Abstract
Homologues of the yeast ubiquitin ligase-associated protein SGT1 are required for disease resistance in plants mediated by nucleotide-binding site/leucine-rich repeat (NBS-LRR) proteins. Here, by silencing SGT1 in Nicotiana benthamiana, we extend these findings and demonstrate that SGT1 has an unexpectedly general role in disease resistance. It is required for resistance responses mediated by NBS-LRR and other R proteins in which pathogen-derived elicitors are recognized either inside or outside the host plant cell. A requirement also exists for SGT1 in nonhost resistance in which all known members of a host species are resistant against every characterized isolate of a pathogen. Our findings show that silencing SGT1 affects diverse types of disease resistance in plants and support the idea that R protein-mediated and nonhost resistance may involve similar mechanisms.
Plant resistance (R) proteins are thought to recognize pathogen avirulence (Avr) determinants and trigger signal transduction cascades that lead to defense responses (1). Candidate resistance signaling components include EDS1, NDR1, and RAR1 that are each required for resistance mediated by R proteins with a central nucleotide-binding site (NBS) and carboxyl-terminal leucine-rich repeats (LRR) (2–6). In general, the requirement for EDS1 or NDR1 is exclusive and NBS-LRR proteins with amino-terminal Toll and interleukin-1 receptor homology (TIR domain) use EDS1, whereas those with coiled-coil (CC) domains signal through NDR1 (7). In contrast, RAR1 is required for resistance mediated by both TIR-NBS-LRR and CC-NBS-LRR proteins (5, 6).
Recently, SGT1 was identified as a RAR1-interacting protein in a yeast two-hybrid screen (8). The involvement of SGT1 in disease resistance was confirmed in barley, where SGT1 silencing compromised powdery mildew resistance mediated by the CC-NBS-LRR protein Mla6 (8). Furthermore, mutation analysis in Arabidopsis revealed that SGT1 is required for resistance against Perenospora parasitica mediated by several TIR-NBS-LRR proteins (9, 10). Originally, SGT1 was defined in yeast, where it interacts with SKP1, a component of the Skp1/Cdc53/F-box protein (SCF) ubiquitin ligase complex (11). The SGT1-SKP1 interaction is conserved in planta, suggesting that ubiquitylation may be involved in regulation of plant disease resistance responses (8).
We used virus-induced gene silencing (VIGS) to investigate further the function of SGT1 in plant disease resistance. This approach was validated in an earlier study where VIGS of Nicotiana EDS1 compromised tobacco mosaic virus (TMV) resistance mediated by N, a TIR-NBS-LRR protein (12). Here we show that SGT1 is required for resistance responses specified by NBS-LRR and multiple other types of R protein. Furthermore, we demonstrate that SGT1 is involved in nonhost resistance, for which little genetic or molecular information exists about the plant components involved (13). Thus, in contrast to previously identified resistance-signaling components, SGT1 may be a general factor of disease resistance.
Materials and Methods
Plant Material and VIGS Constructs.
The transgenic Nicotiana benthamiana plants, their cultivation conditions, and the tobacco rattle virus (TRV):N, TRV:Rx, and TRV:Prf constructs have been described (12). (Requests for materials should be sent to www.sainsbury-laboratory.ac.uk.) For TRV:SGT, PCR primers (5′-TCG CCG TTG ACC TGT ACA CTC AAG C-3′ and 5′-GCA GGT GTT ATC TTG CCA AAC AAC CTA GG-3′) based on tomato SGT1 (The Institute for Genomic Research: TC85297, www.tigr.org/tdb/lgi/) were used with N. benthamiana cDNA (at annealing temperature = 50°C) in independent reactions to generate 634-bp fragments of both NbSGT1.1 (GenBank accession no. AF516180) and NbSGT1.2 (GenBank accession no. AF516181). The NbSGT1.1 fragment was inserted into the TRV vector (14).
Pathogen Isolates.
Viruses: TMV:GFP (GFP, green fluorescent protein) (12), potato virus X (PVX):GFP (12), and cauliflower mosaic virus (CaMV) Cabb B-JI (15), kindly provided by N. Al-Kaff (John Innes Centre), are described elsewhere. Bacteria were Pseudomonas syringae pv. tabaci (avrPto) (12), Pseudomonas syringae pv. maculicola (m2) (16), Xanthomonas axonopodis pv. vesicatoria (82–8) (17), and Xanthomonas campestris pv. campestris (8004) (17). For growth determinations, bacteria were resuspended in 10 mM MgCl2 (10,000-fold dilution from OD600 = 1) and infiltrated into leaves by using a syringe. For hypersensitive response (HR) assays bacteria were infiltrated at concentrations described in the figure legends.
Agrobacterium-Mediated Transient Expression (Agroinfiltration) in N. benthamiana.
Viruses: TRV vectors, TMV:GFP, and PVX:GFP were inoculated by agroinfiltration (12). HR assays: all proteins were expressed transiently by Agrobacterium. The constructs that express Rx (18), the PVX coat protein (elicitor of Rx) (18), Cf-4+Avr4 (19), Cf-9+Avr9 (19) have been described. RPW8.1 and RPW8.2 were expressed from a construct that contained a genomic fragment encoding both proteins. The Pto HR was elicited by using a construct that contains 35S promoter-driven Pto and avrPto. The Inf1 and AvrRpt2 HRs were elicited by using constructs that contain 35S:Inf1 (S. Kamoun, Ohio State University, personal communication) and 35S:AvrRpt2 (20).
Gel Blot Analysis.
Protein: The SGT1 antibody that reacts with the SGS domain was described (8) and the GFP antibody used was monoclonal B34 (Babco, Richmond, CA). RNA: TMV:GFP and PVX:GFP RNA levels were determined with a GFP-specific probe as described (12). DNA: Dot-blot analysis of CaMV was performed as described (15).
Results and Discussion
Virus-Induced Gene Silencing of N. benthamiana SGT1.
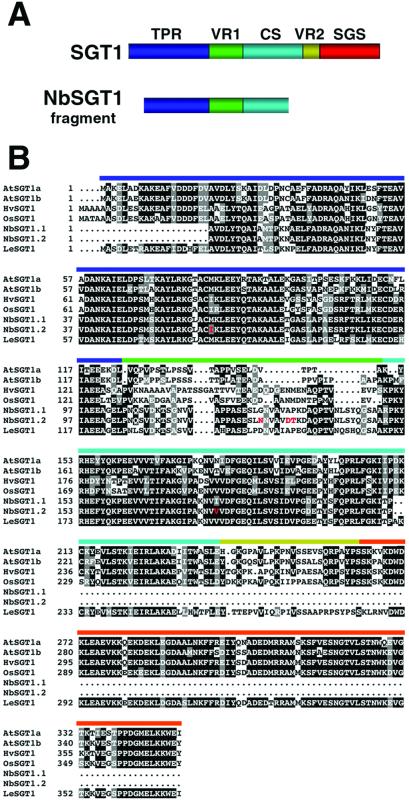
To investigate the role of SGT1 in resistance-signaling pathways, we performed VIGS (21) of SGT1 homologues in N. benthamiana. Plants were inoculated with a virus vector carrying a fragment of N. benthamiana SGT1 (NbSGT1) so that the symptoms in the infected plants would resemble loss-of-function mutations in NbSGT1. Fragments of two NbSGT1 cDNAs (NbSGT1.1 and NbSGT1.2) were produced by PCR. They encoded 97% identical proteins characteristic of the amino-terminal region of barley and Arabidopsis SGT1 (Fig. 1).
Fig 1.
Sequence of NbSGT1 and other plant SGT1 proteins. (A) Structural organization of SGT1 sequences (8): TPR, tetratricopeptide repeats; VR1 and VR2, variable domains; CS motif, CHORD protein and SGT1 specific; SGS, SGT1-specific motif. The domains encoded by NbSGT1.1 and NbSGT1.2 are shown. (B) Sequence alignment of SGT1 proteins. At, Arabidopsis thaliana; Hv, barley; Os, rice; Nb, N. benthamiana; Le, tomato. Black shading, identical residues; gray shading, similar residues. NbSGT1.1 and NbSGT1.2 are 97% identical (amino acid differences are highlighted in red), and the closest Arabidopsis homologues are AtSGT1a (60% identical and 69% similar) and AtSGT1b (58% identical and 68% similar). The colored overlines indicate the domains described in A.
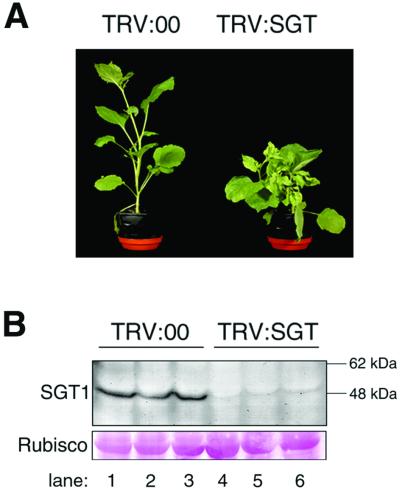
N. benthamiana plants infected with a TRV vector (14) carrying the fragment of NbSGT1.1 (TRV:SGT) were shorter and more branched than the control plants infected with the empty TRV vector (TRV:00) (Fig. 2A). This growth phenotype may reflect a compromised auxin response because of reduced degradation of Aux/IAA proteins (22). Degradation of these proteins is part of the auxin response pathway and requires ubiquitylation by a ubiquitin ligase complex (SCFTIR1) (23) that may contain SGT1 (11). To confirm silencing, extracts of the infected plants were assayed with an antibody that reacts with the SGT1-specific domain (8) (Fig. 1A). Western blot analysis of extracts taken from the upper noninoculated leaves at 21 days post inoculation (dpi) showed that NbSGT1 was substantially less abundant in TRV:SGT-infected plants than in controls infected with an empty TRV vector (TRV:00) (Fig. 2B).
Fig 2.
TRV:SGT infection causes silencing of NbSGT1. (A) Viral symptoms 28 days after TRV:00 or TRV:SGT inoculation. (B) NbSGT1 protein levels in upper leaves 21 days after TRV vector inoculation. Antibodies were raised in rats by using the SGS domain of AtSGT1a and are effective against SGT1 in Arabidopsis and barley (8); consequently, they are expected to be effective against all NbSGT1 proteins. Molecular sizes are indicated. Ponceau S staining of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) was for confirmation of equal loading in each lane.
NbSGT1 Is Required for N-,Rx-, and Pto-Mediated Resistance.
To assess the role of NbSGT1 in R gene-mediated disease resistance, we inoculated TRV:SGT to transgenic plants carrying various R genes. These genes were N from Nicotiana glutinosa that encodes a TIR-NBS-LRR protein and confers TMV resistance; Rx from potato encoding a CC-NBS-LRR protein that mediates PVX resistance; and Pto from tomato that encodes a serine-threonine kinase and mediates resistance against Pseudomonas syringae (24). Next, 21 days after TRV:SGT inoculation, the transgenic plants were challenge-inoculated with the pathogen corresponding to the R gene (12). As controls to demonstrate that N and Rx resistance could be suppressed by silencing, we used TRV constructs carrying a fragment of the respective resistance genes (TRV:N and TRV:Rx) (12). The control for Pto was TRV:Prf in which the insert is from a gene (Prf) that is required for Pto-mediated resistance (12). TRV:00 was a control to indicate any nonspecific effects of TRV on disease resistance.
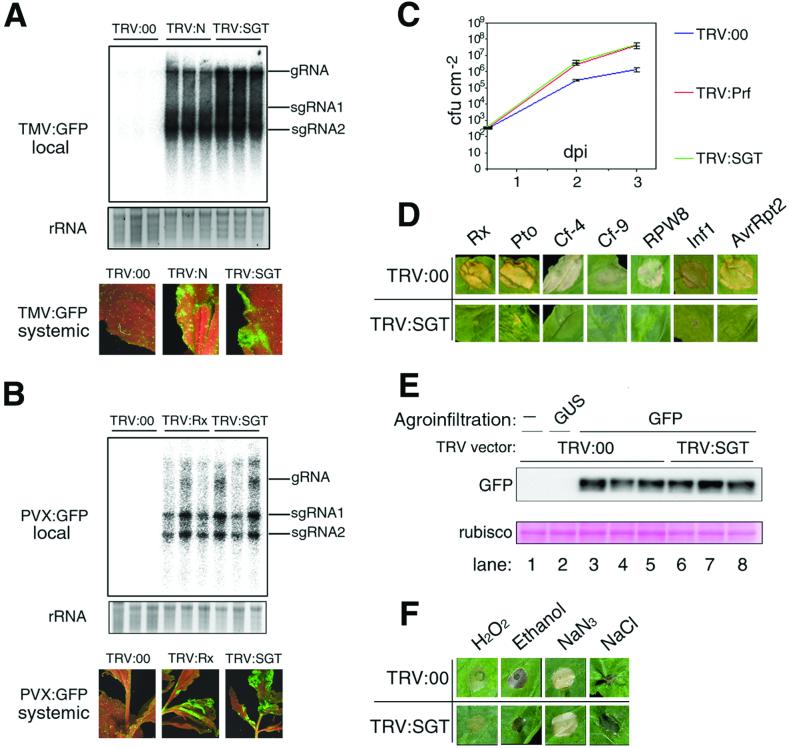
With each of these R genes, silencing of NbSGT1 compromised the disease resistance. Thus, in the N plants, 500-fold more TMV:GFP RNA occurred in the inoculated leaves of TRV:SGT- and TRV:N-infected plants than in the TRV:00 control (Fig. 3A). Correspondingly, by 15 days after the challenge inoculation, systemic spread of TMV:GFP occurred in TRV:SGT- and TRV:N- but not TRV:00-infected plants (Fig. 3A). Similarly, the Rx plants supported accumulation of PVX:GFP in inoculated and upper leaves of the TRV:SGT- and TRV:Rx-infected plants but not in the TRV:00 controls (Fig. 3B). In the Pto plants, the growth of P. syringae pv. tabaci (avrPto) was 25-fold higher during 3 days in the TRV:SGT- and TRV:Prf-infected leaves than in the TRV:00 control (Fig. 3C). Thus, NbSGT1 is required for disease resistance mediated by R proteins of the TIR-NBS-LRR (N), CC-NBS-LRR (Rx), and unrelated (Pto) classes. In N. benthamiana at least two forms of SGT1 exist (Fig. 1B), and at present, we cannot determine whether one or both of these forms are responsible for the silencing phenotypes. The cDNA fragments of NbSGT1.1 and NbSGT1.2 share 98% sequence identity, and both genes would have been silenced by the TRV:SGT construct.
Fig 3.
NbSGT1 silencing compromises a wide range of R gene-specified defense responses. (A) N resistance against TMV. (Top) RNA gel blot showing accumulation of TMV:GFP RNA at 6 dpi on inoculated leaves (local) of N-transgenic TRV:00, TRV:N, or TRV:SGT plants. Genomic (gRNA) and subgenomic (sgRNA) TMV:GFP RNAs are indicated. Approximately 500-fold more TMV:GFP RNA occurred in TRV:N and TRV:SGT leaves than in TRV:00 leaves according to dilutions of total RNA from TMV:GFP-infected tissue into total RNA from noninfected tissue. (Middle) Ethidium bromide staining of rRNA to confirm equal loading in each lane. (Bottom) UV illumination showing systemic TMV:GFP at 15 dpi. (B) Rx resistance against PVX in Rx-transgenic plants. Figure layout and experimental procedures are as described for A. (C) Pto resistance against P. syringae pv. tabaci (avrPto) in Pto-transgenic plants. Leaves were infiltrated with P. syringae pv. tabaci (avrPto), and bacterial growth in inoculated leaves was monitored for 3 days. Each data point represents the mean ± SEM of three replicate samples. (D) Appearance of HRs elicited in TRV:00 and TRV:SGT plants. The HRs were caused by Rx, Pto, Cf-4, and Cf-9 expression with their corresponding Avr protein, or RPW8.1 and RPW8.2 coexpression, or expression of Inf1 or AvrRpt2. (E) Protein gel blot analysis showing GFP levels 3 days after Agrobacterium-mediated transient expression (agroinfiltration). Infiltrated cultures of Agrobacterium carried a 35S:GUS (β-glucuronidase) (lane 2) or 35S:GFP (lanes 3–8) construct in the T-DNA. Ponceau S staining of ribulose-1,5-bisphosphate carboxylase/oxygenase (rubisco) for confirmation of equal loading in each lane is shown at the bottom. (F) Chemical-induced cell death in nontransgenic TRV:00 or TRV:SGT plants caused by infiltration of 3% H2O2/20% ethanol/1 mM NaN3/500 mM NaCl. All experiments involved three replicated samples, and were repeated three or more times, with each repeat giving similar results.
NbSGT1 Is Required for the HR Elicited by Multiple Resistance Interactions.
In addition to the effects on pathogen resistance (Fig. 3 A–C), NbSGT1 silencing also compromised the cell death (HR) that is often associated with disease resistance in plants (25). Thus, the HR caused by Agrobacterium-mediated transient coexpression of R proteins Rx, Pto, Cf-4, and Cf-9 and their cognate elicitor was suppressed in NbSGT1-silenced plants (Fig. 3D). Similarly, when elicitor proteins from either Phytophthora infestans (Inf1) (26) or P. syringae (AvrRpt2) (20) were transiently expressed, an HR occurred (M. Mudgett and B. Staskawicz, personal communication) that was suppressed if NbSGT1 was silenced (Fig. 3D). The simultaneous overexpression of Arabidopsis R proteins RPW8.1 and RPW8.2 (27) also causes an HR in N. benthamiana (D.A.W.J., S.X., and M.J.C., unpublished data) that was suppressed in NbSGT1-silenced plants (Fig. 3D).
The Loss of HR and Resistance in the TRV:SGT-Infected Plants Is Unlikely to Reflect Pleiotropic Effects of NbSGT1 Silencing.
We investigated whether the compromised resistance responses in the TRV:SGT-infected plants may have occurred because of the indirect effects of NbSGT1 silencing. For example, the HR suppression in the assays above could be due to an effect on the Agrobacterium-mediated transient expression. However, that is not likely because GFP was transiently expressed at the same level in leaves of TRV:00- and TRV:SGT-infected plants (Fig. 3E). Furthermore, the NbSGT1 VIGS phenotype was not specific to elicitors delivered by Agrobacterium-mediated transient expression. Thus, the HR triggered by delivery of purified Inf1 protein into N. benthamiana leaves was compromised in TRV:SGT- but not TRV:00-infected plants (I.M., unpublished data). It is also unlikely that the loss of HR and resistance in the TRV:SGT-infected plants was due to reduced R protein stability because NbSGT1 silencing did not affect steady-state levels of Rx (P.M., unpublished data). Finally, to exclude the possibility that NbSGT1 is involved in nonspecific cell death, necrosis was induced in TRV:00- and TRV:SGT-infected plants by a low concentration of hydrogen peroxide, ethanol, sodium azide, or sodium chloride. In each instance, the necrosis developed at the same rate in the infiltrated patch of TRV:00- and TRV:SGT-infected leaves (Fig. 3F).
How SGT1 Could Affect R Protein-Mediated Resistance.
The phenotype of the TRV:SGT-infected plants (Fig. 3) indicates that SGT1 proteins may be required for many different types of disease resistance in plants. The SGT1 requirement applies irrespective of whether the resistance was induced by an intracellular or extracellular elicitor; the elicitors of N (28) and Rx (29) are intracellular, whereas Inf1 of Phytophthora infestans (26) and the elicitors associated with Cf-4 and Cf-9 are extracellular (30). Similarly, the NbSGT1 requirement did not correlate with the involvement of NBS-LRR proteins. Of the resistance systems tested, N, Rx, and Pto require NBS-LRR proteins, whereas Cf-4, Cf-9, and RPW8 do not (24). Cf-4 and Cf-9 are membrane-localized proteins with extracellular LRRs, whereas the RPW8 proteins contain a transmembrane and CC domain (27, 30). The simplest way to explain this general requirement is to invoke convergence of multiple resistance pathways into a common branch that is affected by SGT1. Alternatively, parallel resistance pathways may each be influenced by SGT1 proteins. To understand further how SGT1 would function it will be necessary to find out whether its role in resistance requires association with a ubiquitin ligase complex (11). If that is the case, then the targets for ubiquitylation may be regulators of resistance (8, 9).
NbSGT1 Is Involved in Nonhost Resistance.
Most of the disease resistance mechanisms we investigated (Fig. 3) represent host resistance that varies within a species. This resistance is effective only in plants with R proteins that correspond to elicitors produced by specific isolates of a pathogen. However, a class of disease resistance also exists in plants that applies across a species and is effective against all known isolates of a pathogen (13). This nonhost resistance accounts for the common observation that most plants are resistant against most pathogens. Several indications show that nonhost and host resistance may share similar mechanisms (13, 31). For example, many nonhost-resistance interactions are associated with the HR, and corresponding pathogen elicitor determinants have been characterized (26, 32–34). Furthermore, the recently identified Arabidopsis nho1 (for nonhost) mutant is compromised for host- and nonhost-resistance against Pseudomonas bacteria (35).
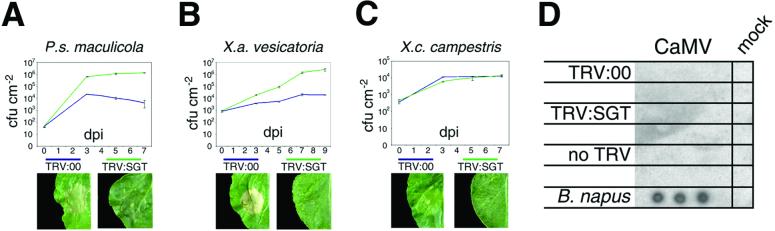
Consistent with the idea that these two classes of resistance share similar mechanisms, silencing of NbSGT1 causes loss of nonhost resistance. For example, in TRV:SGT-infected N. benthamiana the accumulation of P. syringae pv. maculicola, a pathogen of Brassicaceae, was approximately 100-fold greater during 7 days than in TRV:00-infected plants (Fig. 4A). A similar difference was seen with Xanthomonas axonopodis pv. vesicatoria, which is a pathogen of pepper (Fig. 4B). In addition, NbSGT1 silencing suppressed the HR-like cell death that is induced when these bacteria are inoculated at a high titer to N. benthamiana plants (Fig. 4 A and B). The cell death was delayed by NbSGT1 silencing until 72 h after inoculation for P. syringae pv. maculicola, whereas it was prevented during the 9 days of the experiment for X. axonopodis pv. vesicatoria.
Fig 4.
NbSGT1 is required for some nonhost resistance responses. (A) P. syringae pv. maculicola. (Upper) Growth of bacteria in TRV:00 and TRV:SGT plants. Leaves were infiltrated with low inoculum levels and bacterial growth in inoculated leaves was monitored for 7 days. Each data point represents the mean ± SEM of three replicate samples. (Lower) leaves infiltrated with high inoculum (OD600 = 0.05) 48 h after infiltration. (B) X. axonopodis pv. vesicatoria. Experimental details as described for A, except bacterial growth was monitored for 9 days and high inoculum concentration was OD600 = 0.1. (C) X. campestris pv. campestris. Experimental details as described for A, except high inoculum concentration was OD600 = 0.3. (D) Accumulation of CaMV at 14 dpi in upper noninoculated leaves of N. benthamiana plants that were TRV:00-, TRV:SGT-, or non-TRV-infected. CaMV sap was inoculated mechanically on lower leaves. Brassica napus Westar-10 are susceptible to CaMV and were used as controls. Upper leaves from three CaMV- and one mock-treated plant were subjected to DNA blot analysis. All experiments involved three replicated samples, and were repeated three or more times, with each repeat giving similar results.
However, not all examples of nonhost resistance were suppressed in NbSGT1-silenced plants. The growth of X. campestris pv. campestris, a pathogen of Brassicaceae, was unaffected by NbSGT1 silencing (Fig. 4C). Similarly the crucifer pathogen CaMV was unable to accumulate in N. benthamiana irrespective of whether NbSGT1 was silenced (Fig. 4D). Resistance against these pathogens was not associated with a visible HR (Fig. 4C and data not shown).
The finding that NbSGT1 silencing compromised resistance against P. syringae pv. maculicola and X. axonopodis pv. vesicatoria but not against X. campestris pv. campestris and CaMV shows that nonhost resistance can be divided into SGT1-dependent and SGT1-independent classes. SGT1-independent nonhost resistance may rely on preformed antimicrobial compounds (36) or the lack of suitable host and pathogenicity factors. In contrast, SGT1-dependent nonhost resistance may share similar mechanisms to host resistance, including the involvement of R proteins (32, 33, 37). Why then is SGT1-dependent nonhost resistance more durable than host resistance that is frequently lost through changes in the pathogen elicitor determinant? It could be that R proteins involved in SGT1-dependent nonhost resistance recognize an essential pathogen determinant that is widely conserved (38). Alternatively, multiple R proteins may correspond to multiple components of the pathogen (31, 33, 37, 39). Many questions about nonhost resistance clearly remain. However, our demonstration that SGT1 silencing affects this durable type of disease resistance may provide a step toward its molecular characterization.
Acknowledgments
We are grateful to The Gatsby Charitable Foundation for support of this work. Mary Mudgett and Brian Staskawicz (University of California, Berkeley) made the observation that AvrRpt2 elicits a HR in N. benthamiana and kindly provided the 35S:AvrRpt2 construct. The 35S:Inf1 construct was kindly provided by Sophien Kamoun (Ohio State University). Recombinant viruses and transgenic plants were contained in greenhouses under Department for Environment, Food, and Rural Affairs License PHL 161/4080.
Abbreviations
Avr, avirulence
NBS, nucleotide-binding site
LRR, leucine-rich repeat
TIR, Toll and interleukin-1
CC, coiled-coil
GFP, green fluorescent protein
PVX, potato virus X
CaMV, cauliflower mosaic virus
VIGS, virus-induced gene silencing
TRV, tobacco rattle virus
TMV, tobacco mosaic virus
HR, hypersensitive response
dpi, days post inoculation
References
- 1.Bonas U. & Lahaye, T. (2002) Curr. Opin. Microbiol. 5, 44-50. [DOI] [PubMed] [Google Scholar]
- 2.Falk A., Feys, B. J., Frost, L. N., Jones, J. D. G., Daniels, M. J. & Parker, J. E. (1999) Proc. Natl. Acad. Sci. USA 96, 3292-3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Century K. S., Shapiro, A. D., Repetti, P. P., Dahlbeck, D., Holub, E. & Staskawicz, B. (1997) Science 278, 1963-1965. [DOI] [PubMed] [Google Scholar]
- 4.Shirasu K., Lahaye, T., Tan, M.-W., Zhou, F., Azevedo, C. & Schulze-Lefert, P. (1999) Cell 99, 355-366. [DOI] [PubMed] [Google Scholar]
- 5.Muskett P. R., Kahn, K., Austin, M. J., Moisan, L. J., Sadanandom, A., Shirasu, K., Jones, J. D. G. & Parker, J. E. (2002) Plant Cell 14, 979-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tornero P., Merritt, P., Sadanandom, A., Shirasu, K., Innes, R. W. & Dangl, J. L. (2002) Plant Cell 14, 1005-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aarts N., Metz, M., Holub, E., Staskawicz, B. J., Daniels, M. J. & Parker, J. E. (1998) Proc. Natl. Acad. Sci. USA 95, 10306-10311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azevedo C., Sadanandom, A., Kitagawa, K., Freialdenhoven, A., Shirasu, K. & Schulze-Lefert, P. (2002) Science 295, 2073-2076. [DOI] [PubMed] [Google Scholar]
- 9.Austin M. J., Muskett, P., Kahn, K., Feys, B. J., Jones, J. D. G. & Parker, J. E. (2002) Science 295, 2077-2080. [DOI] [PubMed] [Google Scholar]
- 10.Tor M., Gordon, P., Cuzick, A., Eulgem, T., Sinapidou, E., Mert-Turk, F., Can, C., Dangl, J. L. & Holub, E. B. (2002) Plant Cell 14, 993-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitagawa K., Skowyra, D., Elledge, S. J., Harper, J. W. & Hieter, P. (1999) Mol. Cell 4, 21-33. [DOI] [PubMed] [Google Scholar]
- 12.Peart J. R., Cook, G., Feys, B. J., Parker, J. E. & Baulcombe, D. C. (2002) Plant J. 29, 569-579. [DOI] [PubMed] [Google Scholar]
- 13.Heath M. C. (2000) Curr. Opin. Plant Biol. 3, 315-319. [DOI] [PubMed] [Google Scholar]
- 14.Ratcliff F., Martin-Hernandez, A. M. & Baulcombe, D. C. (2001) Plant J. 25, 237-245. [DOI] [PubMed] [Google Scholar]
- 15.Alkaff N. S. & Covey, S. N. (1995) Plant Pathol. 44, 516-526. [Google Scholar]
- 16.Debener T., Lehnackers, H., Arnold, M. & Dangl, J. L. (1991) Plant J. 1, 289-302. [DOI] [PubMed] [Google Scholar]
- 17.Newman M. A., von Roepenack-Lahaye, E., Parr, A., Daniels, M. J. & Dow, J. M. (2002) Plant J. 29, 487-495. [DOI] [PubMed] [Google Scholar]
- 18.Bendahmane A., Kanyuka, K. & Baulcombe, D. C. (1999) Plant Cell 11, 781-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas C. M., Tang, S. J., Hammond-Kosack, K. & Jones, J. D. G. (2000) Mol. Plant–Microbe Interact. 13, 465-469. [DOI] [PubMed] [Google Scholar]
- 20.Mudgett M. B. & Staskawicz, B. J. (1999) Mol. Microbiol. 32, 927-941. [DOI] [PubMed] [Google Scholar]
- 21.Baulcombe D. C. (1999) Curr. Opin. Plant Biol. 2, 109-113. [DOI] [PubMed] [Google Scholar]
- 22.Worley C. K., Zenser, N., Ramos, J., Rouse, D., Leyser, O., Theologis, A. & Callis, J. (2000) Plant J. 21, 553-562. [DOI] [PubMed] [Google Scholar]
- 23.Schwechheimer C., Serino, G., Callis, J., Crosby, W. L., Lyapina, S., Deshaies, R. J., Gray, W. M., Estelle, M. & Deng, X. W. (2001) Science 292, 1379-1382. [DOI] [PubMed] [Google Scholar]
- 24.Dangl J. L. & Jones, J. D. G. (2001) Nature (London) 411, 826-833. [DOI] [PubMed] [Google Scholar]
- 25.Lam E., Kato, N. & Lawton, M. (2001) Nature (London) 411, 848-853. [DOI] [PubMed] [Google Scholar]
- 26.Kamoun S., vanWest, P., Vleeshouwers, V., deGroot, K. E. & Govers, F. (1998) Plant Cell 10, 1413-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao S., Ellwood, S., Calis, O., Patrick, E., Li, T., Coleman, M. & Turner, J. G. (2001) Science 291, 118-120. [DOI] [PubMed] [Google Scholar]
- 28.Erickson F. L., Holzberg, S., Calderon-Urrea, A., Handley, V., Axtell, M., Corr, C. & Baker, B. (1999) Plant J. 18, 67-75. [DOI] [PubMed] [Google Scholar]
- 29.Bendahmane A., Köhm, B. A., Dedi, C. & Baulcombe, D. C. (1995) Plant J. 8, 933-941. [DOI] [PubMed] [Google Scholar]
- 30.Joosten M. & de Wit, P. (1999) Annu. Rev. Phytopathol. 37, 335-367. [DOI] [PubMed] [Google Scholar]
- 31.Kamoun S. (2001) Curr. Opin. Plant Biol. 4, 295-300. [DOI] [PubMed] [Google Scholar]
- 32.Whalen M. C., Stall, R. E. & Staskawicz, B. J. (1988) Proc. Natl. Acad. Sci. USA 85, 6743-6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi D. Y., Tamaki, S. J. & Keen, N. T. (1989) Proc. Natl. Acad. Sci. USA 86, 157-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lauge R., Goodwin, P. H., de Wit, P. J. & Joosten, M. H. (2000) Plant J. 23, 735-745. [DOI] [PubMed] [Google Scholar]
- 35.Lu M., Tang, X. & Zhou, J. M. (2001) Plant Cell 13, 437-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papadopoulou K., Melton, R. E., Leggett, M., Daniels, M. J. & Osbourn, A. E. (1999) Proc. Natl. Acad. Sci. USA 96, 12923-12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tosa Y. (1989) Genome 32, 918-924. [Google Scholar]
- 38.Felix G., Duran, J. D., Volko, S. & Boller, T. (1999) Plant J. 18, 265-276. [DOI] [PubMed] [Google Scholar]
- 39.Heath M. C. (2001) Physiol. Mol. Plant Pathol. 58, 53-54. [Google Scholar]