Abstract
Studies have shown that clusterin (also called apolipoprotein J) can influence the structure and toxicity of amyloid-β (Aβ) in vitro. To determine whether endogenous clusterin plays a role in influencing Aβ deposition, structure, and toxicity in vivo, we bred PDAPP mice, a transgenic mouse model of Alzheimer's disease, to clusterin−/− mice. By 12 months of age, PDAPP, clusterin−/− mice had similar levels of brain Aβ deposition as did PDAPP, clusterin+/+ mice. Although Aβ deposition was similar, PDAPP, clusterin−/− mice had significantly fewer fibrillar Aβ (amyloid) deposits than PDAPP mice expressing clusterin. In the absence of clusterin, neuritic dystrophy associated with the deposited amyloid was markedly reduced, resulting in a dissociation between fibrillar amyloid formation and neuritic dystrophy. These findings demonstrate that clusterin markedly influences Aβ structure and neuritic toxicity in vivo and is likely to play an important role in Alzheimer's disease pathogenesis.
Amyloid-β (Aβ) peptides are predominantly 39–43 aa in length and are derived from the amyloid precursor protein (APP) through endoproteolytic cleavage. Abundant evidence suggests that the conversion of Aβ from soluble to insoluble forms in the brain is a key event in the pathogenesis of Alzheimer's disease (AD). Genetic and biochemical evidence supporting this idea is that all known mutations that cause early-onset forms of familial AD or Αβ-related cerebral amyloid angiopathy map to three genes [APP, presenilin-1 (PS-1), and PS-2] (1, 2). Most of these mutations result in relative overproduction of Aβ42, a particularly amyloidogenic form of Aβ, which over time increases the probability of Aβ aggregation. Some mutations in APP within the Aβ-coding region that result in familial cerebral amyloid angiopathy do not seem to increase Aβ production but increase its propensity for aggregation and toxicity (3, 4). Although these mutations have given insight into a central role for Aβ in both AD and cerebral amyloid angiopathy, cases of “sporadic,” late-onset AD (age >60 years), which accounts for most AD cases, are not associated with increased Aβ production or altered Aβ sequence.
The probability that Aβ will aggregate into different insoluble forms in the brain can be influenced by Aβ-binding proteins, a process which occurs after Aβ generation. One example of a protein that seems to influence Aβ in this way is apolipoprotein E (apoE). The apoE4 isoform of apoE is the only proven genetic risk factor for both late-onset AD and cerebral amyloid angiopathy, and studies suggest that apoE influences Aβ structure, clearance, and neuritic toxicity both in vitro and in vivo with no clear effect on Aβ production (5–7). Whether apolipoproteins other than apoE influence Aβ aggregation and toxicity in vivo is unknown, although a good candidate for such effects is apolipoprotein J, also known as clusterin.
The two most abundantly expressed apolipoproteins in the central nervous system that are present at similar concentrations are apoE and clusterin (8–12). Both apoE and clusterin are expressed by glia and are present in predominantly distinct high-density lipoproteins (13, 14). Studies have shown that clusterin is present in plaques (15, 16), up-regulated in the AD brain (15), associated with soluble Aβ in cerebrospinal fluid (17), and can facilitate Aβ transport across the blood–brain barrier (18, 19). In vitro studies have shown that purified clusterin can interact with Aβ (20) and influence fibril formation (21, 22) as well as acute Aβ neurotoxicity (21, 23, 24). Although these studies suggest that clusterin–Aβ interactions may be relevant to AD, whether clusterin plays a direct role in the formation of AD pathology in vivo is not clear.
To evaluate further the role of clusterin in AD pathology, we bred PDAPP mice, a transgenic mouse model that develops AD-like neuropathology to clusterin−/− mice. Our findings demonstrate that clusterin expression facilitates but is not required for Aβ fibril (amyloid) formation. In addition, amyloid deposits that form in the absence of clusterin expression are associated with far fewer dystrophic neurites. Despite similar levels of Aβ accumulation in the brain, the absence of clusterin was also associated with alterations in the levels of soluble brain Aβ. Together, these studies suggest a role for clusterin in influencing amyloid deposition and the associated neuritic toxicity in vivo.
Methods and Materials
Animals and Tissue Preparation.
PDAPP mice, homozygous (+/+) for the APPV717F transgene (25, 26), were bred to clusterin knockout mice (27, 28) to yield PDAPP+/−, clusterin+/− progeny. The progeny were then bred to each other and animals that were PDAPP+/+, clusterin+/− identified by PCR and breed testing. PDAPP+/+, clusterin+/− mice were then bred together to yield PDAPP+/+ littermate mice with the following clusterin genotypes: clusterin+/+, clusterin+/−, and clusterin−/−. Tissue analysis was performed exactly as described (29). Animals were analyzed at 3, 6, 9, 12, and 15 months of age.
Histological Analysis.
Tissue sections were cut in the coronal plane at 40 μm on a freezing sliding microtome from the genu of the corpus callosum through the caudal extent of the hippocampus. The percent surface area covered by Aβ-immunoreactive deposits (% Aβ Load) as identified with a rabbit pan-Aβ antibody (BioSource International, Camarillo, CA) was quantified according to stereological principles as described (30). Thioflavine-S staining was performed as described (26). Aβ and thioflavine-S load were determined in the cingulate cortex and hippocampus in three sections, each separated by 300 μm. The de Olmos silver stain was performed as described (31), and the number of dystrophic neurites present in three sections determined.
Aβ Quantification.
AβTotal and Aβ42 levels in guanidine lysates were assayed as described (32). In brief, hippocampal or cortical tissue was homogenized in a denaturing buffer containing 5 M guanidine plus protease inhibitors. The extracts were diluted and analyzed in denaturing ELISAs containing a final concentration of 500 mM guanidine for AβTotal (m266 as capture antibody and biotinylated m3D6 as reporter antibody) or Aβ42 (m21F12 as capture antibody and biotinylated m3D6 as reporter antibody). To evaluate the potential soluble pools of brain Aβ, we also performed a carbonate extraction (100 mM carbonate/50 mM NaCl/protease inhibitors, pH 11.5) of hippocampal and cortical tissue (1:20, wt/vol) on ice. Tissue samples were Dounce homogenized and spun in a microcentrifuge at 14,000 rpm for 15 min at 4°C. The supernatant was placed in a fresh tube on ice and the pH of the lysate was neutralized to 7.4 with 1 M Tris (pH 6.8). The carbonate soluble pool of AβTotal was determined with denaturing (described above) and nondenaturing (lacking guanidine) ELISAs. An additional Aβ ELISA was developed to identify possible oligomeric species of Aβ. A monoclonal antibody directed against the first five residues of human Aβ was used for both capturing (m3D6) and detecting (biotinylated m3D6) Aβ.
Acid Gels.
We modified our denaturing polyacrylamide gel system (33) to identify individual Aβ peptides present in the tissue lysates. The carbonate-soluble extract was denatured in formic acid (final concentration, 70%) and reduced with β-mercaptoethanol (1%). The denatured extracts were run (anode to cathode) in a 1 M acetic acid buffer through a 1.6 M acetic acid/6 M urea step gel of the following polyacrylamide percentages: 4% stacker (2.5% N,N,N′,N′-tetramethylethylenediamine), 10% step (2.5% N,N,N′,N′-tetramethylethylenediamine), and a 22% resolving gel (1.875% N,N,N′,N′-tetramethylethylenediamine). The acidic pH of the gel was neutralized before transfer to nitrocellulose. Standard Western blotting procedures were then performed as described (33).
Statistical Analysis.
All statistical analysis was performed by using PRISM V.3.00 (GraphPad, San Diego) for WINDOWS (Microsoft).
Results
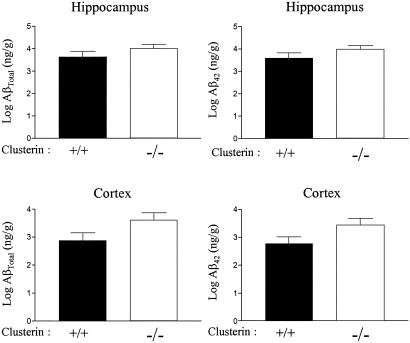
To determine whether clusterin influenced the age of onset or amount of Aβ deposition in PDAPP+/+ mice, we examined PDAPP+/+ mice that were clusterin+/+ and clusterin−/− between 3 and 15 months of age. Aβ-immunoreactive deposits were not observed in any of the mice until after ≈6 months of age. No difference occurred in the onset of Aβ deposition between the groups. By 12 months of age, ≈60% of the clusterin+/+ (13 of 19) and clusterin−/− (15 of 26) mice had developed Aβ deposition with no difference in the ratios between the groups. The variability in Aβ load at this age is consistent with other recent analyses of PDAPP+/+ mice (33, 34). In all mice that had developed Aβ deposition, we analyzed the amount of insoluble Aβ by ELISA in both the cortex and hippocampus. No significant difference in the level of insoluble AβTotal or Aβ42 occurred between clusterin+/+ and clusterin−/− mice (Fig. 1). Although AβTotal levels were on average 25-fold lower in mice lacking plaques, no difference occurred in AβTotal levels in clusterin+/+ versus clusterin−/− mice without Aβ deposition. Thus, the absence of clusterin did not influence either the age of onset of Aβ deposition or the amount of Aβ accumulation in PDAPP+/+ mice.
Fig 1.
Clusterin expression does not alter the mass of deposited Aβ in PDAPP mice. Levels of AβTotal or Aβ42 as assessed by ELISA on guanidine extracts from hippocampus and cortex did not reveal significant differences between 12-month-old PDAPP+/+, clusterin+/+ (n = 13) versus PDAPP+/+, clusterin−/− mice (n = 14). Data reported are means ± SEM.
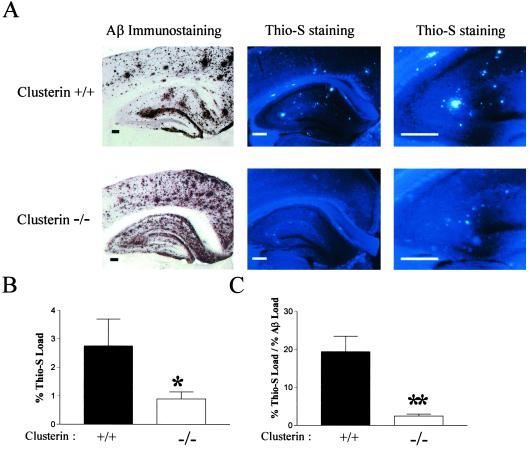
We next asked whether clusterin influenced the anatomical distribution of Aβ deposits and the Aβ structure itself. The anatomical distribution of Aβ deposition in clusterin+/+ and clusterin−/− mice was similar in general, although subtle differences seemed to exist (Fig. 2 A and B). Aβ deposits were prominent in the hippocampus, especially within the molecular layer of the dentate gyrus in the presence or absence of clusterin. In addition, Aβ immunoreactivity was prominent in the cingulate cortex of both groups. Aβ immunoreactivity was, however, more diffuse in appearance in the absence of clusterin with fewer “compact” plaques (Fig. 2A). In contrast to the pattern of Aβ immunoreactivity, marked differences occurred between the groups in the absolute amount and area occupied (percent load) of thioflavine-S-positive Aβ deposits (amyloid) by 12 months of age. Although 77% (10 of 13) clusterin+/+ mice with Aβ deposition had thioflavine-S-positive deposits (fibrillar Aβ or amyloid) in the cortex, only 20% (3 of 15) PDAPP+/+, clusterin−/− mice had detectable cortical thioflavine-S-positive deposits (P < 0.0026, χ2; Fig. 2A). In addition to promoting cortical amyloid deposition, clusterin also promoted the formation of hippocampal amyloid (Fig. 2A). Although all PDAPP+/+, clusterin−/− mice (n = 15) analyzed at 12 months had thioflavine-S-positive deposits in the hippocampus, these mice had significantly less hippocampal amyloid burden (0.89 vs. 2.76% thioflavine load, P = 0.05), as well as a decrease in the percent of Aβ-immunoreactive deposits that were thioflavine-S-positive (2.46 vs. 19.4% thioflavine load/Aβ load, P < 0.0001; Fig. 2 A and B) as compared with clusterin+/+ mice. Qualitatively, clusterin+/− mice had levels of amyloid deposition intermediate between clusterin+/+ and clusterin−/− mice (data not shown). Thus, as for apoE (26, 29, 30, 35), clusterin facilitates the conversion of Aβ to fibrillar forms in vivo.
Fig 2.
Clusterin facilitates the conversion of Aβ into amyloid in vivo. The 12-month-old PDAPP+/+, clusterin+/+ and PDAPP+/+, clusterin−/− mice containing Aβ-immunoreactive deposits were analyzed for the presence of thioflavine-S (Thio-S)-positive plaques (amyloid). (A) Serial brain sections were either immunostained with either a polyclonal antibody against Aβ (Left) or with the dye thioflavine-S (Center and Right). (Scale bars: Left, 500 μm; Center, 250 μm; Right, 125 μm.) (B) PDAPP+/+, clusterin−/− mice (n = 15) had significantly less hippocampal thioflavine-S load than littermate PDAPP+/+, clusterin+/+ mice (n = 13). *, P = 0.05. (C) The percentage of deposited Aβ that was thioflavine-S-positive (fibrillar) was significantly decreased in PDAPP+/+, clusterin−/− mice. **, P < 0.0001. Data in B and C are means ± SEM.
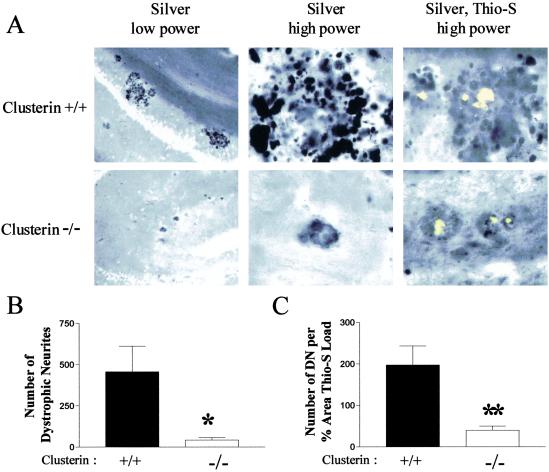
In APP transgenic mice and in human AD, thioflavine-S-positive deposits of fibrillar Aβ (amyloid) are surrounded by enlarged, distorted dendrites and axons (neuritic plaques/dystrophy; refs. 30 and 36), which suggests that the amyloid fibrils themselves (or some form of Aβ associated with amyloid plaques) lead to local neuritic toxicity. To determine the effect of clusterin on both amyloid deposition and neuritic dystrophy, we performed double-labeling of brain sections from PDAPP+/+, clusterin+/+ and PDAPP+/+, clusterin−/− mice by using both thioflavine-S and the de Olmos silver stain. As we have shown in PDAPP mice (30), all thioflavine-S-positive deposits in PDAPP+/+, clusterin+/+ mice were surrounded by multiple enlarged, dystrophic neurites (Fig. 3A). Although fewer thioflavine-S-positive deposits occurred in the absence of clusterin (see above), amyloid deposition was readily demonstrated. The neuritic dystrophy surrounding amyloid deposits in PDAPP+/+, clusterin−/− mice, however, was markedly reduced with many deposits having few to no detectable dystrophic neurites (Fig. 3A). Quantitatively, a 10-fold reduction occurred in dystrophic neurites in the hippocampus of PDAPP+/+, clusterin+/+ vs. clusterin−/− mice (Fig. 3B), and a 5-fold reduction in the number of dystrophic neurites per amyloid deposit (Fig. 3B). We also assessed PDAPP+/+, clusterin−/− mice at 15 months of age. Thioflavine-S-positive amyloid load in the hippocampus increased from 0.89% at 12 months to 2.25 ± 0.48% (mean ± SEM, n = 8) at 15 months. Despite this increase, the number of dystrophic neurites per amyloid deposit did not increase from 12 months (42.9 ± 13.8, n = 15) to 15 months (35.7 ± 19.4, n = 8). Thus, although clusterin promotes amyloid formation, it also facilitates the neuritic toxicity associated with the amyloid formed in its presence.
Fig 3.
Dissociation between amyloid plaques and neurite toxicity in PDAPP+/+, clusterin−/− mice. (A) Brain sections from 12-month-old PDAPP+/+, clusterin+/+ and PDAPP+/+, clusterin−/− mice were labeled with the de Olmos silver stain with or without thioflavine-S (Thio-S) to identify the neuritic dystrophy associated with the fibrillar amyloid. Vast numbers of dystrophic neurites (DN) were observed in the locale of thioflavine-S-positive deposits in PDAPP+/+, clusterin+/+ mice (Upper) at low and high power. Little neuritic dystrophy surrounded thioflavine-S-positive deposits in the PDAPP+/+, clusterin−/− mice (Lower). (B) PDAPP+/+, clusterin−/− mice had significantly fewer dystrophic neurites (mean ± SEM: 42.9 ± 13.8, n = 15) in three equally spaced sections than PDAPP+/+, clusterin+/+ mice (456.6 ± 155.2, n = 13). *, P = 0.0083. (C) The number of dystrophic neurites normalized to the percent area of the hippocampus covered by thioflavine-S was significantly decreased (5-fold) in PDAPP+/+, clusterin−/− mice (mean ± SEM: 40.0 ± 10.1, n = 15) compared with the PDAPP+/+, clusterin+/+ mice (197.3 ± 45.8, n = 13). **, P = 0.0014.
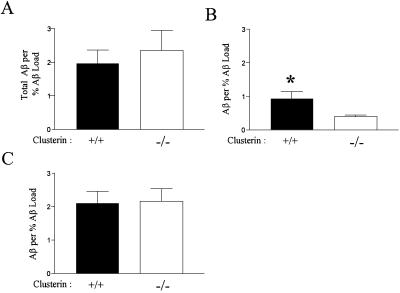
The dissociation between amyloid formation and neuritic dystrophy in PDAPP+/+, clusterin−/− mice suggested that clusterin might be influencing a soluble “toxic” species/form of Aβ during or after the process of Aβ deposition. To address this possibility, we assessed the amount of carbonate-soluble brain Aβ by ELISA in cortical brain homogenates under both denaturing and nondenaturing conditions. By using an ELISA for AβTotal under denaturing conditions, the levels of carbonate-soluble Aβ in brain lysates of PDAPP+/+, clusterin+/+ and clusterin−/− mice were very low (≈8.6 ng/mg protein) and were not different among the groups in animals lacking Aβ deposition. In PDAPP+/+, clusterin+/+ and clusterin−/− mice with Aβ deposition, levels of carbonate-soluble Aβ increased by 5- to 10-fold and no significant difference between genotypes was observed, even when the values were normalized to the percent Aβ load (Fig. 4A). Normalization of the data were required because of the extreme variability in Aβ deposition coupled to an observed highly significant correlation between levels of deposited and soluble Aβ. However, when performing the same ELISA under nondenaturing conditions, we observed that the amount of AβTotal/% Aβ load was significantly higher in the presence vs. the absence of clusterin (0.94 vs. 0.39, P = 0.0111; Fig. 4B). A similar trend was observed in the nondepositing animals (data not shown). The amount of AβTotal detected by nondenaturing ELISA was 2- to 3-fold lower than that observed under denaturing conditions, which suggests that an appreciable fraction of soluble Aβ may be present in different conformations, such as small oligomeric forms, leading to antigenic masking under certain conditions. To assess this issue further, we used the same N-terminal monoclonal anti-Aβ antibody (3D6) to both capture and detect Aβ in our sandwich ELISA. This format should only detect Aβ present in oligomeric forms. By using this assay on carbonate-soluble homogenates under nondenaturing conditions, we observed that the levels of Aβ present were very similar to those found in the same lysates when assayed under denaturing conditions (which disrupt oligomer formation). No statistical difference existed in the amount of Aβ in the presence or absence of clusterin in this assay, which suggests that the increase in soluble Aβ in clusterin+/+ mice detected under nondenaturing conditions may be monomeric. To determine whether differences existed in the species of soluble brain Aβ in the presence or absence of clusterin in plaque-bearing mice, we ran soluble brain lysates in completely denaturing acid-urea gels followed by Western blotting for Aβ. We found that most soluble brain Aβ in clusterin+/+ and clusterin−/− mice was Aβ42 (Fig. 5). A small amount of Aβ40 and lower molecular weight Aβ species were also detected in all clusterin+/+ mice analyzed in this fashion (n = 4); however, Aβ40 was not detected in the soluble brain homogenates of the clusterin−/− mice assayed (n = 4). Taken together, the data suggest that clusterin is influencing not only the form but also the species of Aβ associated with the soluble brain fraction.
Fig 4.
Clusterin expression alters the soluble pool of brain Aβ. (A) Carbonate-soluble hippocampal extracts from 12-month-old PDAPP+/+, clusterin+/+ (n = 13) and PDAPP+/+, clusterin−/− (n = 15) mice had similar levels of AβTotal when assayed by ELISA under denaturing conditions. The AβTotal concentrations (nanograms per milligram of protein) were normalized to the percent Aβ load to correct for the variability in deposition between animals. (B) When the carbonate extracts were analyzed under nondenaturing conditions with the same ELISA, mice expressing clusterin had a significant 2-fold increase in the soluble pool of AβTotal. *, P = 0.01. (C) Carbonate extracts analyzed under nondenaturing conditions with an ELISA by using the same capture and detecting antibody (resulting in an assay that should be specific for oligomeric forms of Aβ) did not detect a significant difference between PDAPP+/+, clusterin+/+ and PDAPP+/+, clusterin−/− mice. Data in B and C are means ± SEM.
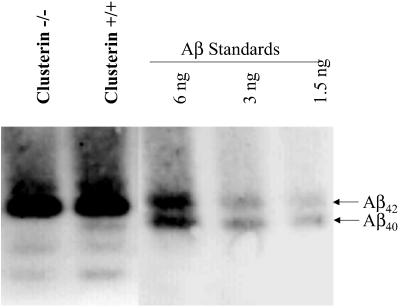
Fig 5.
The subtle difference in the Aβ peptide composition of the soluble pool of brain Aβ is revealed by acid-urea polyacrylamide gels. The Aβ peptide composition of the carbonate-soluble hippocampal extracts from 12-month-old PDAPP+/+, clusterin+/+ and PDAPP+/+, clusterin−/− mice was determined by acid-urea gel analysis (n = 4 per group). Human Aβ40 and Aβ42 synthetic peptides are used for mass and migration comparisons. Soluble extracts analyzed under the completely denaturing conditions demonstrate that human Aβ42 is the predominant Aβ peptide present. Human Aβ40 was readily detectable in extracts from all PDAPP+/+, clusterin+/+ mice examined, whereas human Aβ40 could not be detected in PDAPP+/+, clusterin−/− extracts.
Discussion
We have developed and characterized PDAPP+/+ transgenic animals that were either clusterin-expressing or -deficient to test whether clusterin plays any role in vivo in the cascade of amyloid deposition and toxicity. Although clusterin did not have a significant effect on the absolute levels of deposited brain Aβ at 12 months of age, its presence was associated with increased fibril formation. Moreover, we observed that the thioflavine-S-positive amyloid that deposits in the absence of clusterin is associated with far less neuritic dystrophy than amyloid present in clusterin-expressing PDAPP mice. Although the brain of clusterin−/− mice seems to develop and age normally, it is possible that the absence of clusterin results in a compensatory change in other molecules that results in the phenotype we observed. However, our data combined with prior in vitro data suggest that clusterin is directly involved in Aβ metabolism in vivo. It was found that purified clusterin can bind to soluble Aβ40 with a dissociation constant characteristic of a high-affinity interaction (Kd of 2 nM) (20). In addition, multiple laboratories have shown that clusterin prevents aggregation and polymerization of synthetic Aβ in vitro (21, 22). Other studies have shown that clusterin may be an important regulator of soluble central nervous system Aβ levels. Studies by Zlokovic and colleagues have shown that Aβ–clusterin complexes can be transported across the blood–brain barrier by a high-affinity receptor-mediated process involving transcytosis (18, 19). In addition, cell culture experiments have demonstrated that Aβ uptake and degradation is facilitated by the presence of clusterin (37). Although these in vitro studies highlight possible physiologically relevant interactions between clusterin and Aβ, it was unknown whether clusterin had any role in the process of amyloidogenesis in vivo.
We demonstrate that expression of murine clusterin in some way facilitates the conversion of a larger percentage of aggregated Aβ into thioflavine-S-positive amyloid as compared with clusterin-deficient mice. Some in vitro studies have demonstrated that at certain concentrations, purified clusterin can interact with Aβ and result in an inhibition of fibril formation (21, 22). Although these results may seem contradictory to our in vivo findings, it is always difficult to know a priori whether observations obtained from a two-component, cell-free in vitro assay will be the same as those found in the complex in vivo situation. Many factors (multiple binding proteins, proteolytic events, and clearance mechanisms) function in vivo, complicating interpretation/extrapolation of in vitro findings. We have also identified a highly significant dissociation between amyloid (fibrillar Aβ) and neuritic toxicity in PDAPP+/+, clusterin−/− mice. Even after normalizing for the amount of amyloid plaque (thioflavine-S-positive Aβ deposits) in the clusterin-deficient mice, a 5-fold decrease occurred in the number of dystrophic neurites. The mechanism underlying the increased neuritic toxicity we observed in clusterin-expressing PDAPP mice is unclear. One possibility is that the thioflavine-S-positive amyloid formed in the presence vs. the absence of clusterin is structurally different. Another possibility is that clusterin is promoting the formation of toxic soluble oligomeric forms of Aβ (21, 24, 38, 39). Oda et al., for example, demonstrated that clusterin decreased aggregation of Aβ42 and that subsequent incubation of the less aggregated material to PC12 cells significantly increased oxidative stress (21). Studies by Lambert et al. showed that small diffusible oligomers of Aβ42 induced by the presence of clusterin were associated with increased neuronal toxicity in organotypic central nervous system cultures at nanomolar concentrations (24). In addition, soluble oligomers of Aβ42 were found to be deleterious for long-term potentiation in the dentate gyrus of rat hippocampal slices (39), and protofibrillar intermediates of Aβ induced acute electrophysiological and toxic changes to cortical neurons (40).
A systematic biochemical examination of carbonate-soluble brain extracts with a nondenaturing ELISA that should be specific for oligomeric forms of Aβ in carbonate-soluble lysates detected no significant differences between PDAPP+/+, clusterin+/+ and clusterin−/− mice. However, by using Aβ ELISAs under both denaturing and nondenaturing conditions, we did identify a significant 2-fold increase in the pool of Aβ which may be monomeric in mice expressing clusterin. Whether an Aβ monomeric pool was present in the oligomeric Aβ populations used in some previous in vitro studies (21, 24, 38, 39) is unknown. Analysis of carbonate-soluble brain extracts by acid-urea gels revealed the presence of several Aβ peptides, with Aβ42 being the predominant form present irrespective of clusterin genotype. We noted the complete absence of human Aβ40 in the soluble lysates from PDAPP+/+, clusterin−/− mice. Although the exact meaning of this clusterin-dependent alteration of soluble Aβ is unknown, these data are further direct evidence that clusterin modifies Aβ metabolism and/or structure to influence amyloid deposition.
How might the clusterin-dependent dissociation between fibrillar amyloid and neuritic toxicity relate to the observed differences in the concentrations of soluble pools Aβ that we detected? The answer may lie in an emerging literature documenting the role of clusterin as a secreted chaperone protein (41–44). Several recent studies have shown that clusterin can “solubilize” a very broad spectrum of proteins that contain exposed hydrophobic patches (41, 42). This chaperone-like activity has been attributed to a molten globule-like region located in the clusterin protein itself (43, 44). It is possible that the chaperone-like properties of clusterin may be inducing local alterations in the equilibrium between deposited and soluble monomeric Aβ and, as a consequence, unmask toxic epitopes present in either fibrillar amyloid or soluble Aβ, thereby increasing neuritic toxicity and facilitating further expansion of the fibrillar amyloid. Our results demonstrate that clusterin is a critical component in vivo for the development of amyloid as well as its associated neuritic toxicity. The data also suggest that modifying clusterin expression is likely to have important effects on AD pathology and that further study of the mechanism underlying these clusterin-mediated effects on Aβ structure and toxicity may yield important insights for therapeutic intervention.
Acknowledgments
We thank Eric Foss and Malca Kierson for their technical assistance and Barbara Cordell and Patrick May for helpful comments. We also acknowledge the support of Eli Lilly and Scios, Inc. This work was supported by National Institutes of Health Grants AG05681, AG13956, and AG11355 (to D.M.H.).
Abbreviations
Aβ, amyloid-β
APP, amyloid precursor protein
AD, Alzheimer's disease
apoE, apolipoprotein E
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Selkoe D. J. (2001) Physiol. Rev. 81, 741-766. [DOI] [PubMed] [Google Scholar]
- 2.Sisodia S. S. (1999) J. Clin. Invest. 104, 1169-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Nostrand W. E., Melchor, J. P., Cho, H. S., Greenberg, S. M. & Rebeck, G. W. (2001) J. Biol. Chem. 276, 32860-32866. [DOI] [PubMed] [Google Scholar]
- 4.Nilsberth C., Westlind-Danielsson, A., Eckman, C. B., Condron, M. M., Axelman, K., Forsell, C., Stenh, C., Luthman, J., Teplow, D. B., Younkin, S. G., et al. (2001) Nat. Neurosci. 4, 887-893. [DOI] [PubMed] [Google Scholar]
- 5.Strittmatter W. J., Saunders, A. M., Schmechel, D., Pericak-Vance, M., Enghild, J., Salvesen, G. S. & Roses, A. D. (1993) Proc. Natl. Acad. Sci. USA 90, 1977-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wisniewski T., Ghiso, J. & Frangione, B. (1997) Neurobiol. Dis. 4, 313-328. [DOI] [PubMed] [Google Scholar]
- 7.Holtzman D. M. (2001) J. Mol. Neurosci. 17, 147-155. [DOI] [PubMed] [Google Scholar]
- 8.Roheim P. S., Carey, M., Forte, T. & Vega, G. L. (1979) Proc. Natl. Acad. Sci. USA 76, 4646-4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hochstrasser A.-C., James, R. W., Martin, B. M., Harrington, M., Hochstrasser, D., Pometta, D. & Merril, C. R. (1988) Appl. Theoret. Electrophoresis 1, 73-76. [PubMed] [Google Scholar]
- 10.May P. C. & Finch, C. E. (1992) Trends Neurol. Sci. 15, 391-396. [DOI] [PubMed] [Google Scholar]
- 11.Aronow B. J., Lund, S. D., Brown, T. L., Harmony, J. A. K. & Witte, D. P. (1993) Proc. Natl. Acad. Sci. USA 90, 725-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borghini I., Barja, F., Pometta, D. & James, R. W. (1995) Biochim. Biophys. Acta 1255, 192-200. [DOI] [PubMed] [Google Scholar]
- 13.LaDu M. J., Gilligan, S. M., Lukens, S. R., Cabana, V. G., Reardon, C. A., Van Eldik, L. J. & Holtzman, D. M. (1998) J. Neurochem. 70, 2070-2081. [DOI] [PubMed] [Google Scholar]
- 14.DeMattos R. B., Brendza, R. P., Heuser, J. E., Kierson, M., Cirrito, J. R., Fryer, J. D., Sullivan, P. M., Fagan, A. M., Han, X. & Holtzman, D. M. (2001) Neurochem. Int. 39, 415-425. [DOI] [PubMed] [Google Scholar]
- 15.May P. C., Lampert-Etchells, M., Johnson, S. A., Poirier, J., Masters, J. N. & Finch, C. E. (1990) Neuron 5, 831-839. [DOI] [PubMed] [Google Scholar]
- 16.Calero M., Rostagno, A., Matsubara, E., Zlokovic, B., Frangione, B. & Ghiso, J. (2000) Microsc. Res. Tech. 50, 305-315. [DOI] [PubMed] [Google Scholar]
- 17.Ghiso J., Matsubara, E., Koudinov, A., Choi-Miura, N. H., Tomita, M., Wisniewski, T. & Frangione, B. (1993) Biochem. J. 293, 27-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zlokovic B. V., Martel, C. L., Mackic, J. B., Matsubara, E., Wisniewski, T., McComb, J. G., Frangione, B. & Ghiso, J. (1994) Biochem. Biophys. Res. Commun. 205, 1431-1437. [DOI] [PubMed] [Google Scholar]
- 19.Zlokovic B. V., Martel, C. L., Matsubara, E., McComb, J. G., Zheng, G., McCluskey, R. T., Frangione, B. & Ghiso, J. (1996) Proc. Natl. Acad. Sci. USA 93, 4229-4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsubara E., Frangione, B. & Ghiso, J. (1995) J. Biol. Chem. 270, 7563-7567. [DOI] [PubMed] [Google Scholar]
- 21.Oda T., Wals, P., Osterburg, H. H., Johnson, S. A., Pasinetti, G. M., Morgan, T. M., Rozovsky, I., Stine, W. B., Snyder, S. W., Holzman, T. F., et al. (1995) Exp. Neurol. 136, 22-31. [DOI] [PubMed] [Google Scholar]
- 22.Matsubara E., Soto, C., Governale, S., Frangione, B. & Ghiso, J. (1996) Biochem. J. 316, 671-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boggs L. N., Fuson, K. S., Baez, M., Churgay, L., McClure, D., Becker, G. & May, P. C. (1996) J. Neurochem. 67, 1324-1327. [DOI] [PubMed] [Google Scholar]
- 24.Lambert M. P., Barlow, A. K., Chromy, B. A., Edwards, C., Freed, R., Liosatsos, M., Morgan, T. E., Rozovsky, I., Trommer, B., Viola, K. L., et al. (1998) Proc. Natl. Acad. Sci. USA 95, 6448-6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Games D., Adams, D., Alessandrini, R., Barbour, R., Berthelette, P., Blackwell, C., Carr, T., Clemens, J., Donaldson, T., Gillespie, F., et al. (1995) Nature (London) 373, 523-527. [DOI] [PubMed] [Google Scholar]
- 26.Bales K. R., Verina, T., Dodel, R. C., Du, Y., Altstiel, L., Bender, M., Hyslop, P., Johnstone, E. M., Little, S. P., Cummins, D. J., et al. (1997) Nat. Genet. 17, 263-264. [DOI] [PubMed] [Google Scholar]
- 27.Mclaughlin L., Zhu, G., Mistry, M., Ley-Ebert, C., Stuart, W. D., Florio, C. J., Groen, P. A., Witt, S. A., Kimball, T. R., Witte, D. P., et al. (2000) J. Clin. Invest. 106, 1105-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han B. H., DeMattos, R. B., Dugan, L. L., Kim-Han, J. S., Brendza, R., Fryer, J. D., Kierson, M., Cirrito, J., Quick, K., Harmony, J. A. K., et al. (2001) Nat. Med. 7, 338-343. [DOI] [PubMed] [Google Scholar]
- 29.Holtzman D. M., Fagan, A. M., Mackey, B., Tenkova, T., Sartorius, L., Paul, S. M., Bales, K., Ashe, K. H., Irizzary, M. C. & Hyman, B. T. (2000) Ann. Neurol. 47, 739-747. [PubMed] [Google Scholar]
- 30.Holtzman D. M., Bales, K. R., Tenkova, T., Fagan, A. M., Parsadanian, M., Sartorius, L. J., Mackey, B., Olney, J., McKeel, D., Wozniak, D. & Paul, S. M. (2000) Proc. Natl. Acad. Sci. USA 97, 2892-2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wozniak D. F., Brosnan-Watters, G., Nardi, A., McEwen, M., Corso, T. D., Olney, J. W. & Fix, A. S. (1996) Brain Res. 707, 165-179. [DOI] [PubMed] [Google Scholar]
- 32.Johnson-Wood K., Lee, M., Motter, R., Hu, K., Gordon, G., Barbour, R., Khan, K., Gordon, M., Tan, H., Games, D., et al. (1997) Proc. Natl. Acad. Sci. USA 94, 1550-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeMattos R. B., Bales, K. R., Cummins, D. J., Dodart, J.-C., Paul, S. M. & Holtzman, D. M. (2001) Proc. Natl. Acad. Sci. USA 98, 8850-8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fishman C. E., Cummins, D. J., Bales, K. R., DeLong, C. A., Esterman, M. A., Hanson, J. C., White, S. L., Paul, S. M. & Jordan, W. H. (2001) J. Neurosci. Methods 108, 145-152. [DOI] [PubMed] [Google Scholar]
- 35.Bales K. R., Verina, T., Cummins, D. J., Du, Y., Dodel, J. C., Saura, J., Fishman, C. E., DeLong, C. A., Piccardo, P., Petegnief, V., et al. (1999) Proc. Natl. Acad. Sci. USA 96, 15233-15238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masliah E., Sisk, A., Mallory, M., Mucke, L., Schenk, D. & Games, D. (1996) J. Neurosci. 16, 5795-5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hammad S. M., Ranganathan, S., Loukinova, E., Twal, W. O. & Argraves, W. S. (1997) J. Biol. Chem. 272, 18644-18649. [DOI] [PubMed] [Google Scholar]
- 38.Klein W. L., Krafft, G. A. & Finch, C. E. (2001) Trends Neurosci. 24, 219-224. [DOI] [PubMed] [Google Scholar]
- 39.Wang H.-W., Pasternak, J. F., Kuo, H., Ristic, H., Lambert, M. P., Chromy, B., Viola, K. L., Klein, W. L., Stine, W. B., Krafft, G. A. & Trommer, B. L. (2002) Brain Res. 924, 133-140. [DOI] [PubMed] [Google Scholar]
- 40.Hartley D. M., Walsh, D. M., Ye, C. P., Diehl, T., Vasquez, S., Vassilev, P. M., Teplow, D. B. & Selkoe, D. J. (1999) J. Neurosci. 19, 8876-8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Humphreys D. T., Carver, J. A., Easterbrook-Smith, S. B. & Wilson, M. R. (1999) J. Biol. Chem. 274, 6875-6881. [DOI] [PubMed] [Google Scholar]
- 42.Poon S., Easterbrook-Smith, S. B., Rybchyn, M. S., Carver, J. A. & Wilson, M. R. (2000) Biochemistry 39, 15953-15960. [DOI] [PubMed] [Google Scholar]
- 43.Bailey R. W., Dunker, A. K., Brown, C. J., Garner, E. C. & Griswold, M. D. (2001) Biochemistry 40, 11828-11840. [DOI] [PubMed] [Google Scholar]
- 44.Dunker A. K., Lawson, J. D., Brown, C. J., Williams, R. M., Romero, P., Oh, J. S., Oldfield, C. J., Campen, A. M., Ratliff, C. M., Hipps, K. W., et al. (2001) J. Mol. Graphics Modell. 19, 26-59. [DOI] [PubMed] [Google Scholar]