Abstract
Prion diseases are transmissible neurodegenerative disorders of humans and animals for which no effective treatment is available. Conformationally altered, protease-resistant forms of the prion protein (PrP) termed PrPSc are critical for disease transmissibility and pathogenesis, thus representing a primary target for therapeutic strategies. Based on previous findings that tetracyclines revert abnormal physicochemical properties and abolish neurotoxicity of PrP peptides in vitro, we tested the ability of these compounds to interact with PrPSc from patients with the new variant of Creutzfeldt–Jakob disease (vCJD) and cattle with bovine spongiform encephalopathy (BSE). The incubation with tetracycline hydrochloride or doxycycline hyclate at concentrations ranging from 10 μM to 1 mM resulted in a dose-dependent decrease in protease resistance of PrPSc. This finding prompted us to investigate whether tetracyclines affect prion infectivity by using an animal model of disease. Syrian hamsters were injected intracerebrally with 263K scrapie-infected brain homogenate that was coincubated with 1 mM tetracycline hydrochloride, 1 mM doxycycline hyclate, or vehicle solution before inoculation. Hamsters injected with tetracycline-treated inoculum showed a significant delay in the onset of clinical signs of disease and prolonged survival time. These effects were paralleled by a delay in the appearance of magnetic-resonance abnormalities in the thalamus, neuropathological changes, and PrPSc accumulation. When tetracycline was preincubated with highly diluted scrapie-infected inoculum, one third of hamsters did not develop disease. Our data suggest that these well characterized antibiotics reduce prion infectivity through a direct interaction with PrPSc and are potentially useful for inactivation of BSE- or vCJD-contaminated products and prevention strategies.
Prion diseases are a group of transmissible neurodegenerative disorders including scrapie of sheep, spongiform encephalopathy of cattle (BSE) and Creutzfeldt–Jakob disease (CJD), fatal insomnia, and Gerstmann–Sträussler–Scheinker disease in humans. The pathogenic mechanism underlying these conditions is a conformational conversion of the cellular prion protein into disease-specific species (PrPSc) that possess abnormal chemicophysical properties such as insolubility and protease resistance (1) and accumulate in the brain in the form of amorphous aggregates and amyloid fibrils (2). Evidence indicates that PrPSc is responsible for neuronal degeneration and glial activation (3) and is critical for disease transmissibility by converting the cellular isoform of PrP into a likeness of itself (4, 5). Accordingly, PrPSc represents a primary target for therapeutic strategies.
The urgency to identify and develop therapeutic compounds has increased remarkably after the epidemic of BSE and the appearance of a new variant of CJD (vCJD) that seem to be causally linked (6–8). Since 1995, more than 110 vCJD patients have been identified in Europe, and there is concern about a future outbreak of this condition (9).
Several compounds have been found to antagonize prion propagation in cellular and/or animal models of disease. These molecules include polyanions (10–14), polyene antibiotics (15–17), Congo red (18, 19), iododoxorubicin (20), tetrapyrroles (21, 22), branched polyamines (23, 24), and modified PrP peptides (25). Unfortunately, the suitability of these compounds for therapy is limited, primarily because of inability to cross the blood–brain barrier and/or severe toxicity. Recently, Korth and coworkers screened a variety of drugs already used for treatment of unrelated human disorders and known to penetrate the blood–brain barrier. They found that tricyclic derivatives of acridine and phenothiazine inhibit PrPSc formation in scrapie-infected neuroblastoma cells (26), confirming earlier reports on antiprion activity of these compounds (27–29).
In a previous study, we found that the well characterized antibiotic tetracycline binds to synthetic PrP peptides, hinders assembly of these peptides into amyloid fibrils, reverts the protease resistance of PrPSc extracted from brain tissue of patients with sporadic CJD, and prevents neuronal death and astrocyte proliferation induced by PrP peptides in vitro (30). These findings prompted us to investigate whether tetracyclines alter the chemicophysical properties of PrPSc from vCJD and BSE and reduce prion infectivity.
Materials and Methods
In Vitro Conversion of Protease-Resistant PrP to a Protease-Sensitive Form.
PrPSc was partially purified from brain-tissue samples of three patients with vCJD and one cattle with BSE after a procedure described previously (31) with minor modifications. Sample aliquots containing ≈2 ng of PrPSc were incubated at 37°C for 48 h either in the absence or presence of tetracycline hydrochloride or doxycycline hyclate at concentrations ranging from 1 μM to 1 mM and then subjected to proteinase K digestion (vCJD: 100 μg/ml; BSE: 20 μg/ml) at 37°C for 1 h. The digestion was terminated by the addition of PMSF (1 mM final concentration). Similar experiments were carried out by using whole-brain homogenates instead of partially purified PrPSc.
To investigate whether tetracycline could be more effective during PrPSc folding, partially purified PrPSc from vCJD was subjected to reversible denaturation with guanidine isothiocyanate (1 M final concentration) at 45°C for 1 h. The mixture then was diluted to 0.75 M guanidine isothiocyanate by using TBS (10 mM Tris⋅HCl, pH 7.5/150 mM NaCl) supplemented with 1.5 mM cetylpyridinium chloride, and tetracycline was added to a final concentration of 20 nM. The samples were incubated at 37°C for 48 h, diluted further to 0.375 M guanidine isothiocyanate with TBS, and digested with proteinase K (100 μg/ml, 37°C, 1 h). After the addition of 1 mM PMSF followed by 270 nM thyroglobulin, the proteins were precipitated with 4 vol of methanol and resuspended in 20 μl of Laemmli sample buffer.
The amount of PrP remaining after proteolysis was assessed by Western blot analysis using the monoclonal antibody 3F4 (1:50,000) for vCJD and the rabbit antiserum PrP 95-108 (1:10,000) for BSE (32, 33). Immunoreactive bands were visualized by enhanced chemiluminescence (Amersham Pharmacia), and their average signal intensity was quantified by densitometry as described (30). Values were expressed as percentage of signal intensity of samples nontreated with tetracyclines, and the significance of difference was assessed by Dunnett's test. Control experiments included (i) the incubation of samples with 1 μM to 1 mM gentamicin instead of tetracycline compounds, (ii) the addition of tetracycline or doxycycline to the samples immediately before proteinase K digestion, and (iii) the treatment of proteinase K-digested samples with 3 M guanidine isothiocyanate, pH 2.5, for 10 min, followed by the addition of BSA to a final concentration of 200 μg/ml and methanol precipitation, before SDS/PAGE and immunoblot (34).
The effect of the compounds also was tested on PrPSc from 263K scrapie-infected hamsters. Tetracycline or doxycycline was added to the samples (10−4 brain homogenates) to a final concentration of 1 mM. After incubation at 37°C for 24 h, 350-μl aliquots were digested with proteinase K (50 μg/ml, 37°C, 30 min). The proteins then were precipitated with methanol, resuspended in sample buffer, and analyzed by Western blot using the antibody 3F4.
Experimental Scrapie.
Golden Syrian hamsters were injected intracerebrally with the 263K strain of scrapie that had been incubated with either the test compound or vehicle solution before inoculation. Three experiments were carried out in which the inocula were prepared as 10−4 (experiment 1), 10−5 (experiment 2), and 10−6 (experiment 3) dilutions of brain homogenate from scrapie-infected hamsters at the terminal stage of disease. Tetracycline hydrochloride (experiments 1 and 3) or doxycycline hyclate (experiment 2) was added to the homogenates at a final concentration of 1 mM. After incubation at 20°C for 24 h, 30 μl of homogenate were injected into the right cerebral hemisphere of experimental animals. Control groups included hamsters inoculated with (i) scrapie-infected brain homogenate preincubated at 20°C for 24 h with vehicle solution instead of tetracycline compounds (positive controls) or (ii) noninfected hamster brain homogenate (negative controls). The animals were observed once a week to detect the onset and progression of clinical signs of disease. The behavioral analysis included the evaluation of reactivity to tactile and acoustic stimulation, posture, balance and coordination, and the presence of tremors as described previously (20). In experiments 1 and 3, three animals per group (randomly selected before inoculation) were killed for neuropathological and biochemical examination when clinical signs of disease were first apparent in the positive controls. In experiment 3, these animals were subjected to magnetic resonance imaging (MRI) before being killed. All remaining hamsters were analyzed at the terminal stage of the disease.
MRI.
Hamsters were anesthetized with chloral hydrate, placed in a Plexiglas frame to avoid movements, and examined on a 1.5 T Philips ACS Magneton with a soft flexible coil, usually used for imaging orbit. The sequences were as follows: coronal T1-weighted images (fast-field echo: relaxation time 33, echo time 11, flip angle 30°, thickness 2 mm); coronal T2-weighted images (turbo-spin echo: relaxation time 1,800, echo time 90, flip angle 90°, turbo factor 9, thickness 1.5 mm).
Neuropathology and Western Blot Analysis.
At autopsy, the right cerebral hemisphere, the brainstem, and cerebellum were dissected at standard levels, fixed in Carnoy solution, and embedded in paraplast, whereas the left cerebral hemisphere was frozen and stored at −80°C. Eight-micrometer-thick serial sections from paraplast-embedded blocks were stained with hematoxylin/eosin and thioflavine S or incubated with a monoclonal antibody to glial fibrillary acidic protein (Dako) (1:100 dilution) or the anti-PrP antibody 3F4 (1:1,000 dilution). Immunoreactions were revealed by the Envision system for mouse immunoglobulins (Dako) using 3–3′-diaminobenzidine as chromogen. Negative control sections were incubated with normal mouse serum as primary antibody. The specificity of PrP immunoreactivity was verified by absorption. The antibody 3F4 was incubated with a synthetic peptide homologous to residues 101–119 of human PrP (10 mM) for 1 h at 37°C and then overnight at 4°C. After centrifugation at 15,000 × g for 15 min, the supernatant was used as primary antibody.
For immunoblot analysis, a 10% (wt/vol) homogenate of the left cerebral hemisphere from each hamster was prepared in 10 mM Tris, pH 7.4/100 mM NaCl/10 mM EDTA/0.5% Nonidet P-40/0.5% sodium deoxycholate. After centrifugation at 1,000 × g for 10 min, the protein concentration in the supernatant was determined by the bicinchoninic acid assay (Pierce). Samples equivalent to 100 μg of protein were mixed with equal volumes of twice the concentration of Laemmli sample buffer and incubated with proteinase K (50 μg/ml) at 37°C for 1 h. Proteolysis was terminated by the addition of PMSF (1 mM final concentration). The samples were fractionated on 12.5% SDS/polyacrylamide minigels under reducing conditions, electrophoretically transferred to poly(vinylidene difluoride) membranes (Immobilon, Millipore), and probed with the antibody 3F4 (1:50,000). Immunoreactive bands were visualized with enhanced chemiluminescence, quantified by densitometry, and analyzed as described above.
Results
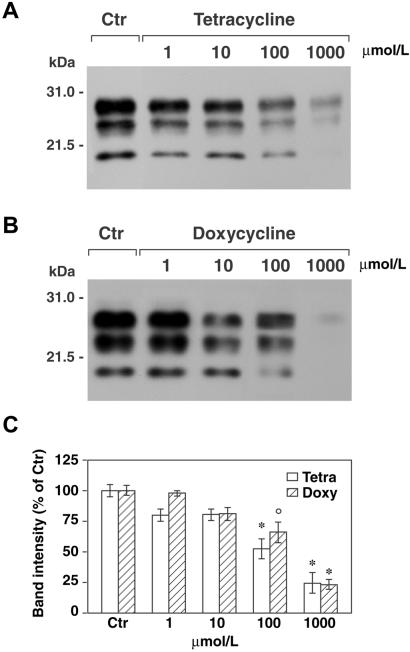
A distinctive feature of PrPSc is the partial resistance to proteinase K digestion under conditions in which the cellular isoform of PrP is degraded completely (1). This property likely reflects a change in conformation and/or aggregation state and is thought to underlie the accumulation of PrPSc in the brain, leading to the disease state. To investigate whether tetracycline compounds are able to affect protease resistance of PrPSc from vCJD, the protein was partially purified from cerebral cortex of three patients, incubated with tetracycline hydrochloride or doxycycline hyclate at a concentration ranging from 10 μM to 1 mM, and then treated with proteinase K and analyzed by Western blot. The incubation of PrPSc with either compound for 48 h resulted in decreased protease resistance (Fig. 1 A and B). This effect was dose-dependent, reaching almost 80% at the highest drug concentration (Fig. 1C). Conversely, no changes were observed when tetracyclines were added to the samples immediately before proteinase K digestion or after incubation of PrPSc with up to 1 mM gentamycin. To rule out the possibility that these findings could be caused by overstabilization of PrPSc aggregates impeding the entry of the protein into the gel as observed with Congo red (34), some samples were treated with 3 M guanidine isothiocyanate before SDS/PAGE. The results were comparable to those observed without guanidine denaturation. Tetracyclines were effective also on PrPSc partially purified from BSE and on whole-brain homogenates of vCJD, BSE, and 263K scrapie-infected hamsters. In particular, the incubation of a 10−4 dilution of 263K scrapie-infected brain homogenate with 1 mM tetracycline or doxycycline resulted in more than 90% reduction in the protease-resistant core of PrPSc (data not shown).
Fig 1.
Tetracyclines revert the protease resistance of PrPSc from vCJD brains. Immunoblot analysis of partially purified PrPSc from vCJD after incubation in the absence (Ctr) or presence of tetracycline (A) and doxycycline (B), followed by proteinase K digestion. The blots were probed with the antibody 3F4 (1:50,000). Molecular mass markers are indicated to the left. (C) Quantification of the protease-resistant core of PrPSc in tetracycline- (Tetra) and doxycycline-treated (Doxy) samples. The values were obtained by densitometric analysis of immunoblots and are expressed as percentage of signal intensity of samples nontreated with tetracyclines. The data are the mean ± SEM of six experiments from three different patients. °, P < 0.05, and *, P < 0.01 versus the relevant control group (Dunnett's test).
To investigate whether tetracycline could be more efficient when the drug-to-protein interaction occurred during PrPSc folding, PrPSc from vCJD was subjected to reversible denaturation with 1 M guanidine isothiocyanate followed by renaturation in the presence or absence of 20 nM tetracycline. Western blot analysis of proteinase K-digested samples showed that the fraction of PrPSc after tetracycline treatment was reduced to 56.4 ± 2.4% of control values.
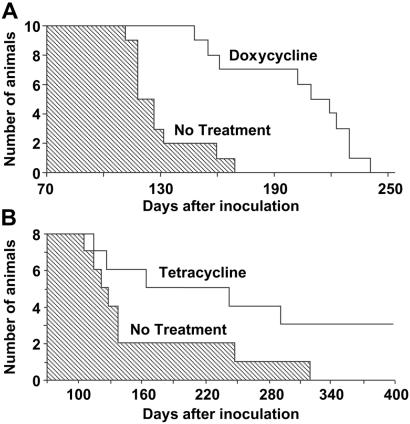
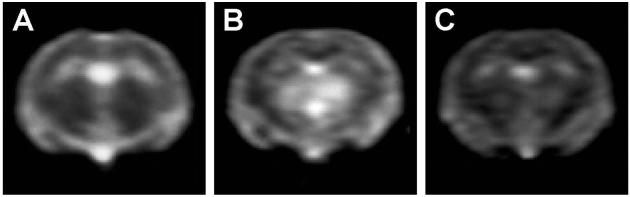
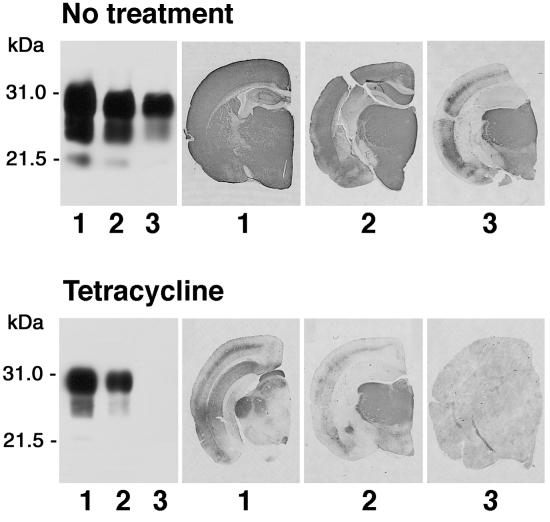
These findings prompted us to examine whether the physicochemical changes of PrPSc induced by tetracyclines were associated with a decrease in prion infectivity. 263K scrapie-infected brain homogenates from hamsters at the terminal stage of disease were incubated with 1 mM tetracycline, 1 mM doxycycline, or vehicle solution for 24 h and then inoculated intracerebrally into Syrian hamsters. Both compounds significantly delayed the onset of clinical signs of disease and prolonged survival time. The magnitude of the effect depended on prion titer. With a 10−4 dilution of 263K scrapie-infected brain homogenate, the survival time of animals injected with untreated (n = 15) or tetracycline-treated (n = 17) inoculum was (mean ± SEM) 128.8 ± 2.3 and 139.4 ± 2.9 days, respectively (P < 0.01 Student's t test). The incubation of a 10−5 brain-homogenate dilution with doxycycline increased mean survival time by 58% (from 130.0 ± 6.0 to 205.0 ± 10.0 days, P < 0.01 Student's t test, Fig. 2A). When a 10−6 brain-homogenate dilution was incubated with tetracycline, one third of the hamsters did not develop disease (Fig. 2B). The difference in survival time reflected a similar difference in incubation time, as revealed by the behavioral analysis designed to detect early clinical signs of scrapie infection such as hyperreactivity to tactile and acoustic stimulation and abnormalities of posture, balance, and coordination. The effects of tetracycline on disease onset were associated with a delay in the appearance of MRI changes. It is known that patients with vCJD have increased signal intensity in the thalamus on T2- and proton density-weighted images (35). Similar changes are present consistently in 263K scrapie-infected hamsters, becoming detectable in the last part of the incubation period, before the onset of clinical symptoms (unpublished data). In experiment 3, three animals per group randomly selected before inoculation were subjected to MRI at day 90, when clinical symptoms first were apparent in the positive controls. The study showed that the T2-signal hyperintensity in the thalamus was remarkably less intense in the tetracycline-treated group that in the positive controls (Fig. 3). After MRI, these animals were killed for a comparative neuropathological and biochemical investigation. PrP immunohistochemistry and Western blot analysis revealed some variability within each experimental group that was similar to the variability observed in the survival time. Overall, however, the extent of PrPSc accumulation in the brain was remarkably more abundant in the positive controls than in tetracycline-treated hamsters, one of which appeared to be unaffected (Fig. 4). This difference between the two groups was paralleled by a difference in the severity of spongiform changes and astrogliosis in the cerebral cortex and subcortical gray structures.
Fig 2.
Effects of tetracyclines on survival of scrapie-infected Syrian hamsters. (A) Survival time of hamsters injected intracerebrally with a 10−5 dilution of 263K scrapie-infected brain homogenate (shaded area) or the same inoculum after incubation with doxycycline (white area). (B) Survival time of hamsters injected intracerebrally with a 10−6 dilution of 263K scrapie-infected brain homogenate (shaded area) or with the same inoculum after incubation with tetracycline (white area).
Fig 3.
Effects of tetracycline on cerebral MRI changes in scrapie-infected hamsters. T2-weighted images of hamsters injected intracerebrally with a 10−6 dilution of normal brain homogenate (A), 263K scrapie-infected brain homogenate (B), or 263K scrapie-infected brain homogenate after incubation with tetracycline (C) are shown. The MRI was carried out 90 days after inoculation. Note the striking difference in thalamic hyperintensity between tetracycline-untreated (B) and tetracycline-treated (C) hamsters.
Fig 4.
Effects of tetracycline on accumulation of PrPSc in the brain of scrapie-infected hamsters. Shown are PrP immunoblot analysis and immunohistochemistry of brain tissue of three hamsters (1–3) from the tetracycline-untreated (Upper) and tetracycline-treated (Lower) group with the antibody 3F4. The animals, randomly selected before infection, were injected intracerebrally with a 10−6 dilution of 263K scrapie-infected brain homogenate (Upper) or 263K scrapie-infected brain homogenate after incubation with tetracycline (Lower) and killed 90 days after inoculation (experiment 3).
Once the disease progressed and hamsters began to die of the infection, the differences between groups in PrPSc accumulation, spongiform changes, and astrogliosis were apparent no longer.
Discussion
This study shows that tetracyclines interact with PrPSc and render it susceptible to proteolytic degradation. This effect was observed on various types of PrPSc associated with spongiform encephalopathies of human and animal origin and was similar in partially purified preparations and whole-brain homogenates. The decrease in protease resistance was accompanied by a reduction in prion infectivity. In fact, preincubation of 263K scrapie-infected brain homogenates with the compounds before inoculation caused a delay in the appearance of clinical symptoms and prolonged survival time in recipient animals. The effectiveness of tetracyclines depended on compound concentration and prion titer; in particular, when tetracycline was added to highly diluted scrapie-infected brain homogenate, one third of hamsters did not develop disease. This observation is relevant particularly for acquired forms of prion disease such as iatrogenic CJD, vCJD, and BSE that occurred after exposure to organic material containing presumably low prion levels. Accordingly, we advance that tetracycline antibiotics with well characterized pharmacokinetic properties and safe toxicological profile could be used for prion inactivation in potentially contaminated products of medical relevance and for prevention strategies.
The prolongation of incubation and survival times in experimental scrapie after tetracycline treatment of prion-infected inoculum was associated with a delay in the accumulation of PrPSc in the brain as revealed by immunohistochemistry and Western blot analysis. This effect was associated with a delay in the appearance of histological signs of disease, i.e., gliosis and spongiform changes. The variability in the extent of PrPSc accumulation was paralleled by variability in survival of the same group of tetracycline-treated hamsters. In particular, five of eight animals developed disease over a large period, whereas the other three were still healthy 500 days after inoculation and did not show signs of prion infection at neuropathological and biochemical examination. In a previous study we found that 263K scrapie-infected hamsters consistently display MRI abnormalities in the thalamus on T2-weighted images (unpublished data). Of note, these changes are similar to those observed in vCJD (35) and occur early, before the onset of clinical symptoms. On this ground, we used MRI to analyze the effects of tetracyclines on the development of prion-related brain modifications in living animals and found a striking difference between tetracycline-treated and tetracycline-untreated hamsters. Accordingly, we suggest that MRI is an effective tool to follow onset and progression of experimental scrapie and evaluate the effectiveness of pharmacological treatments in a noninvasive form.
Tetracycline antibiotics were selected for study based on structural homologies with antiprion compounds, in particular with the aglycone moiety of the anthracycline iododoxorubicin, and the availability of derivatives such as doxycycline and minocycline known to have a favorable distribution in the central nervous system. Tetracyclines show chemical analogies also with other inhibitors of PrPSc formation and prion replication such as Congo red, tetrapyrroles, and acridine derivatives. All these molecules contain an extended hydrophobic core formed by aromatic moieties with a large number of hydrophilic substituents conferring an amphiphilic character. It is conceivable that these characteristics enable strong interactions between such compounds and lipophilic domains of PrPSc and are important structural features for the antiprion activity. This view is supported by a previous study with a synthetic peptide homologous to the amyloid protein purified from Gerstmann–Sträussler–Scheinker brains (i.e., residues 82–146 of human PrP; ref. 36) and a fragment thereof comprising residues 106–126, which is highly amyloidogenic, neurotoxic, and capable of activating astrocytes and microglia cells in vitro (37–40). Tetracycline was found to bind not only to PrP aggregates but also to oligomeric-monomeric forms of the peptides, as revealed by fluorescence microscopy and NMR spectroscopy, respectively. In particular, the NMR data provided evidence for through-space interactions between the compound and hydrophobic peptide domains (30). This binding property was associated with the ability of tetracycline to prevent aggregation and acquisition of protease resistance of PrP peptides, disrupt PrP peptides aggregates, and inhibit neuronal death and astroglial proliferation induced by PrP peptides (30). Further, tetracyclines reduce the protease resistance of PrPSc from CJD, BSE, and 263K scrapie-infected hamster, suggesting that the drug (although at high concentrations) can disrupt PrPSc aggregates and/or destabilize PrPSc conformation. Because the effect on protease resistance was 1,000-fold stronger when PrPSc was subjected to reversible denaturation followed by renaturation in the presence of the compound, it is conceivable that tetracycline also may act on partially unfolded intermediates that are thought to play a central role in the cellular isoform of PrP-to-PrPSc conversion (41). Of note, the active concentration of tetracycline in the denaturation-renaturation experiment was lower than the plasma levels detected in man after a single oral dose of 250 mg (42). Tetracycline was found also to prevent and revert aggregation of β-amyloid peptides that accumulate in Alzheimer brains (43), suggesting that the compound may be effective in other neurodegenerative conditions caused by protein misfolding.
In addition to a direct interaction with misfolded proteins, tetracyclines may exert neuroprotective effects through indirect mechanisms, as observed in a transgenic model of Huntington's disease (44) and an animal model of Parkinson's disease (45). Evidence suggests that the mechanisms of neuroprotection involve inhibition of caspase-1, caspase-3, and inducible nitric oxide (NO) synthase expression and/or NO-mediated toxicity. These properties could be beneficial also in prion-related encephalopathies, because PrP amyloid peptides have the ability to induce caspase activation and NO synthase expression in vitro (46, 47), and a large number of activated caspase-3-immunoreactive cells have been observed in the brain of prion disease patients (A. Migheli, personal communication).
In the light of our data and the results obtained by other studies, tetracyclines should be reconsidered for pharmacological effects independently from the antibiotic activity; in particular, these drugs could be a therapeutic tool for neurodegenerative disorders associated with protein misfolding. In prion-related encephalopathies, tetracyclines are immediate candidates for prion inactivation in potentially contaminated products and prevention strategies relevant to acquired forms of disease.
Acknowledgments
The antibody 3F4 was kindly supplied by R. J. Kascsak (Institute for Basic Research in Developmental Disabilities, Staten Island, NY). This work was supported in part by the Italian Ministry of Health, Department of Social Services Grant RF 2001-96, Italian Ministry of University and Education Grant PRIN 2001, and European Community Grant QRLT 2001-00283.
Abbreviations
BSE, bovine spongiform encephalopathy
CJD, Creutzfeldt–Jakob disease
PrP, prion protein
PrPSc, disease-associated form of PrP
vCJD, new variant of CJD
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Prusiner S. B. (1998) Proc. Natl. Acad. Sci. USA 90, 10962-10966. [Google Scholar]
- 2.DeArmond S. J. & Prusiner, S. B. (1997) in Greenfield's Neuropathology, eds. Graham, D. I. & Lantos, P. L. (Arnold, London), pp. 235–280.
- 3.Tagliavini F., Forloni, G., D'Ursi, P., Bugiani, O. & Salmona, M. (2001) Adv. Protein Chem. 57, 171-202. [DOI] [PubMed] [Google Scholar]
- 4.Kocisko D. A., Come, J. H., Priola, S. A., Chesebro, B., Raymond, G. J., Lansbury, P. T. & Caughey, B. (1994) Nature (London) 370, 471-474. [DOI] [PubMed] [Google Scholar]
- 5.Saborio G. P., Permanne, B. & Soto, C. (2001) Nature (London) 411, 810-813. [DOI] [PubMed] [Google Scholar]
- 6.Will R. G., Ironside, J. W., Zeidler, M., Cousens, S. N., Estibeiro, K., Alperovitch, A., Poser, S., Pocchiari, M., Hoffmann, A. & Smith, P. J. (1996) Lancet 347, 921-925. [DOI] [PubMed] [Google Scholar]
- 7.Collinge J., Sidle, K. C. L., Meads, J., Ironside, J. & Hill, A. F. (1996) Nature (London) 383, 685-690. [DOI] [PubMed] [Google Scholar]
- 8.Bruce M. E., Will, R. G., Ironside, J. W., McConnell, I., Drummond, D., Suttie, A., McCardle, L., Chree, H., Hope, J., Birkett, C., et al. (1997) Nature (London) 389, 498-501. [DOI] [PubMed] [Google Scholar]
- 9.Ghani A. C., Ferguson, N. M., Donnelly, C. A., Hagenaars, T. J. & Anderson, R. M. (1998) Proc. R. Soc. London 265, 2443-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimberlin R. H. & Walker, C. A. (1983) Arch. Virol. 78, 9-18. [DOI] [PubMed] [Google Scholar]
- 11.Ehlers B. & Diringer, H. (1984) J. Gen. Virol. 65, 1325-1330. [DOI] [PubMed] [Google Scholar]
- 12.Farquhar C. F. & Dickinson, A. G. (1986) J. Gen. Virol. 67, 463-473. [DOI] [PubMed] [Google Scholar]
- 13.Kimberlin R. H. & Walker, C. A. (1986) Antimicrob. Agents Chemother. 30, 409-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caughey B. & Raymond, G. J. (1993) J. Virol. 67, 643-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pocchiari M., Schmittinger, S. & Masullo, C. (1987) J. Gen. Virol. 68, 219-223. [DOI] [PubMed] [Google Scholar]
- 16.Xi Y. G., Ingrosso, L., Ladogana, A., Masullo, C. & Pocchiari, M. (1992) Nature (London) 356, 598-601. [DOI] [PubMed] [Google Scholar]
- 17.Adjou K. T., Demaimay, R., Lasmezsas, C., Deslys, J. P., Seman, M. & Dortmond, D. (1995) Antimicrob. Agents Chemother. 39, 2810-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caughey B. & Race, R. E. (1992) J. Neurochem. 59, 768-771. [DOI] [PubMed] [Google Scholar]
- 19.Ingrosso L., Ladogana, A. & Pocchiari, M. (1995) J. Virol. 69, 506-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tagliavini F., McArthur, R. A., Canciani, B., Giaccone, G., Porro, M., Bugiani, M., Lievens, P. M.-J., Bugiani, O., Peri, E., Dall'Ara, P., et al. (1997) Science 276, 1119-1122. [DOI] [PubMed] [Google Scholar]
- 21.Caughey W. S., Raymond, L. D., Horiuchi, M. & Caughey, B. (1998) Proc. Natl. Acad. Sci. USA 95, 12117-12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Priola S. A., Raines, A. & Caughey, W. S. (2000) Science 287, 1503-1506. [DOI] [PubMed] [Google Scholar]
- 23.Supattapone S., Nguyen, H. O., Cohen, F. E., Prusiner, S. B. & Scott, M. R. (1999) Proc. Natl. Acad. Sci. USA 96, 14529-14534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Supattapone S., Wille, H., Uyechi, L., Safar, J., Tremblay, P., Szoka, F. C., Cohen, F. E., Prusiner, S. B. & Scott, M. R. (2001) J. Virol. 75, 3453-3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soto C., Kascsak, R. J., Saborìo, G. P., Aucouturier, P., Wisniewski, T., Prelli, F., Kascsak, R., Mendez, E., Harris, D. A., Ironside, J., et al. (2000) Lancet 355, 192-197. [DOI] [PubMed] [Google Scholar]
- 26.Korth C., May, B. C., Cohen, F. E. & Prusiner, S. B. (2001) Proc. Natl. Acad. Sci. USA 98, 9836-9841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roikhel V. M., Fokina, G. & Pogodina, V. V. (1984) Acta Virol. (Engl. Ed.) 28, 321-324. [PubMed] [Google Scholar]
- 28.Dees C., Wade, W. F., German, T. L. & Marsh, R. F. (1985) J. Gen. Virol. 66, 845-849. [DOI] [PubMed] [Google Scholar]
- 29.Doh-ura K., Iwaki, T. & Caughey, B. (2000) J. Virol. 74, 4894-4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tagliavini F., Forloni, G., Colombo, L., Rossi, G., Girola, L., Canciani, B., Angeretti, N., Giampaolo, L., Peressini, E., Awan, T., et al. (2000) J. Mol. Biol. 300, 1309-1322. [DOI] [PubMed] [Google Scholar]
- 31.Caughey B., Kocisko, D. A., Priola, S. A., Raymond, G. J., Race, R. E., Bessen, R. A., Lansbury, P. T., Jr. & Chesebro, B. (1997) in Methods in Molecular Medicine: Prion Diseases, eds. Baker, H. & Ridley, R. M. (Humana, Totowa, NJ), pp. 285–300.
- 32.Kascsak R. J., Rubenstein, R., Mertz, P. A., Tonna-DeMasi, M., Fersko, R., Carp, R. I., Wisniewski, H. M. & Diringer, H. (1987) J. Virol. 61, 3688-3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piccardo P., Langeveld, J. P., Hill, A. F., Dlouhy, S. R., Young, K., Giaccone, G., Rossi, G., Bugiani, M., Bugiani, O., Meloen, R. H., et al. (1998) Am. J. Pathol. 152, 1415-1420. [PMC free article] [PubMed] [Google Scholar]
- 34.Caspi S., Halimi, M., Yanai, A., Ben Sasson, S., Taraboulos, A. & Gabizon, R. (1998) J. Biol. Chem. 273, 3484-3489. [DOI] [PubMed] [Google Scholar]
- 35.Collie D. A., Sellar, R. J., Zeidler, M., Colcheter, A. C., Knight, R. & Will, R. G. (2001) Clin. Radiol. 56, 726-739. [DOI] [PubMed] [Google Scholar]
- 36.Tagliavini F., Lievens, P. M.-J., Tranchant, C., Warter, J.-M., Mohr, M., Giaccone, G., Perini, F., Rossi, G., Salmona, M., Piccardo, P., et al. (2001) J. Biol. Chem. 276, 6009-6015. [DOI] [PubMed] [Google Scholar]
- 37.Forloni G., Angeretti, N., Chiesa, R., Monzani, E., Salmona, M., Bugiani, O. & Tagliavini, F. (1993) Nature (London) 362, 543-545. [DOI] [PubMed] [Google Scholar]
- 38.Forloni G., Del Bo, R., Angeretti, N., Chiesa, R., Smiroldo, S., Doni, R., Ghibaudi, E., Salmona, M., Porro, M., Verga, L., et al. (1994) Eur. J. Neurosci. 6, 1415-1422. [DOI] [PubMed] [Google Scholar]
- 39.Tagliavini F., Prelli, F., Verga, L., Giaccone, G., Sarma, R., Gorevic, P., Ghetti, B., Passerini, F., Ghibaudi, E., Forloni, G., et al. (1993) Proc. Natl. Acad. Sci. USA 90, 9678-9682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown D. R., Schmidt, B. & Kretzschmar, H. A. (1996) Nature (London) 380, 345-347. [DOI] [PubMed] [Google Scholar]
- 41.Weissmann C. (1991) Nature (London) 349, 569-571. [DOI] [PubMed] [Google Scholar]
- 42.Kapusnik-Uner J. E., Sande, M. A. & Chambers, F. (1996) in The Pharmacological Basis of Therapeutics, eds. Harman, J. G., Limbird, L. E., Molinof, P. B. & Ruddon, R. W. (McGraw–Hill, New York), pp. 1123–1130.
- 43.Forloni G., Colombo, L., Gerola, L., Tagliavini, F. & Salmona, M. (2001) FEBS Lett. 487, 404-407. [DOI] [PubMed] [Google Scholar]
- 44.Chen M., Ona, V. O., Ferrante, R. Y., Fink, K. B., Zhu, S., Bian, J., Guo, L., Farrell, L. A., Hersch, S. M., Hobbs, W., et al. (2000) Nat. Med. 6, 797-801. [DOI] [PubMed] [Google Scholar]
- 45.Du Y., Ma, Z., Lin, S., Dodel, R. C., Gao, F., Bales, K. R., Triarhou, L. C., Chernet, E., Perry, K. W., Nelson, D. L., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 14669-14674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saez-Valero J., Angeretti, N. & Forloni, G. (2000) Neurosci. Lett. 293, 207-210. [DOI] [PubMed] [Google Scholar]
- 47.Fabrizi C., Silei, V., Menegazzi, M., Salmona, M., Tagliavini, F., Suzuki, H. G. & Lauro, G. M. (2001) J. Biol. Chem. 276, 25692-25696. [DOI] [PubMed] [Google Scholar]