Abstract
A proton–sucrose symporter mediates the key step in carbon export from leaves of most plants. Sucrose transport activity and steady-state mRNA levels of BvSUT1, a sugar beet leaf sucrose symporter, are negatively regulated specifically by sucrose. Results reported here show that BvSUT1 mRNA was localized to companion cells of the leaf's vascular system, which supports its role in the systemic distribution of photoassimilate. Immunoblot analysis showed that decreased transport activity was caused by a reduction in the abundance of symporter protein. RNA gel blot analysis of the leaf symporter revealed that message levels also declined, and nuclear run-on experiments demonstrated that this was the result of decreased transcription. Further analysis showed that symporter protein and message are both degraded rapidly. Taken together, these data show that phloem loading is regulated by means of sucrose-mediated changes in transcription of a phloem-specific sucrose symporter gene in a regulatory system that may play a pivotal role in balancing photosynthetic activity with resource utilization.
Plants are photoautotrophic organisms composed of both heterotrophic (sink) and autotrophic (source) tissues. Resources acquired via photosynthesis in source leaves are loaded into the phloem of the plant's elaborate vascular system and distributed among the sink tissues in a process called “assimilate partitioning”. Because up to 80% of all fixed carbon is exported to sinks (1), regulation of assimilate partitioning is vital to plant growth and development, and it can have dramatic affects on crop yield and productivity (2). Long-distance transport in the phloem cells of the plant vascular system is mediated by a positive hydrostatic pressure difference between the source and sink tissues that drives mass flow of solution. Positive pressure in the leaf phloem results from hyperaccumulation of an osmotically active solute. In most plants this solute is sucrose, which is loaded into the phloem by an electrogenic secondary active proton–sucrose symporter (3–5). Recently, the sucrose transport activity of a sugar beet leaf proton–sucrose symporter (BvSUT1) was found to be negatively regulated by sucrose and not by hexoses or changes in osmotic potential (6), but the mechanism and implications of this regulatory pathway remained unresolved. Here we report that sucrose transport activity in the phloem is regulated via sucrose-mediated changes in transcription of the proton–sucrose symporter gene in a regulatory system that plays a pivotal role in balancing photosynthetic activity with resource utilization.
Materials and Methods
Tissue Fixation and Embedding.
Leaf tissue from mature sugar beets (Beta vulgaris Linnaeus) was fixed on ice for 6 h under vacuum in 4% wt/vol paraformaldehyde in potassium phosphate buffer (pH 7.4). Tissue was dehydrated through a 10% step-graded ethanol series beginning at 20% and ending at 90% and allowed to incubate overnight at 4°C. After three 100% ethanol washes, the tissue was substituted with tertiary-buytl alcohol, infiltrated with Paraplast Plus-saturated tertiary-butyl alcohol, and embedded in 100% wt/vol Paraplast Plus (Sherwood Medical, St. Louis). Sections (7 μm) were affixed to Superfrost Plus (Fisher Scientific, Pittsburgh) slides.
In Situ Hybridization.
In situ hybridizations were performed essentially as described by Harrington et al. (7). Briefly, after removing the wax and a 2.5-min digestion in 0.01 mg/ml proteinase K (Promega, Madison, WI), slides were incubated for 1 h at 55°C in hybridization solution then for 24 h at 55°C in hybridization solution plus 9% wt/vol dextran sulfate and 1600 ng/ml dioxygenin-labeled (DIG) cRNA sense or antisense BvSUT1 (GenBank accession no. U64967) probes. Slides were washed twice in 2× SSC, incubated with 0.02 g/ml RNase A in STE (500 mM NaCl/20 mM Tris·HCl, pH 7.5/1 mM EDTA) for 30 min, placed in 50% formamide in 2× SSC at 50°C for 30 min, then washed at room temperature for 5 min in 1× SSC and 0.5× SSC. Slides were blocked for 2 h with 1% wt/vol BSA/Tris-buffered saline (TBS), probed with anti-DIG serum (Roche Molecular Biochemicals) diluted 1:300 in TBS/1% wt/vol BSA for 2 h, washed three times in TBS/1% wt/vol BSA for 25 min and three times in TBS for 5 min, and incubated in a nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate solution at 4°C for 12 h.
Plasma Membrane Isolation.
Plasma membrane vesicles (PMV) were purified by using an aqueous two-phase partitioning method as described (8). The purified plasma membrane vesicles were suspended at 3–5 mg of protein per milliliter of suspension buffer.
Transport Measurements.
Proton motive force-dependent sucrose and alanine transport was measured in isolated plasma membrane vesicles as described by Bush (8).
Antibody Preparation.
Polyclonal antibodies were prepared in rabbit by Sigma-Genosys (The Woodlands, TX) against a synthetic oligopeptide from the C terminus (PPPEAKIGGSMGGH) of BvSUT1 that was conjugated to keyhole limpet hemocyanin.
Immunoblots.
PMV proteins (7.5 or 15 μg) were separated on a 10% (wt/vol) SDS/polyacrylamide gel and transferred to Immobilon-P poly(vinylidene difluoride) membrane (Millipore) with a SemiDry Transfer Cell (Bio-Rad). Membranes were blocked for 1 h in 3% wt/vol BSA/TBST (10 mM Tris⋅HCl, pH 7.4/140 mM NaCl/0.1% Tween-20), rinsed with distilled water, and incubated in a 4°C 1:500 dilution of BvSUT1 C-terminal antiserum in 1% wt/vol BSA/TBST for 18 h. Blots were washed four times for 5 min in TBST, incubated with a 1:2,000 dilution of alkaline-phosphatase-conjugated goat anti-rabbit secondary antiserum (Bio-Rad) in 1% wt/vol BSA/TBST for 1.5 h, washed four times for 5 min in TBST, and developed in a nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate solution (Sigma) for 15 min. A diffuse band between 43 and 49 kDa was detected by serum directed against the C-terminal peptide. Specificity of this band was demonstrated by performing immunoblots with 1:500 dilution of antiserum that had been precompeted with 5 μg/ml BSA-conjugated C-terminal peptide for 8 h at 4°C. Recognition of the 43–49 kDa band was completely abolished, but other nonspecific bands remained.
RNA Isolation and Gel Blot Analysis.
RNA was isolated by using the RNAeasy kit according to the manufacturer's directions (Qiagen, Valencia, CA). Total RNA (15 μg) from each sample was fractionated on a 1.0% agarose-formaldehyde gel transferred to a Hybond N+ (Amersham Pharmacia) nylon membrane. BvSUT1 (GenBank accession no. U64967) and EF1A (GenBank accession no. AF030517) [α-32P]-dCTP probes were made by using the Megaprime DNA Labeling System (Amersham Pharmacia). Hybridizations were performed in Ultrahyb solution (Ambion, Austin, TX) for 18 h at 42°C. Blots were washed twice in 42°C 5× SSC/0.1% wt/vol SDS for 15 min, twice in 42°C 0.1× SSC/0.1% wt/vol SDS for 30 min, and exposed to x-ray film. Band intensities were measured by using a digital densitometer.
Isolation of Nuclei.
Frozen leaf tissue was crushed under liquid nitrogen, added to grind solution (2.5% wt/vol Ficoll 400/5.1% wt/vol Dextran T-40/25 mM Tris⋅HCl, pH 8.5/2.5% vol/vol Triton X-100/4.6 mM MgCl2/0.44 M sucrose/0.007% 2-mercaptoethanol/0.2 g/liter spermine), and homogenized by using a Brinkmann Polytron at setting 1 for 45 s. The homogenate was filtered through 6 layers of rinsed, chilled cheesecloth and centrifuged at 4°C for 8 min at 6,000 rpm in a Sorvall SS-34 rotor. The pellet was suspended in grind solution (minus spermine) and centrifuged. This step was repeated once. Pellets were suspended in ice-cold wash buffer (50 mM Tris⋅HCl, pH 8.5/5 mM MgCl2/20% vol/vol glycerol) and centrifuged as described. Finally, pellets were suspended in 500 μl ice-cold storage buffer (50 mM Tris⋅HCl, pH 8.5/5 mM MgCl2/50% vol/vol glycerol), flash-frozen, and stored at −80°C. An aliquot from each isolation was counted in a hemacytometer.
Nuclear Run-On Analysis.
Run-on transcription was performed as described in Liu (9). Transcription reactions containing [α−32P]-UTP-labeled mRNA were extracted in phenol/chloroform/isoamyl alcohol (25:24:1, pH 4.6) followed by chloroform and the RNA was ethanol-precipitated overnight at −20°C. RNA pellets were suspended in diethyl pyrocarbonate-treated water. RNA samples (1 × 106 cpm/ml) were incubated with membranes containing 5 μg spots of BvSUT1, Arabidopsis ACT2 (GenBank accession no. U41998), rice EF1A, and pBluescript-KS (Stratagene) in 2 ml Ultrahyb solution at 42°C for 24 h. Blots were washed twice in 42°C 2× SSC, 0.1% wt/vol SDS for 5 min and twice for 20 min in 42°C 0.1× SSC, 0.1% wt/vol SDS, and exposed to x-ray film for 1 to 2 days. Signal intensities were determined by using a digital densitometer. To determine relative transcription rates, BvSUT1 signal was expressed as the ratio between its signal intensity and the intensity of the control gene EF1A. These values were then compared between blots to derive relative BvSUT1 transcription.
Results
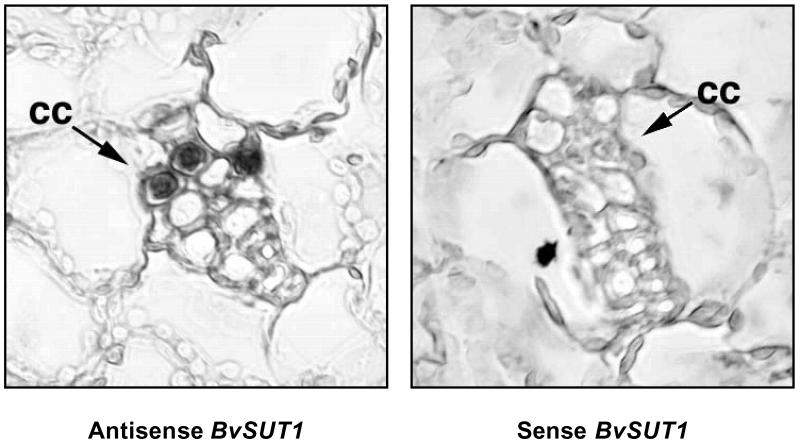
Sucrose transporters are expressed not only in the phloem, but also in other cell types and tissues where they fulfill diverse physiological roles (10, 11). In sugar beet leaves, BvSUT1 mRNA expression corresponds to the onset of photosynthetic maturity, supporting a role in photoassimilate transport (12). However, the prospect that this transporter might also be found in other leaf cell types complicated interpretation of its sucrose-mediated regulation. When in situ hybridization of paraffin-embedded leaf tissues was used, the BvSUT1 messenger RNA was localized exclusively in the phloem companion cells of minor and higher-order veins in mature leaves (Fig. 1). No signals were detected in mesophyll cells, even when we processed slides with extended hybridization and development times (not shown). Although we cannot rule out low levels of symporter expression in the mesophyll that are below the level of detection, we can conclude that the sucrose-regulated symporter message is not located in the mesophyll. If the sucrose-sensitive message was present in the mesophyll rather than the companion cells, then at least 70% of the symporter mRNA would be found in the mesophyll (6). Using an experimentally based estimate of mesophyll to companion cell cytoplasmic volume ratio of 9 to 1 (13), under control conditions the relative message abundance ratio would be 0.7 to 3, respectively. This is less than a 5-fold difference between the two cytoplasms and thus the mesophyll abundance would have been more than adequate to allow BvSUT1 mRNA detection in our experiment. Therefore, we conclude that sucrose-sensitive symporter expression is limited to the companion cells (Fig. 1). Taken together, demonstration of sucrose transport activity, expression with the onset of photosynthetic activity, and localization in companion cells strongly support the hypothesis that BvSUT1 is a key contributor to phloem loading in sugar beet leaves.
Fig 1.
Companion cell-specific localization of BvSUT1 mRNA. In sections of mature sugar beet leaf hybridized with antisense BvSUT1 cRNA probe, staining was localized to cytoplasmically dense companion cells proximal to the sieve elements, whereas no cells were stained in sections hybridized with the sense probe. The veins shown are directly embedded in the mesophyll and lack supporting parenchyma, indicating they are minor veins involved in phloem loading.
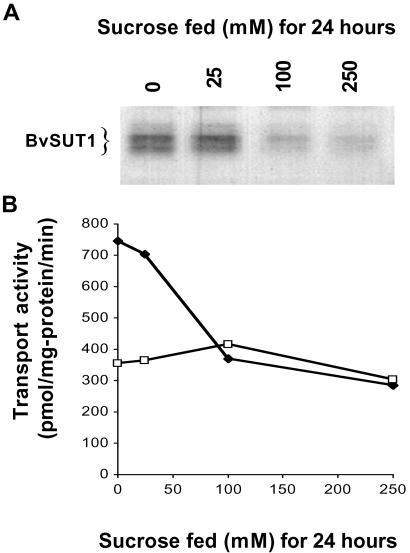
Kinetic analysis of proton–sucrose symporter transport activity in purified PMV isolated from water- or sucrose-fed leaves revealed a sucrose-dependent decrease in the Vmax for sucrose transport (6). Such a change could be the result of inactivation of some percentage of the pool of symporter proteins or by a decrease in symporter protein abundance. Immunoblots of the BvSUT1 protein showed that symporter abundance decreased in a sucrose concentration-dependent manner in PMV isolated from leaves fed 0, 25, 100, or 250 mM sucrose for 24 h (Fig. 2A). Sucrose transport activity declined in the same vesicles, whereas alanine transport showed little change (Fig. 2B). The drop in sucrose transport activity was proportional to the observed decline in symporter abundance, suggesting that sucrose transport is regulated by changes in the amount of BvSUT1 protein.
Fig 2.
BvSUT1 protein abundance and sucrose transport activity as modulated by sucrose feeding. (A) In immunoblots of PMV isolated from leaves fed increasing concentrations of sucrose for 24 h. (B) Proton motive force-dependent sucrose (♦) and alanine transport (□) in the same PMV.
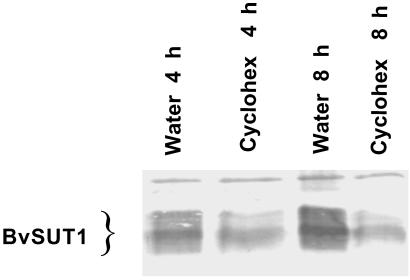
Proteins that catalyze the first or rate-limiting step of metabolic pathways are often rapidly turned over and substrate flux is regulated, at least in part, by modulating the synthesis of these key enzymes (14, 15). Because sucrose transport activity was proportional to BvSUT1 protein abundance, it was important to measure the stability of the symporter protein. BvSUT1 abundance was measured after inhibiting translation with cycloheximide (Fig. 3). BvSUT1 abundance decreased to 36% ± 5% of water-fed controls in immunoblots performed on PMV isolated from leaves fed with cycloheximide for 4 h (n = 4), which is consistent with the rapid degradation reported for the potato sucrose symporter, StSUT1 (16). Based on these results and assuming initial rates of linear decay, the approximate half-life of the symporter protein in the absence of active translation was estimated to be 2.7 h. Thus, the BvSUT1 protein is rapidly turned over, which is consistent with a regulatory role for this sucrose symporter in phloem loading.
Fig 3.
Sucrose symporter BvSUT1 protein stability in leaves treated with cycloheximide (cyclohex). Immunoblots of PMV isolated from leaves fed water or 100 μm cycloheximide for 4 and 8 h. BvSUT1 protein abundance decreased to 42% and 34% of water-fed control levels, respectively.
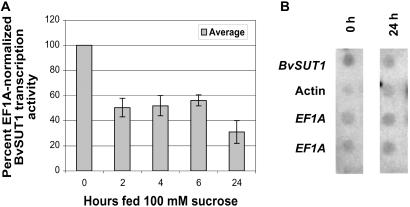
Previous results demonstrated that BvSUT1 transcript levels decreased in parallel with the loss of sucrose transport activity as a function of sucrose accumulation in the leaf (6). This change in mRNA abundance could be the result of decreased transcription or of an increased message degradation rate. Nuclear run-on experiments demonstrated that BvSUT1 gene transcription was repressed significantly in nuclei from leaves fed 100 mM exogenous sucrose (Fig. 4), suggesting that sucrose-dependent modulation of BvSUT1 mRNA levels is mediated by changes in transcription. Moreover, this rapid response to sucrose further supports the notion that phloem loading is a crucial, perhaps even rate-limiting, step in assimilate partitioning. That hypothesis is consistent with complementary observations showing that glucose accumulation in maize mesophyll cells decreases photosynthetic gene expression within 3 h (17).
Fig 4.
BvSUT1 transcription in response to sucrose feeding. (A) Individual excised leaves were fed 100 mM sucrose for 0, 2, 4, 6, or 24 h, then flash-frozen in liquid nitrogen. Nuclei were isolated from each leaf and transcription of BvSUT1, ACT2, and EF1A was assayed by using nuclear run-on analysis. BvSUT1 and ACT2 transcription activity was normalized within each assay using EF1A transcription activity as an internal standard. Normalized BvSUT1 transcription activity for each time point was then expressed as a percentage of normalized BvSUT1 transcription activity in the 0-h leaf. Normalized BvSUT1 transcription decreased to around 50% of 0-h levels after just 2 h of sucrose feeding and remained depressed for up to 24 h. Error bars represent ± SE, and each time point is the mean of 3–5 independent experiments. (B) In this representative autoradiograph from a nuclear run-on assay performed on leaves fed 100 mM sucrose for 0 or 24 h, EF1A-normalized BvSUT1 transcription activity in the 24-h sucrose-fed leaf was 31% ± 9% of 0-h BvSUT1 transcription activity. Normalized ACT2 transcription activity was not affected by sucrose feeding.
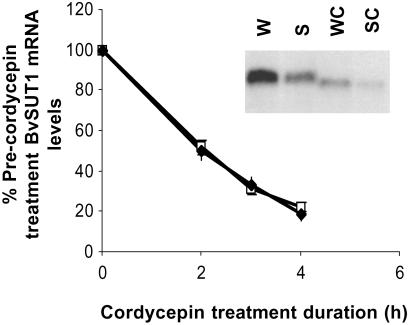
In addition to transcriptional regulation, differential mRNA stability can play a significant role in regulating steady-state mRNA levels (18, 19). For example, the sugar-dependent repression of rice α-amylase Amy3D is mediated by decreased gene transcription and by a parallel decrease in mRNA half-life from 6 to 1.5 h (20–22). The rapid decline of BvSUT1 message in response to sucrose feeding (M.W.V., unpublished data) suggested that sucrose-mediated destabilization of the symporter mRNA could contribute to the decrease in its steady-state mRNA levels. To address this possibility, the rate of BvSUT1 mRNA degradation was assayed in leaves fed water or 100 mM sucrose for a short period and then treated with the transcription inhibitor cordycepin. RNA gel blot analysis of BvSUT1 mRNA levels was performed on samples collected before and after cordycepin treatment. The mRNA decay curves were equivalent between water- and sucrose-fed leaves (Fig. 5). Furthermore, the half-lives for BvSUT1 message calculated for water-fed (1.9 ± 0.1 h) and sucrose-fed (1.8 ± 0.1 h) samples did not differ significantly (P = 0.47, n = 3). Based on these observations, we conclude that the BvSUT1 mRNA has a constant high turnover rate, and that the observed sucrose-dependent decrease in BvSUT1 mRNA is the result of reduced transcriptional activity.
Fig 5.
Effect of sucrose feeding on BvSUT1 mRNA stability. BvSUT1 mRNA stability was examined in leaves fed water or sucrose for 3 h, then treated with 150 μg/ml cordycepin. BvSUT1 mRNA levels were measured before and after cordycepin treatment. BvSUT1 message decayed at about the same rate in sucrose-fed (♦) as in water-fed (□) leaves. (Inset) RNA gel blot analysis of BvSUT1 mRNA abundance in leaves fed water (W) or sucrose (S) for 3 h followed by cordycepin for 3 h (WC and SC).
Discussion
The rapid degradation of BvSUT1 message and protein documented here suggests that regulation of its transcription by sucrose is a pivotal regulatory step in phloem loading and assimilate partitioning. Sink strength, or the ability of heterotrophic tissues to use photoassimilate, is a primary factor determining the rate of carbohydrate export from, and ultimately the photosynthetic activity of, source leaves (23, 24), but mechanisms by which this occurs have not been elucidated.
Sucrose-regulation of sucrose symporter activity could be a key control point that maintains tight coordination between photosynthetic activity and sink utilization of photoassimilate. Sucrose symporters located in the plasma membrane of phloem cells provide the driving force for mass flow of solutes from source to sink tissue by loading sucrose to high concentrations in the phloem. The resulting elevated osmotic potential gives rise to an influx of water that leads to a high hydrostatic pressure that drives a mass flow of solutes away from the loading site. At the other end of the transport pathway, sucrose is released from the phloem into import-dependent sink tissues. Any changes in phloem unloading can affect the pressure gradient in the phloem and ultimately, the concentration of sucrose at the loading site. For example, nutrient deficiency can lead to reduced growth rates in sinks and decreased rates of sucrose metabolism. This results in diminished phloem unloading, a smaller hydrostatic pressure difference driving mass flow in the phloem and, in the presence of steady rates of phloem loading, higher sucrose concentrations in the leaf phloem (24).
We hypothesize that a sucrose sensor in the phloem is a dynamic regulator of symporter transcription and that the capacity for phloem loading is directly proportional to transcriptional activity because of the high rate of symporter message and protein turnover. Our working model suggests a sucrose sensor in the phloem responds to increased sucrose by down-regulating transcription of the sucrose symporter gene that, because of rapid symporter protein and mRNA degradation, directly lowers phloem loading capacity. Initially, photosynthesis will continue and carbohydrate will back up in the mesophyll, favoring starch synthesis. However, as decreased phloem loading is maintained, feedback inhibition will decrease photosynthesis via hexose-mediated repression of photosynthetic gene expression (17). Increased sink demand would have the opposite effect. In this way, sucrose-mediated transcriptional regulation of the sucrose symporter can play a fundamental role in integrating photosynthetic activity in source leaves with demand for photoassimilate in the heterotrophic tissues of the plant.
Acknowledgments
We thank Dr. Kris Lambert for use of his microscopy facilities and Dr. Wendy Ransom-Hodgkins for help with membrane isolation and useful comments regarding the manuscript. This research was supported by grants from the U.S. Department of Agriculture–Agricultural Research Service and the U.S. Department of Energy, Energy Biosciences.
Abbreviations
PMV, plasma membrane vesicles
This paper was submitted directly (Track II) to the PNAS office.
To whom reprint requests should be addressed. E-mail: dbush@uiuc.edu.
References
- 1.Kalt-Torres W., Kerr, P., Usuda, H. & Huber, S. (1987) Plant Physiol. 83, 283-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gifford R. M., Thorne, J. H., Hitz, W. D. & Giaquinta, R. T. (1984) Science 225, 801-808. [DOI] [PubMed] [Google Scholar]
- 3.Giaquinta R. T. (1983) Annu. Rev. Plant Physiol. 34, 347-387. [Google Scholar]
- 4.Bush D. R. (1993) Annu. Rev. Plant Physiol. Plant Mol. Biol. 44, 513-542. [Google Scholar]
- 5.Kuhn C., Barker, L., Burkle, L. & Frommer, W. B. (1999) J. Exp. Bot. 50, 935-953. [Google Scholar]
- 6.Chiou T. J. & Bush, D. R. (1998) Proc. Natl. Acad. Sci. USA 95, 4784-4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrington G. N., Nussbaumer, Y., Wang, X. D., Tegeder, M., Franceschi, V. R., Frommer, W. B., Patrick, J. W. & Offler, C. E. (1997) Protoplasma 200, 35-50. [Google Scholar]
- 8.Bush D. R. (1989) Plant Physiol. 89, 1318-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu H., (1999) Ph.D. thesis (University of Illinois at Urbana-Champaign, Urbana).
- 10.Bush D. R. (1999) Curr. Opin. Plant Biol. 2, 187-191. [DOI] [PubMed] [Google Scholar]
- 11.Williams L. E., Lemoine, R. & Sauer, N. (2000) Trends Plant Sci. 5, 283-290. [DOI] [PubMed] [Google Scholar]
- 12.Lu M. Y., (1998) Ph.D. thesis (University of Illinois at Urbana-Champaign, Urbana).
- 13.Winter H., Robinson, D. G. & Heldt, H. W. (1994) Planta 193, 530-535. [Google Scholar]
- 14.Goldberg A. L. & St. John, A. C. (1976) Annu. Rev. Biochem. 45, 747-803. [DOI] [PubMed] [Google Scholar]
- 15.Vierstra R. D. (1993) Annu. Rev. Plant Physiol. Plant Mol. Biol. 44, 385-410. [Google Scholar]
- 16.Kuhn C., Franceschi, V. R., Schulz, A., Lemoine, R. & Frommer, W. B. (1997) Science 275, 1298-1300. [DOI] [PubMed] [Google Scholar]
- 17.Jang J. C. & Sheen, J. (1994) Plant Cell 6, 1665-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Day D. A. & Tuite, M. F. (1998) J. Endocrinol. 157, 361-371. [DOI] [PubMed] [Google Scholar]
- 19.Gutierrez R. A., MacIntosh, G. C. & Green, P. J. (1999) Trends Plant Sci. 4, 429-438. [DOI] [PubMed] [Google Scholar]
- 20.Sheu J. J., Yu, T. S., Tong, W. F. & Yu, S. M. (1996) J. Biol. Chem. 271, 26998-27004. [DOI] [PubMed] [Google Scholar]
- 21.Chan M.-T. & Yu, S.-M. (1998) Proc. Natl. Acad. Sci. USA 95, 6543-6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Umemura T., Perata, P., Futsuhara, Y. & Yamaguchi, J. (1998) Planta 204, 420-428. [DOI] [PubMed] [Google Scholar]
- 23.Gifford R. M. & Evans, L. T. (1981) Annu. Rev. Plant Physiol. 32, 485-509. [Google Scholar]
- 24.Coruzzi G. & Bush, D. R. (2001) Plant Physiol. 125, 65-68. [DOI] [PMC free article] [PubMed] [Google Scholar]