Abstract
1. Nitrobenzylthioinosine is a potent and specific inhibitor of nucleoside translocation in animal cells. Kinetic and inhibitor binding studies were undertaken to clarify how this inhibitor interacts with the nucleoside transporter from human and nucleoside-permeable type sheep erythrocytes.
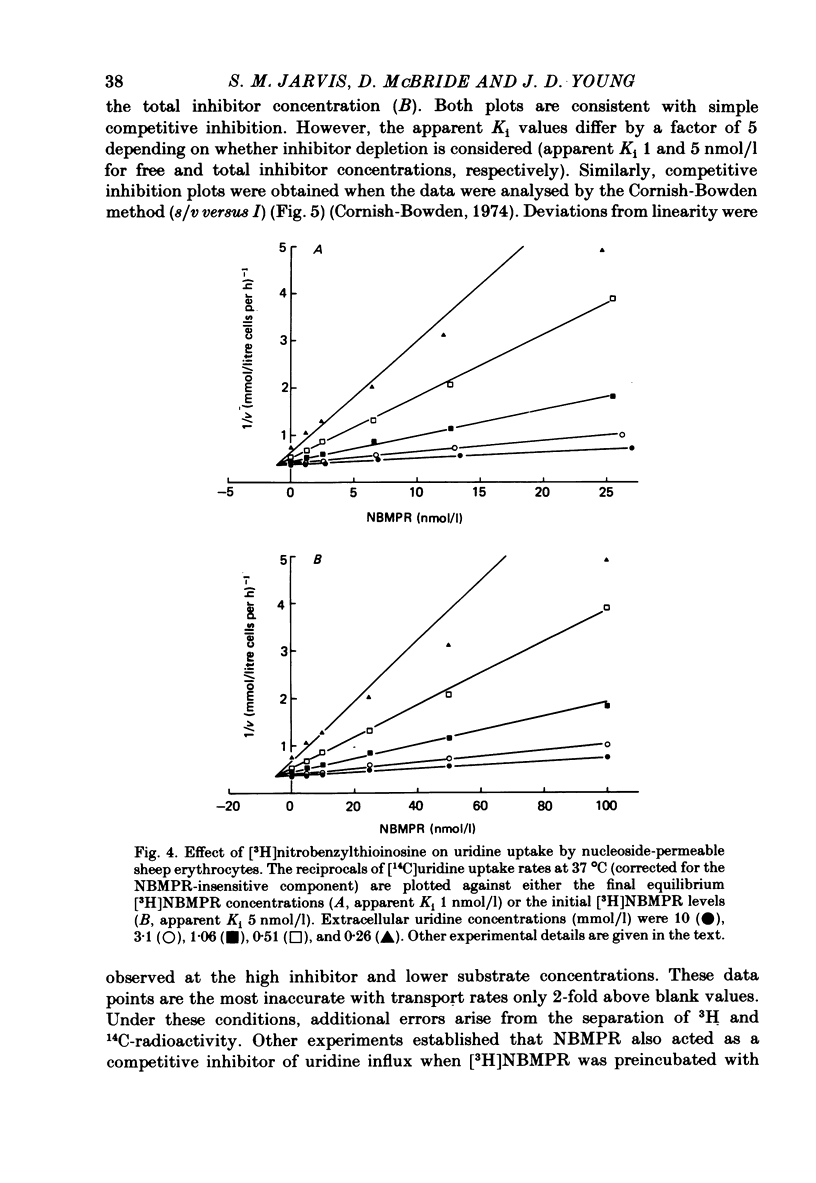
2. [3H]nitrobenzylthioinosine inhibition of zero-trans [U-14C]uridine influx into nucleoside-permeable type sheep cells was consistent with simple competitive inhibition (apparent Ki 1 nmol/l). Analysis of results using total inhibitor levels instead of cell-free inhibitor concentrations did not affect the inhibition pattern, but increased the apparent Ki value by 5-fold.
3. In contrast, [3H]nitrobenzylthioinosine was a non-competitive inhibitor of zero-trans [U-14C]uridine efflux (apparent Ki 1·5 nmol/l). Dipyridamole, another potent inhibitor of nucleoside translocation, also inhibited zero-trans [U-14C]uridine influx in a competitive manner (apparent Ki 20-40 nmol/l).
4. [3H]nitrobenzylthioinosine bound to high-affinity sites on cell membranes from human and nucleoside-permeable type sheep cells (apparent KD values ≃ 1 nmol/l). Binding of inhibitor to these sites was competitively blocked by uridine, a well characterized substrate for the nucleoside transporter (apparent Ki 1·25 and 0·9 mmol/l, respectively). These apparent Ki values are close to the apparent Km for uridine equilibrium exchange in human erythrocytes.
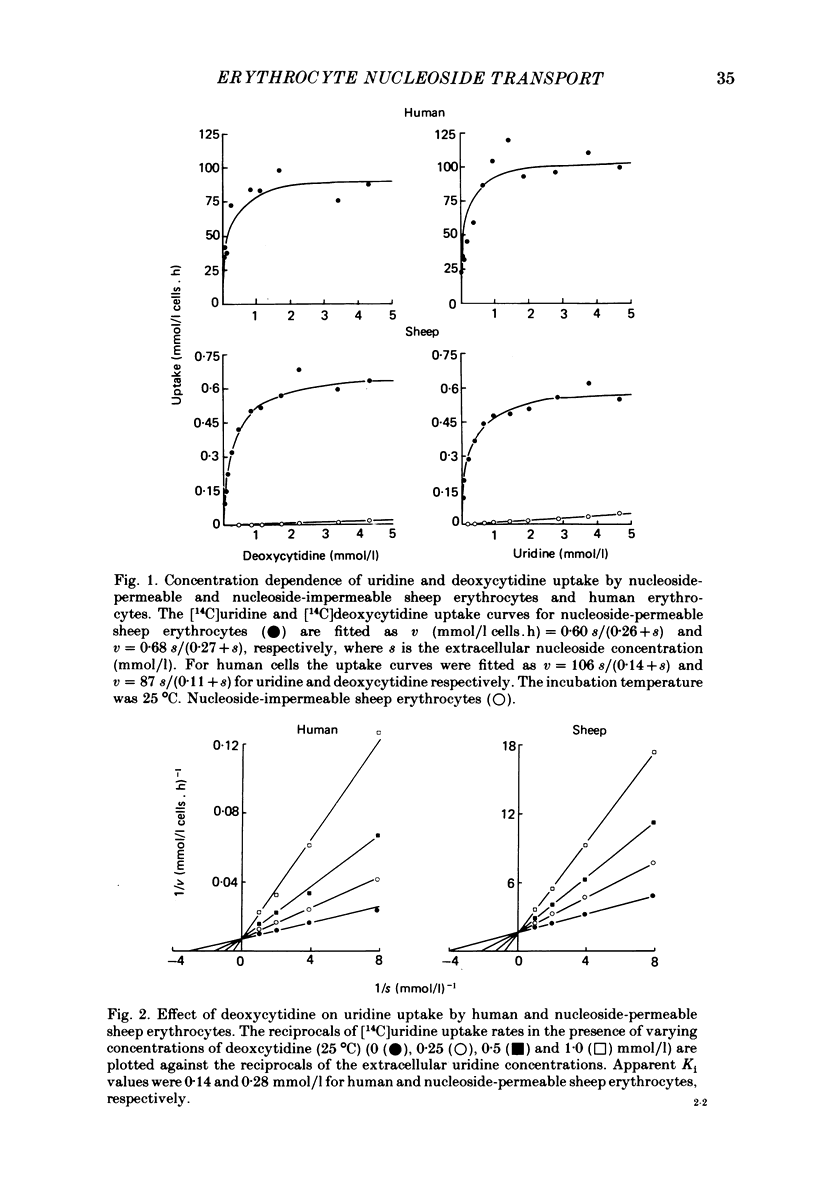
5. Similarly, deoxycytidine was found to be a competitive inhibitor of high-affinity [3H]nitrobenzylthioinosine binding activity (apparent Ki 1·0 and 1·2 mmol/l for human and nucleoside-permeable type sheep cell membranes, respectively). This contrasts with a previous report that this nucleoside had no effect on inhibitor binding activity. Transport studies confirmed that deoxycytidine is a substrate for the erythrocyte nucleoside transporter. Apparent Km and Vmax values for [U-14C]-deoxycytidine zero-trans influx into human and nucleoside-permeable type sheep cells were comparable to those obtained for [U-14C]uridine.
6. It is suggested from these results that nitrobenzylthioinosine competes directly with nucleosides for the permeation site of the nucleoside transporter, but that inhibitor binds preferentially to the external membrane surface.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basketter D. A., Widdas W. F. Asymmetry of the hexose transfer system in human erythrocytes. Comparison of the effects of cytochalasin B, phloretin and maltose as competitive inhibitors. J Physiol. 1978 May;278:389–401. doi: 10.1113/jphysiol.1978.sp012311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabantchik Z. I., Ginsburg H. Transport of uridine in human red blood cells. Demonstration of a simple carrier-mediated process. J Gen Physiol. 1977 Jan;69(1):75–96. doi: 10.1085/jgp.69.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass C. E., Gaudette L. A., Paterson A. R. Mediated transport of nucleosides in human erythrocytes. Specific binding of the inhibitor nitrobenzylthioinosine to nucleoside transport sites in the erythrocyte membrane. Biochim Biophys Acta. 1974 Apr 12;345(1):1–10. doi: 10.1016/0005-2736(74)90239-9. [DOI] [PubMed] [Google Scholar]
- Cass C. E., Paterson A. R. Inhibition of thymidine uptake in asynchronous HeLa cells by nitrobenzylthioinosine. Exp Cell Res. 1977 Mar 15;105(2):427–435. doi: 10.1016/0014-4827(77)90139-2. [DOI] [PubMed] [Google Scholar]
- Cass C. E., Paterson A. R. Mediated transport of nucleosides in human erythrocytes. Accelerative exchange diffusion of uridine and thymidine and specificity toward pyrimidine nucleosides as permeants. J Biol Chem. 1972 May 25;247(10):3314–3320. [PubMed] [Google Scholar]
- Cass C. E., Paterson A. R. Nitrobenzylthionionosine binding sites in the erythrocyte membrane. Biochim Biophys Acta. 1976 Jan 21;419(2):285–294. doi: 10.1016/0005-2736(76)90354-0. [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden A. A simple graphical method for determining the inhibition constants of mixed, uncompetitive and non-competitive inhibitors. Biochem J. 1974 Jan;137(1):143–144. doi: 10.1042/bj1370143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devés R., Krupka R. M. A new approach in the kinetics of biological transport. The potential of reversible inhibition studies. Biochim Biophys Acta. 1978 Jun 16;510(1):186–200. doi: 10.1016/0005-2736(78)90140-2. [DOI] [PubMed] [Google Scholar]
- Devés R., Krupka R. M. Cytochalasin B and the kinetics of inhibition of biological transport: a case of asymmetric binding to the glucose carrier. Biochim Biophys Acta. 1978 Jul 4;510(2):339–348. doi: 10.1016/0005-2736(78)90034-2. [DOI] [PubMed] [Google Scholar]
- Duhm J. Inosine permeability and purine nucleoside phosphorylase activity as limiting factors for the synthesis of 2,3-diphosphoglycerate from inosine, pyruvate, and inorganic phosphate in erythrocytes of various mammalian species. Biochim Biophys Acta. 1974 Mar 20;343(1):89–100. doi: 10.1016/0304-4165(74)90241-4. [DOI] [PubMed] [Google Scholar]
- Eilam Y., Bibi O. Distinct properties of uridine transport systems in growing, quiescent and serum-stimulated hamster embryo cells. Biochim Biophys Acta. 1977 May 16;467(1):51–64. doi: 10.1016/0005-2736(77)90241-3. [DOI] [PubMed] [Google Scholar]
- Eilam Y., Cabantchik Z. I. Nucleoside transport in mammalian cell membranes: a specific inhibitory mechanism of high affinity probes. J Cell Physiol. 1977 Aug;92(2):185–201. doi: 10.1002/jcp.1040920207. [DOI] [PubMed] [Google Scholar]
- Ferreira H. G., Lew V. L. Use of ionophore A23187 to measure cytoplasmic Ca buffering and activation of the Ca pump by internal Ca. Nature. 1976 Jan 1;259(5538):47–49. doi: 10.1038/259047a0. [DOI] [PubMed] [Google Scholar]
- Jarvis S. M., Young J. D., Ansay M., Archibald A. L., Harkness R. A., Simmonds R. J. Is inosine the physiological energy source of pig erythrocytes? Biochim Biophys Acta. 1980 Mar 27;597(1):183–188. doi: 10.1016/0005-2736(80)90162-5. [DOI] [PubMed] [Google Scholar]
- Jarvis S. M., Young J. D. Direct evidence for the specific interaction of nitrobenzylthioinosine with functional nucleoside transport sites in sheep erythrocyte membranes [proceedings]. J Physiol. 1978 Nov;284:96P–97P. [PubMed] [Google Scholar]
- Jarvis S. M., Young J. D., Ellory J. C. Nucleoside transport in human erythrocytes. Apparent molecular weight of the nitrobenzylthioinosine-binding complex estimated by radiation-inactivation analysis. Biochem J. 1980 Aug 15;190(2):373–376. doi: 10.1042/bj1900373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis S. M., Young J. D. Extraction and partial purification of the nucleoside-transport system from human erythrocytes based on the assay of nitrobenzylthioinosine-binding activity. Biochem J. 1981 Jan 15;194(1):331–339. doi: 10.1042/bj1940331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis S. M., Young J. D. Genetic control of nucleoside transport in sheep erythrocytes. Biochem Genet. 1978 Oct;16(9-10):1035–1043. doi: 10.1007/BF00483754. [DOI] [PubMed] [Google Scholar]
- Jarvis S. M., Young J. D. Nucleoside translocation in sheep reticulocytes and fetal erythrocytes: a proposed model for the nucleoside transporter. J Physiol. 1982 Mar;324:47–66. doi: 10.1113/jphysiol.1982.sp014100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis S. M., Young J. D. Nucleoside transport in human and sheep erythrocytes. Evidence that nitrobenzylthioinosine binds specifically to functional nucleoside-transport sites. Biochem J. 1980 Aug 15;190(2):377–383. doi: 10.1042/bj1900377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolassa N., Plank B., Turnheim K. pH and temperature dependence of adenosine uptake in human erythrocytes. Eur J Pharmacol. 1978 Dec 1;52(3-4):345–351. doi: 10.1016/0014-2999(78)90288-1. [DOI] [PubMed] [Google Scholar]
- Paterson A. R., Babb L. R., Paran J. H., Cass C. E. Inhibition by nitrobenzylthioinosine of adenosine uptake by asynchronous HeLa cells. Mol Pharmacol. 1977 Nov;13(6):1147–1158. [PubMed] [Google Scholar]
- Paterson A. R., Lau E. Y., Dahlig E., Cass C. E. A common basis for inhibition of nucleoside transport by dipyridamole and nitrobenzylthioinosine? Mol Pharmacol. 1980 Jul;18(1):40–44. [PubMed] [Google Scholar]
- Paterson A. R., Naik S. R., Cass C. E. Inhibition of uridine uptake in HeLa cells by nitrobenzylthioinosine and related compounds. Mol Pharmacol. 1977 Nov;13(6):1014–1023. [PubMed] [Google Scholar]
- Paterson A. R., Oliver J. M. Nucleoside transport. II. Inhibition by p-nitrobenzylthioguanosine and related compounds. Can J Biochem. 1971 Feb;49(2):271–274. doi: 10.1139/o71-039. [DOI] [PubMed] [Google Scholar]
- Paterson A. R., Simpson A. I. Inhibition of ribonucleoside metabolism in Ehrlich ascites tumor cells by purine analogue ribonucleosides. Can J Biochem. 1965 Oct;43(10):1701–1710. doi: 10.1139/o65-188. [DOI] [PubMed] [Google Scholar]
- Paterson A. R., Simpson A. I. Inhibitors of nucleoside metabolism. Cancer Res. 1967 May;27(5 Pt 2):353–363. [PubMed] [Google Scholar]
- Paul B., Chen M. F., Paterson A. R. Inhibitors of nucleoside transport. A structure-activity study using human erythrocytes. J Med Chem. 1975 Oct;18(10):968–973. doi: 10.1021/jm00244a003. [DOI] [PubMed] [Google Scholar]
- Pickard M. A., Brown R. R., Paul B., Paterson A. R. Binding of the nucleoside transport inhibitor 4-nitrobenzylthioinosine to erythrocyte membranes. Can J Biochem. 1973 May;51(5):666–672. doi: 10.1139/o73-083. [DOI] [PubMed] [Google Scholar]
- Pickard M. A., Paterson A. R. Use of 4-nitrobenzylthioinosine in the measurement of rates of nucleoside transport in human erythrocytes. Can J Biochem. 1972 Jul;50(7):839–840. doi: 10.1139/o72-116. [DOI] [PubMed] [Google Scholar]
- Roos H., Pfleger K. Kinetics of adenosine uptake by erythrocytes, and the influence of dipyridamole. Mol Pharmacol. 1972 Jul;8(4):417–425. [PubMed] [Google Scholar]
- Shohami E., Koren R. S-(N-dansylaminoethyl)-6-mercaptoguanosine as a fluorescent probe for the uridine transport system in human erythrocytes. Biochem J. 1979 Feb 15;178(2):271–277. doi: 10.1042/bj1780271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnheim K., Plank B., Kolassa N. Inhibition of adenosine uptake in human erythrocytes by adenosine-5'-carboxamides, xylosyladenine, dipyridamole, hexobendine, and p-nitrobenzylthioguanosine. Biochem Pharmacol. 1978;27(18):2191–2197. doi: 10.1016/0006-2952(78)90076-x. [DOI] [PubMed] [Google Scholar]
- Wohlhueter R. M., Marz R., Plagemann P. G. Properties of the thymidine transport system of Chinese hamster ovary cells as probed by nitrobenzylthioinosine. J Membr Biol. 1978 Sep 19;42(3):247–264. doi: 10.1007/BF01870361. [DOI] [PubMed] [Google Scholar]
- Wohlhueter R. M., Marz R., Plagemann P. G. Thymidine transport in cultured mammalian cells. Kinetic analysis, temperature dependence and specificity of the transport system. Biochim Biophys Acta. 1979 May 17;553(2):262–283. doi: 10.1016/0005-2736(79)90231-1. [DOI] [PubMed] [Google Scholar]
- Young J. D. Nucleoside transport in sheep erythrocytes: genetically controlled transport variation and its influence on erythrocyte ATP concentrations. J Physiol. 1978 Apr;277:325–339. doi: 10.1113/jphysiol.1978.sp012274. [DOI] [PMC free article] [PubMed] [Google Scholar]


