Abstract
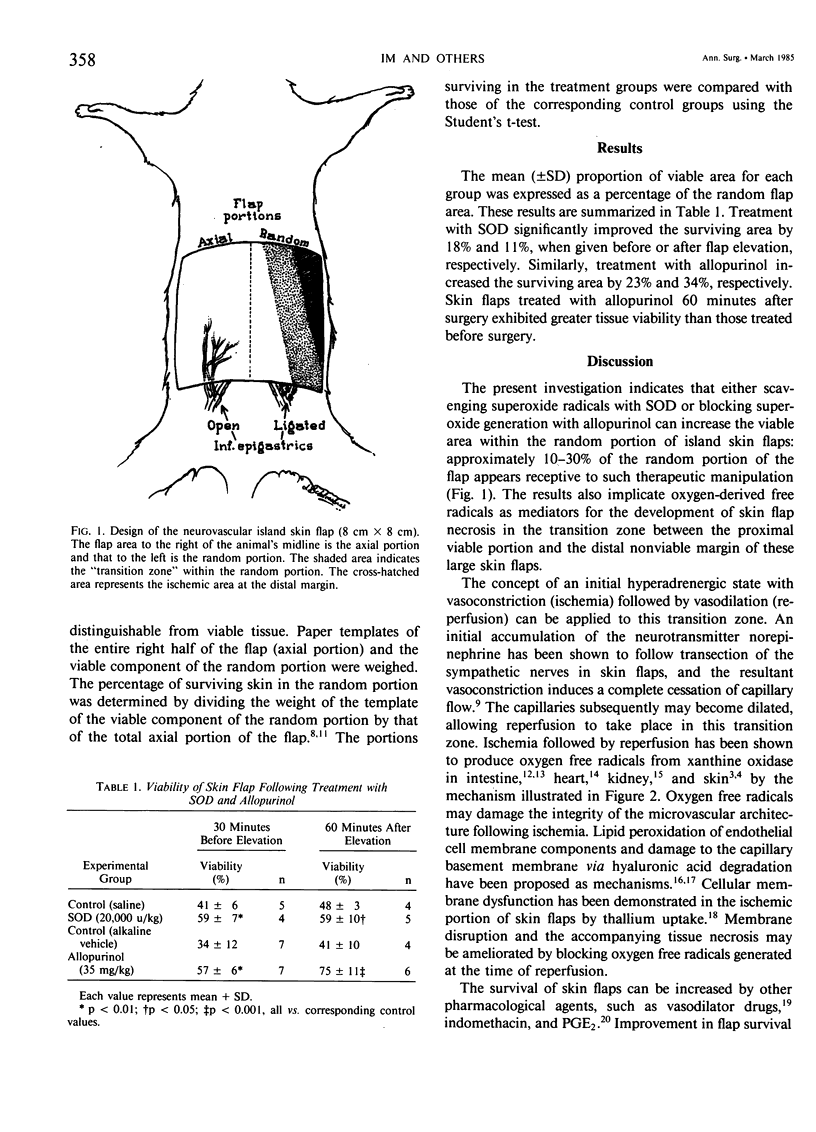
We have demonstrated previously that oxygen-derived free radicals are important mediators of tissue injury following ischemia (total venous occlusion) and reperfusion in small (3 cm X 6 cm) island skin flaps in rats. In this study, we evaluated extension of this concept to regional ischemia in large (8 cm X 8 cm) acute island skin flaps which were constructed to exceed their sole blood supply via unilateral inferior epigastric vessels. Under normal (control) circumstances, a significant portion of the flap would undergo necrosis at the periphery, mimicking the corresponding clinical situation. Blocking the generation of superoxide radicals from xanthine oxidase with a single dose of allopurinol prior to flap elevation significantly improved the area of flap viability from 34 +/- 12% to 57 +/- 6% (p less than 0.01) in the random portion of the flap, contralateral to the source of blood supply. Similarly, the detoxification of superoxide radicals with a single dose of superoxide dismutase improved viability from 41 +/- 6% to 58 +/- 7% (p less than 0.01). Similar results were obtained when either of these agents were administered 60 minutes after flap elevation. These findings suggest that oxygen-derived free radicals play an important role in the development of tissue necrosis in the critical transition zone between well-vascularized and devascularized skin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Del Maestro R., Thaw H. H., Björk J., Planker M., Arfors K. E. Free radicals as mediators of tissue injury. Acta Physiol Scand Suppl. 1980;492:43–57. [PubMed] [Google Scholar]
- Finseth F., Adelberg M. G. Prevention of skin flap necrosis by a course of treatment with vasodilator drugs. Plast Reconstr Surg. 1978 May;61(5):738–743. doi: 10.1097/00006534-197805000-00013. [DOI] [PubMed] [Google Scholar]
- Finseth F., Cutting C. An experimental neurovascular island skin flap for the study of the delay phenomenon. Plast Reconstr Surg. 1978 Mar;61(3):412–420. doi: 10.1097/00006534-197803000-00016. [DOI] [PubMed] [Google Scholar]
- Granger D. N., Rutili G., McCord J. M. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981 Jul;81(1):22–29. [PubMed] [Google Scholar]
- Hoopes J. E., Im M. J. Skin flap necrosis in guinea pigs: limitation of glucose supply and accumulation of lactate. Plast Reconstr Surg. 1978 May;61(5):748–752. doi: 10.1097/00006534-197805000-00015. [DOI] [PubMed] [Google Scholar]
- Im M. J., Hoopes J. E. Distribution of glucose in the pedicle skin flaps of guinea pigs. J Surg Res. 1978 Sep;25(3):269–273. doi: 10.1016/0022-4804(78)90118-x. [DOI] [PubMed] [Google Scholar]
- Im M. J., Shen W. H., Pak C. J., Manson P. N., Bulkley G. B., Hoopes J. E. Effect of allopurinol on the survival of hyperemic island skin flaps. Plast Reconstr Surg. 1984 Feb;73(2):276–278. doi: 10.1097/00006534-198402000-00023. [DOI] [PubMed] [Google Scholar]
- Manson P. N., Anthenelli R. M., Im M. J., Bulkley G. B., Hoopes J. E. The role of oxygen-free radicals in ischemic tissue injury in island skin flaps. Ann Surg. 1983 Jul;198(1):87–90. doi: 10.1097/00000658-198307000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M. Free radicals and inflammation: protection of synovial fluid by superoxide dismutase. Science. 1974 Aug 9;185(4150):529–531. doi: 10.1126/science.185.4150.529. [DOI] [PubMed] [Google Scholar]
- Mes L. G. Improving flap survival by sustaining cell metabolism within ischemic cells: a study using rabbits. Plast Reconstr Surg. 1980 Jan;65(1):56–65. doi: 10.1097/00006534-198001000-00011. [DOI] [PubMed] [Google Scholar]
- Palmer B. Sympathetic denervation and reinnervation of cutaneous blood vessels following surgery. An experimental study on rats by means of a histochemical fluorescence method. Scand J Plast Reconstr Surg. 1970;4(2):93–99. doi: 10.3109/02844317009038451. [DOI] [PubMed] [Google Scholar]
- Parks D. A., Bulkley G. B., Granger D. N. Role of oxygen-derived free radicals in digestive tract diseases. Surgery. 1983 Sep;94(3):415–422. [PubMed] [Google Scholar]
- Pearl R. M. A unifying theory of the delay phenomenon--recovery from the hyperadrenergic state. Ann Plast Surg. 1981 Aug;7(2):102–112. doi: 10.1097/00000637-198108000-00005. [DOI] [PubMed] [Google Scholar]
- Rees R., Powers T., Lynch J. B., O'Leary J. P. Thallium distribution in ischemic skin flaps. J Surg Res. 1982 May;32(5):507–514. doi: 10.1016/0022-4804(82)90133-0. [DOI] [PubMed] [Google Scholar]
- Sasaki G. H., Pang C. Y. Experimental evidence for involvement of prostaglandins in viability and acute skin flaps: effects on viability and mode of action. Plast Reconstr Surg. 1981 Mar;67(3):335–340. doi: 10.1097/00006534-198103000-00010. [DOI] [PubMed] [Google Scholar]
- Sasaki G. H., Pang C. Y. Hemodynamics and viability of acute neurovascular island skin flaps in rats. Plast Reconstr Surg. 1980 Feb;65(2):152–158. doi: 10.1097/00006534-198002000-00005. [DOI] [PubMed] [Google Scholar]
- Su C. T., Im M. J., Hoopes J. E. Tissue glucose and lactate following vascular occlusion in island skin flaps. Plast Reconstr Surg. 1982 Aug;70(2):202–205. doi: 10.1097/00006534-198208000-00014. [DOI] [PubMed] [Google Scholar]
- Tan C. M., Im M. J., Myers R. A., Hoopes J. E. Effects of hyperbaric oxygen and hyperbaric air on the survival of island skin flaps. Plast Reconstr Surg. 1984 Jan;73(1):27–30. doi: 10.1097/00006534-198401000-00005. [DOI] [PubMed] [Google Scholar]


