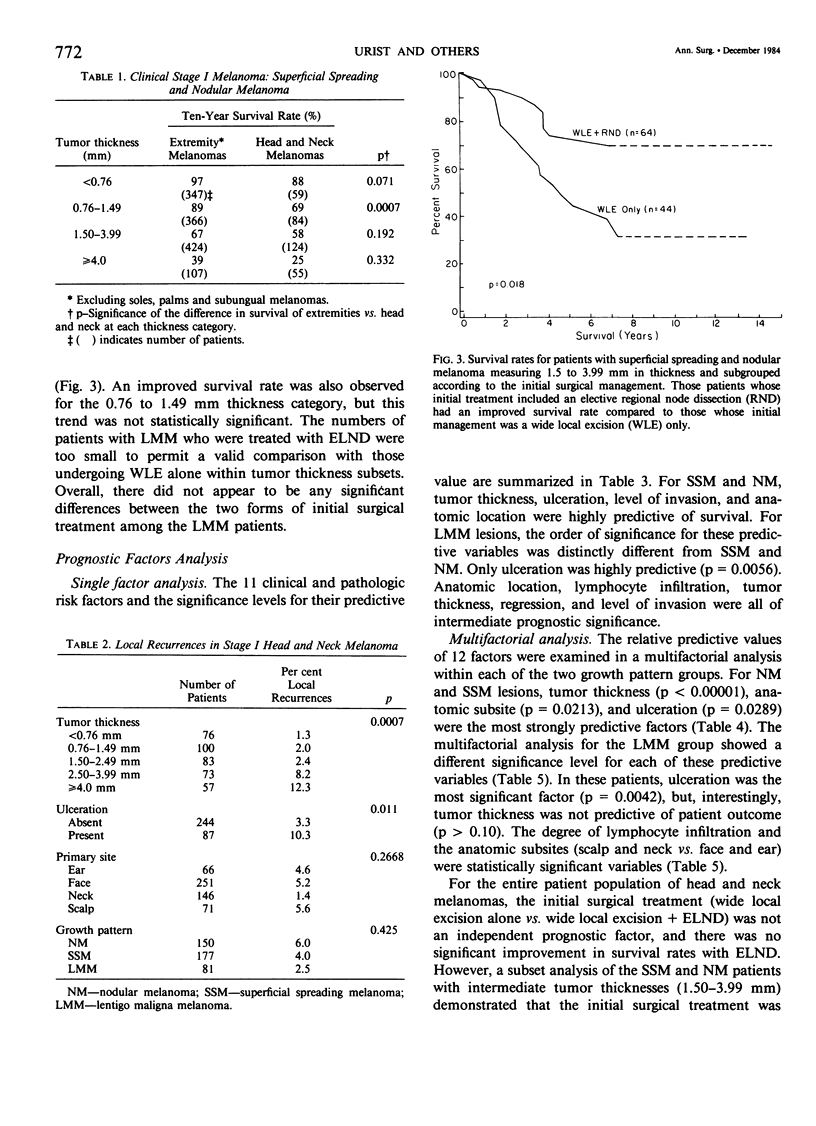
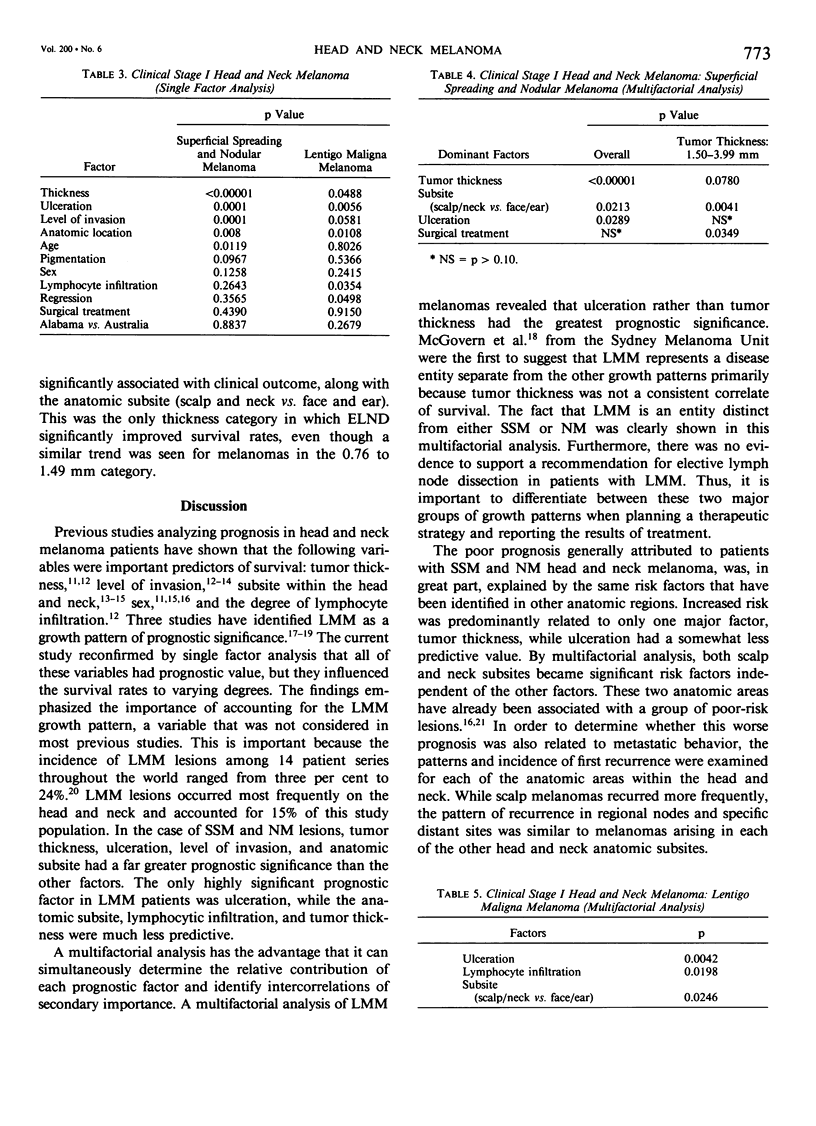
Single and multifactorial analyses were used to evaluate prognosis and results of surgical treatment in 534 clinical Stage I patients with head and neck cutaneous melanoma treated at the University of Alabama in Birmingham (U.S.A.) and the University of Sydney (Australia). This computerized data base was prospectively accumulated in over 90% of cases. Melanomas were about equally distributed between men and women. They were located on the skin of the face in 47%, neck in 27%, scalp in 13%, and the ear in 13% of patients. Both the results of the prognostic factors analyses and the surgical treatment demonstrated that lentigo maligna melanoma (LMM) was distinct from the other two growth patterns, superficial spreading melanoma and nodular melanoma (SSM and NM). In a multifactorial analysis of the 453 patients with SSM and NM, the dominant prognostic variables were tumor thickness (p less than 0.00001), anatomic subsite (p = 0.0213), and ulceration (p = 0.0289). Patients with melanomas on the scalp or neck subsites fared worse than those with tumors located on the face or ear. The results differed for LMM, where thickness was not a significant predictor of survival, and the most dominant prognostic variable was ulceration (p = 0.0042). Local recurrence rates were low, being 2.4% for tumors less than 2.5 mm in thickness, but were 12.3% for tumors greater than or equal to 4.0 mm in thickness. Patients with SSM and NM lesions located on the head and neck had a lower survival rate than those with extremity melanomas in every tumor thickness category, although only those in the 0.76 to 1.49 mm thickness subgroup were significantly different (p = 0.0007). After 5 years of follow-up, patients who underwent an elective lymph node dissection for SSM and NM with a thickness range of 1.5 to 3.99 mm had a better survival (72%) than patients with melanomas of equivalent thickness whose initial treatment was wide excision alone (45%). LMM had a less aggressive biologic behavior compared to SSM or NM and was treated more conservatively. Thus, LMM lesions had an 85% 10-year survival rate with wide excision only, and there was no significant improvement in survival with ELND. Growth patterns, tumor thickness, ulceration, and anatomic subsites should be considered when evaluating risk factors and when making treatment decisions in head and neck melanoma patients.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames F. C., Sugarbaker E. V., Ballantyne A. J. Analysis of survival and disease control in stage I melanoma of the head and neck. Am J Surg. 1976 Oct;132(4):484–491. doi: 10.1016/0002-9610(76)90325-1. [DOI] [PubMed] [Google Scholar]
- Balch C. M., Murad T. M., Soong S. J., Ingalls A. L., Halpern N. B., Maddox W. A. A multifactorial analysis of melanoma: prognostic histopathological features comparing Clark's and Breslow's staging methods. Ann Surg. 1978 Dec;188(6):732–742. doi: 10.1097/00000658-197812000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch C. M., Soong S. J., Milton G. W., Shaw H. M., McGovern V. J., Murad T. M., McCarthy W. H., Maddox W. A. A comparison of prognostic factors and surgical results in 1,786 patients with localized (stage I) melanoma treated in Alabama, USA, and New South Wales, Australia. Ann Surg. 1982 Dec;196(6):677–684. doi: 10.1097/00000658-198212001-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch C. M., Soong S. J., Murad T. M., Ingalls A. L., Maddox W. A. A multifactorial analysis of melanoma. II. Prognostic factors in patients with stage I (localized) melanoma. Surgery. 1979 Aug;86(2):343–351. [PubMed] [Google Scholar]
- Balch C. M., Wilkerson J. A., Murad T. M., Soong S. J., Ingalls A. L., Maddox W. A. The prognostic significance of ulceration of cutaneous melanoma. Cancer. 1980 Jun 15;45(12):3012–3017. doi: 10.1002/1097-0142(19800615)45:12<3012::aid-cncr2820451223>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Ballantyne A. J. Malignant melanoma of the skin of the head and neck. An analysis of 405 cases. Am J Surg. 1970 Oct;120(4):425–431. doi: 10.1016/s0002-9610(70)80001-0. [DOI] [PubMed] [Google Scholar]
- Byers R. M., Smith J. L., Russell N., Rosenberg V. Malignant melanoma of the external ear. Review of 102 cases. Am J Surg. 1980 Oct;140(4):518–521. doi: 10.1016/0002-9610(80)90203-2. [DOI] [PubMed] [Google Scholar]
- Close L. G., Goepfert H., Ballantyne A. J., Jesse R. H. Malignant melanoma of the scalp. Laryngoscope. 1979 Aug;89(8):1189–1196. doi: 10.1002/lary.1979.89.8.1189. [DOI] [PubMed] [Google Scholar]
- Day C. L., Jr, Mihm M. C., Jr, Sober A. J., Harris M. N., Kopf A. W., Fitzpatrick T. B., Lew R. A., Harrist T. J., Golomb F. M., Postel A. Prognostic factors for melanoma patients with lesions 0.76 - 1.69 mm in thickness. An appraisal of "thin" level IV lesions. Ann Surg. 1982 Jan;195(1):30–34. doi: 10.1097/00000658-198201001-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnellan M. J., Seemayer T., Huvos A. G., Miké V., Strong E. W. Clinicopathologic study of cutaneous melanoma of the head and neck. Am J Surg. 1972 Oct;124(4):450–455. doi: 10.1016/0002-9610(72)90065-7. [DOI] [PubMed] [Google Scholar]
- Gupta T. K. Results of treatment of 269 patients with primary cutaneous melanoma: a five-year prospective study. Ann Surg. 1977 Aug;186(2):201–209. doi: 10.1097/00000658-197708000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M. G., McCarten A. B. Tumor thickness and lymphocytic infiltration in malignant melanoma of the head and neck. Am J Surg. 1974 Oct;128(4):557–561. doi: 10.1016/0002-9610(74)90275-x. [DOI] [PubMed] [Google Scholar]
- Harris T. J., Hinckley D. M. Melanoma of the head and neck in Queensland. Head Neck Surg. 1983 Jan-Feb;5(3):197–203. doi: 10.1002/hed.2890050303. [DOI] [PubMed] [Google Scholar]
- McGovern V. J., Shaw H. M., Milton G. W., Farago G. A. Is malignant melanoma arising in a Hutchinson's melanotic freckle a separate disease entity? Histopathology. 1980 May;4(3):235–242. doi: 10.1111/j.1365-2559.1980.tb02918.x. [DOI] [PubMed] [Google Scholar]
- McGovern V. J., Shaw H. M., Milton G. W., Farago G. A. Prognostic significance of the histological features of malignant melanoma. Histopathology. 1979 Sep;3(5):385–393. doi: 10.1111/j.1365-2559.1979.tb03020.x. [DOI] [PubMed] [Google Scholar]
- McGovern V. J., Shaw H. M., Milton G. W., McCarthy W. H. Ulceration and prognosis in cutaneous malignant melanoma. Histopathology. 1982 Jul;6(4):399–407. doi: 10.1111/j.1365-2559.1982.tb02737.x. [DOI] [PubMed] [Google Scholar]
- Olson R. M., Woods J. E., Soule E. H. Regional lymph node management and outcome in 100 patients with head and neck melanoma. Am J Surg. 1981 Oct;142(4):470–473. doi: 10.1016/0002-9610(81)90377-9. [DOI] [PubMed] [Google Scholar]
- Roses D. F., Harris M. N., Grunberger I., Gumport S. L. Selective surgical management of cutaneous melanoma of the head and neck. Ann Surg. 1980 Nov;192(5):629–632. doi: 10.1097/00000658-198011000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim F. H., Taylor W. F., Ivins J. C., Pritchard D. J., Soule E. H. A prospective randomized study of the efficacy of routine elective lymphadenectomy in management of malignant melanoma. Preliminary results. Cancer. 1978 Mar;41(3):948–956. doi: 10.1002/1097-0142(197803)41:3<948::aid-cncr2820410324>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Stage I melanoma of the skin: the problem of resection margins. W.H.O. Collaborating Centres for Evaluation of Methods of Diagnosis and Treatment of Melanoma. Eur J Cancer. 1980 Aug;16(8):1079–1085. [PubMed] [Google Scholar]
- Veronesi U., Adamus J., Bandiera D. C., Brennhovd I. O., Caceres E., Cascinelli N., Claudio F., Ikonopisov R. L., Javorskj V. V., Kirov S. Inefficacy of immediate node dissection in stage 1 melanoma of the limbs. N Engl J Med. 1977 Sep 22;297(12):627–630. doi: 10.1056/NEJM197709222971202. [DOI] [PubMed] [Google Scholar]


