Abstract
1. Nucleoside transport by fetal erythrocytes from nucleoside-permeable and nucleoside-impermeable type new-born lambs and by reticulocytes from adult sheep was compared with that of mature erythrocytes from adult sheep of the two phenotypes.
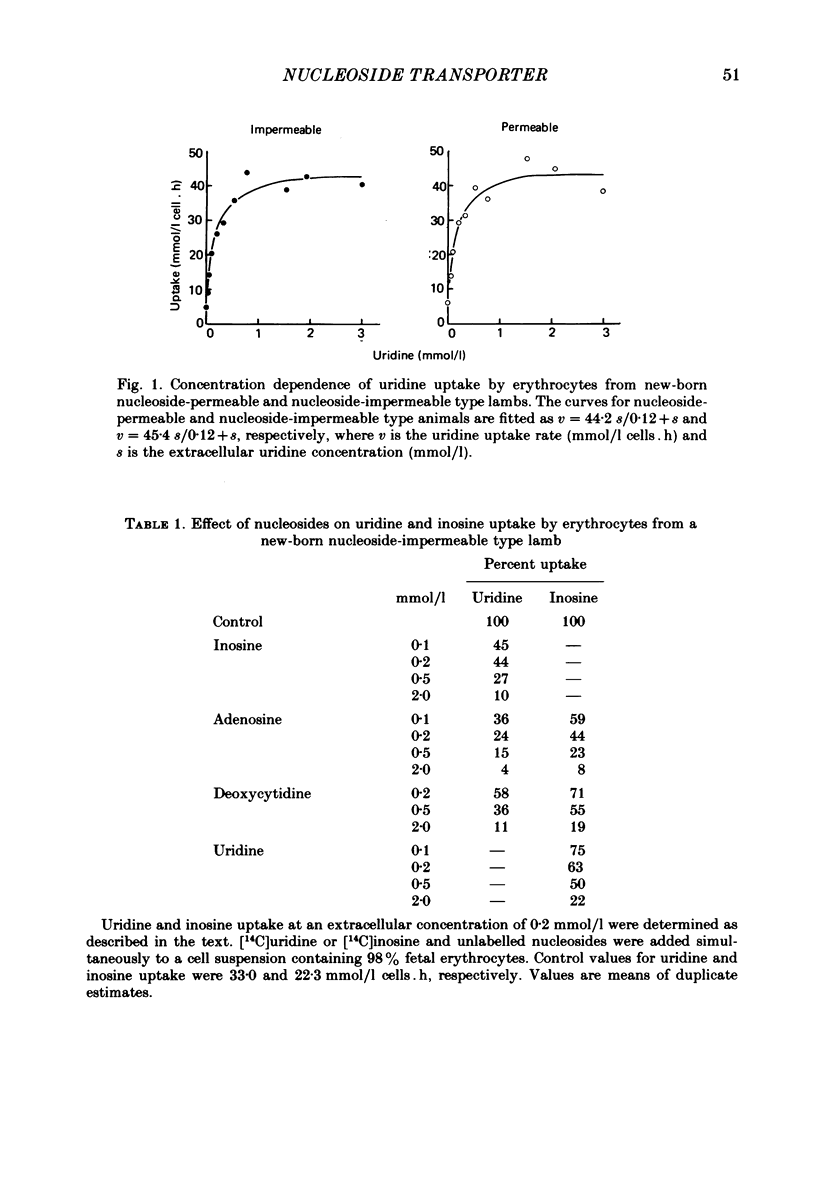
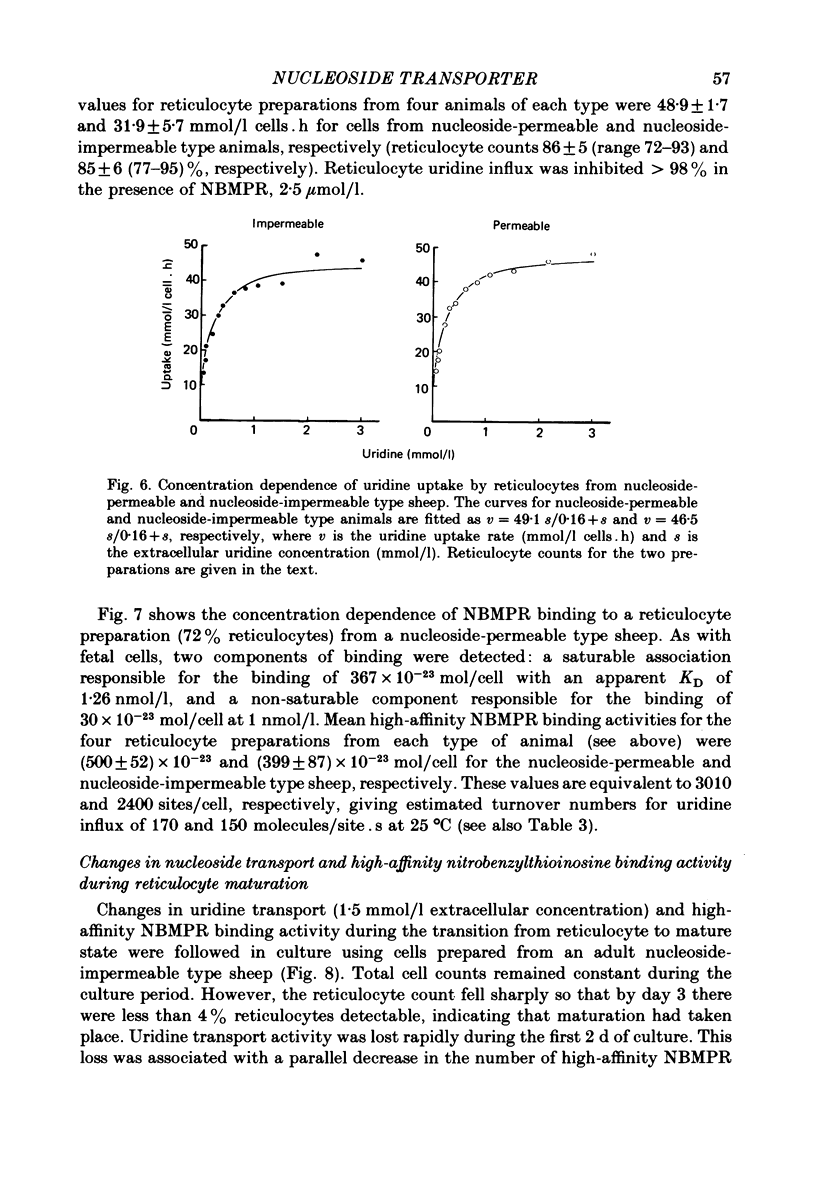
2. Fetal cells and reticulocytes transported [U-14C]uridine rapidly, with little difference between cells from the two types of sheep. Transport occurred by a saturable uptake mechanism with similar properties to that present in mature cells from adult nucleoside-permeable type animals, except for an approximately 100-fold higher Vmax.
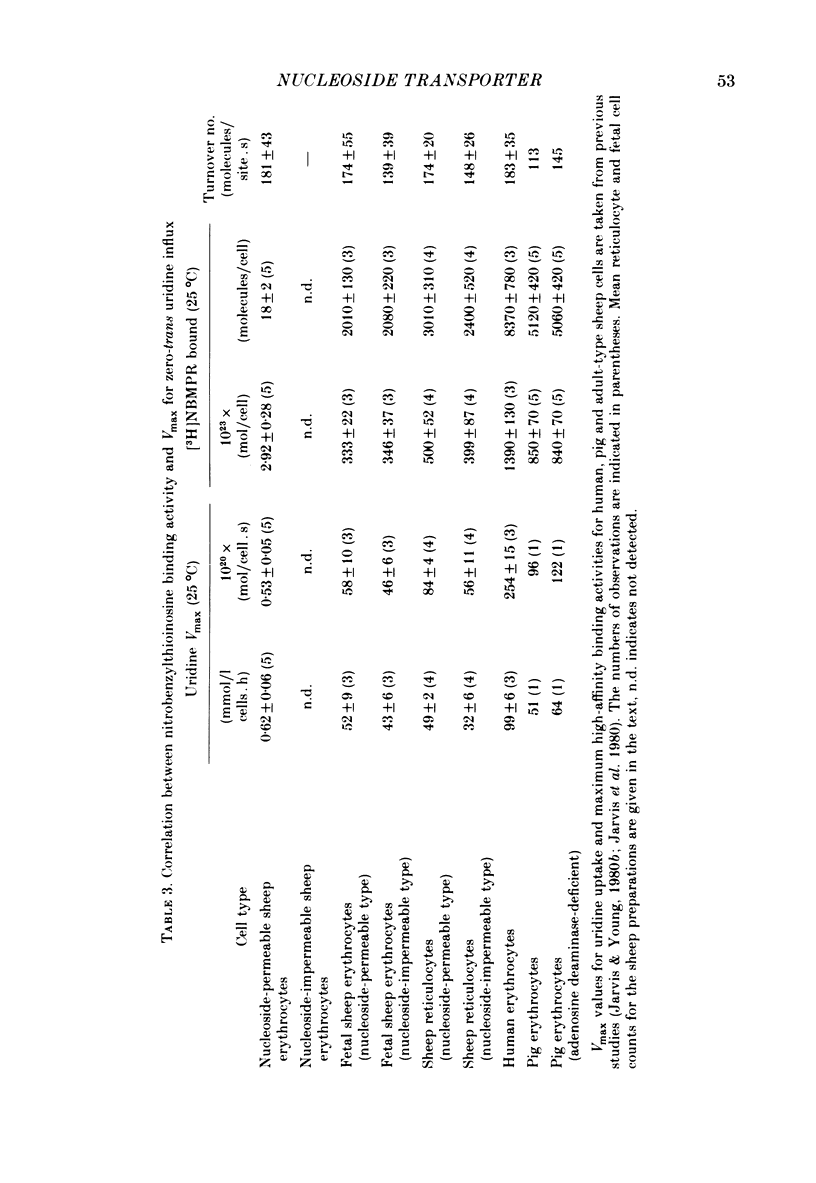
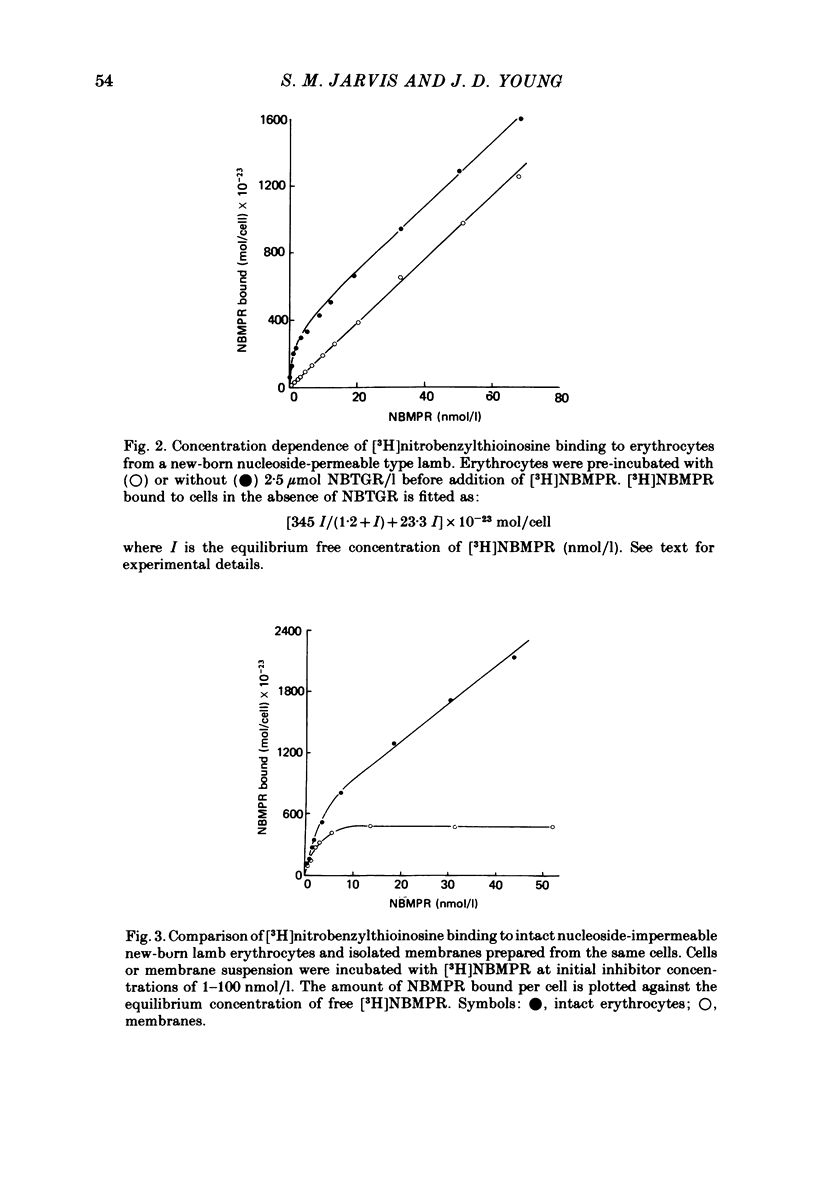
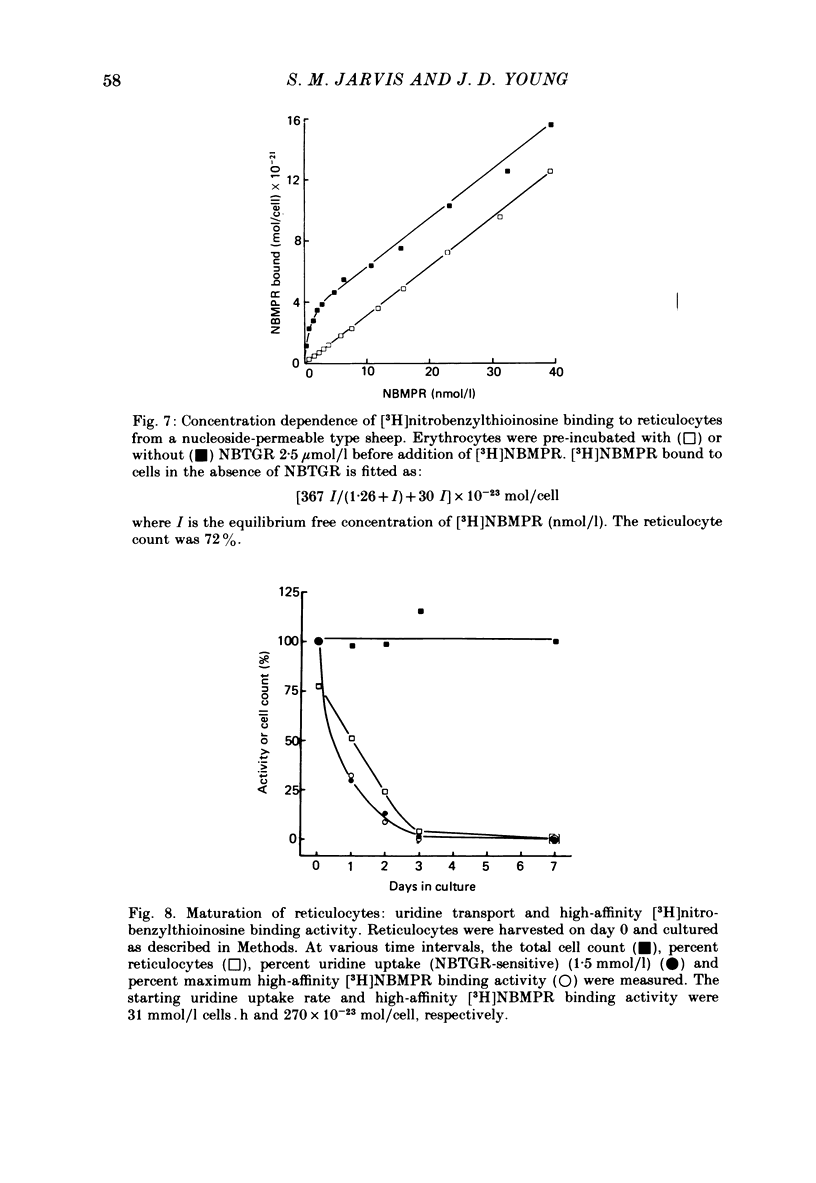
3. This increased translocation capacity was associated with increased numbers of high-affinity [3H]nitrobenzylthioinosine binding sites (∼ 2000-3000 sites/cell compared with approximately 20 sites/cell for mature nucleoside-permeable sheep erythrocytes).
4. The calculated transport capacity for each nucleoside translocation site is therefore similar in all cell types (140-180 molecules/site. s at 25 °C, assuming that each transport site binds a single molecule of inhibitor). These values compare favourably with turnover estimates for the nucleoside transporter from human and pig erythrocytes.
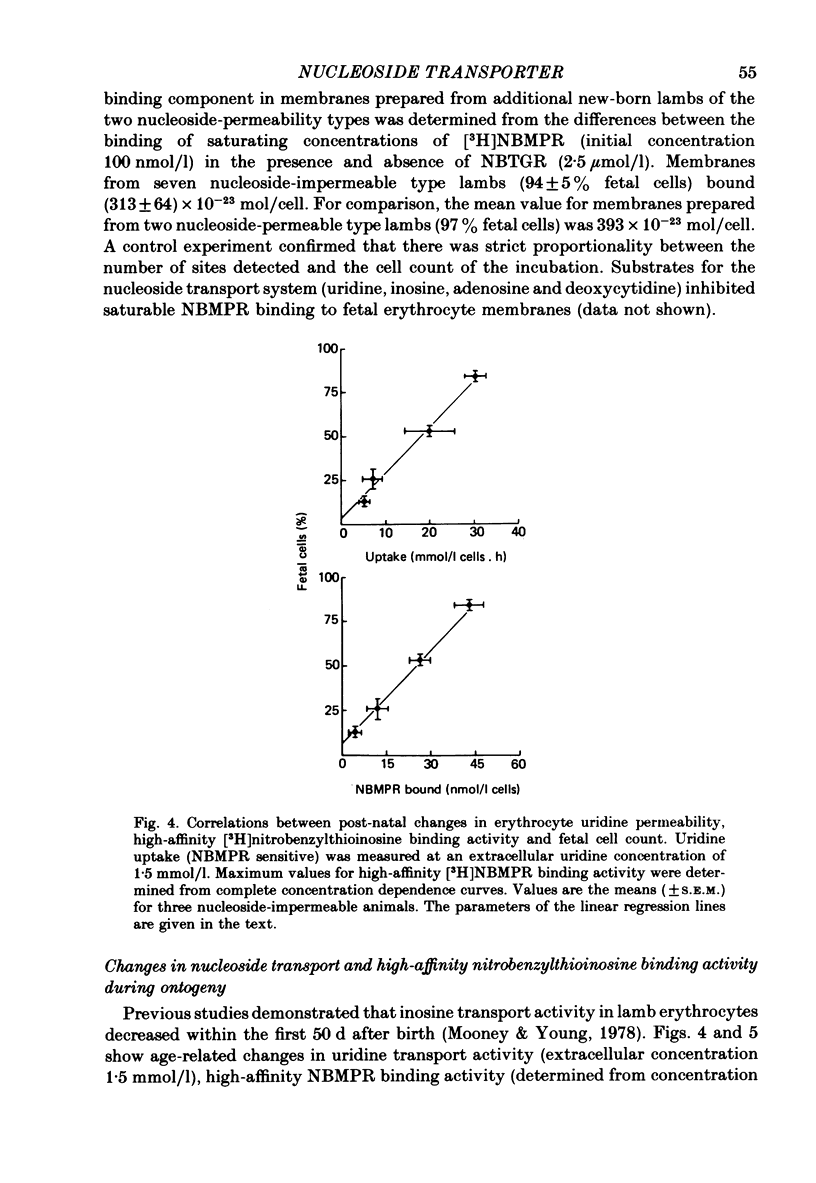
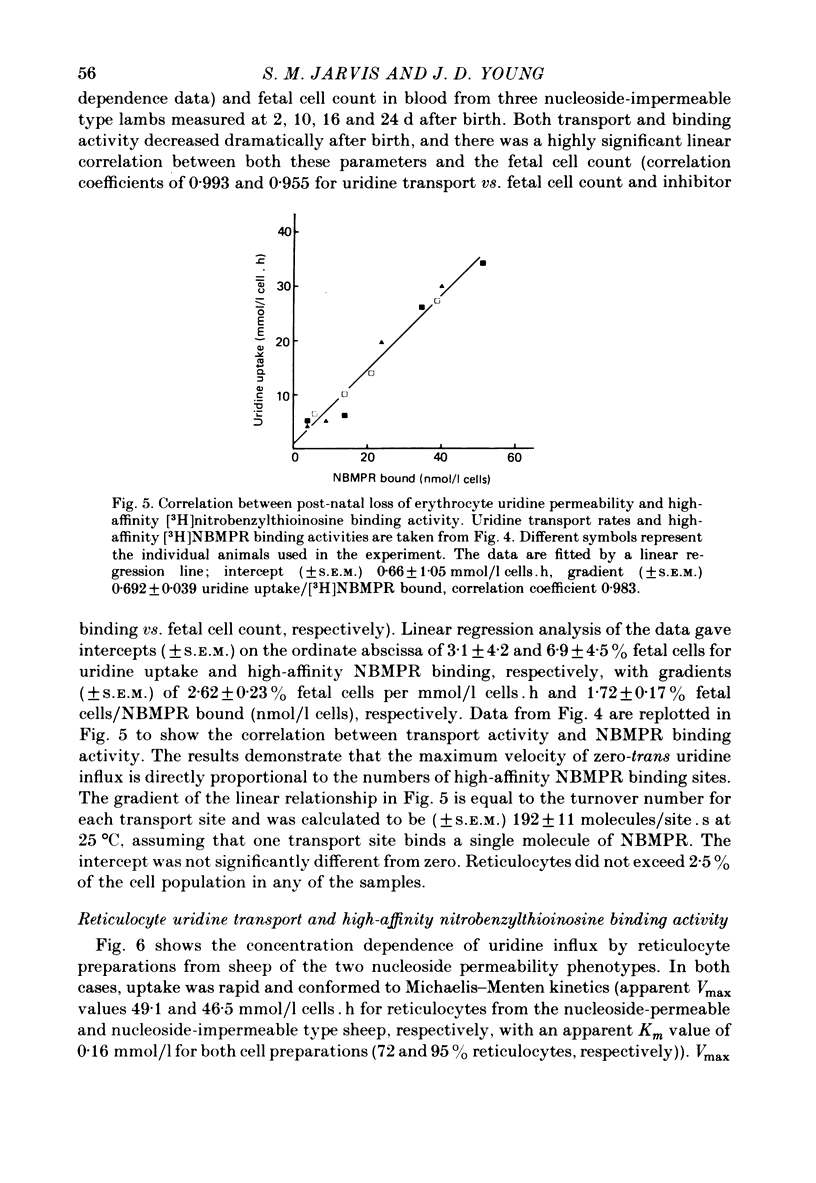
5. Loss of nucleoside transport activity after birth closely paralleled loss of [3H]nitrobenzylthioinosine binding sites and the progressive loss of fetal cells from the circulation. Similarly, reticulocyte maturation in vitro was also associated with rapid loss of both nucleoside transport capacity and inhibitor binding activity.
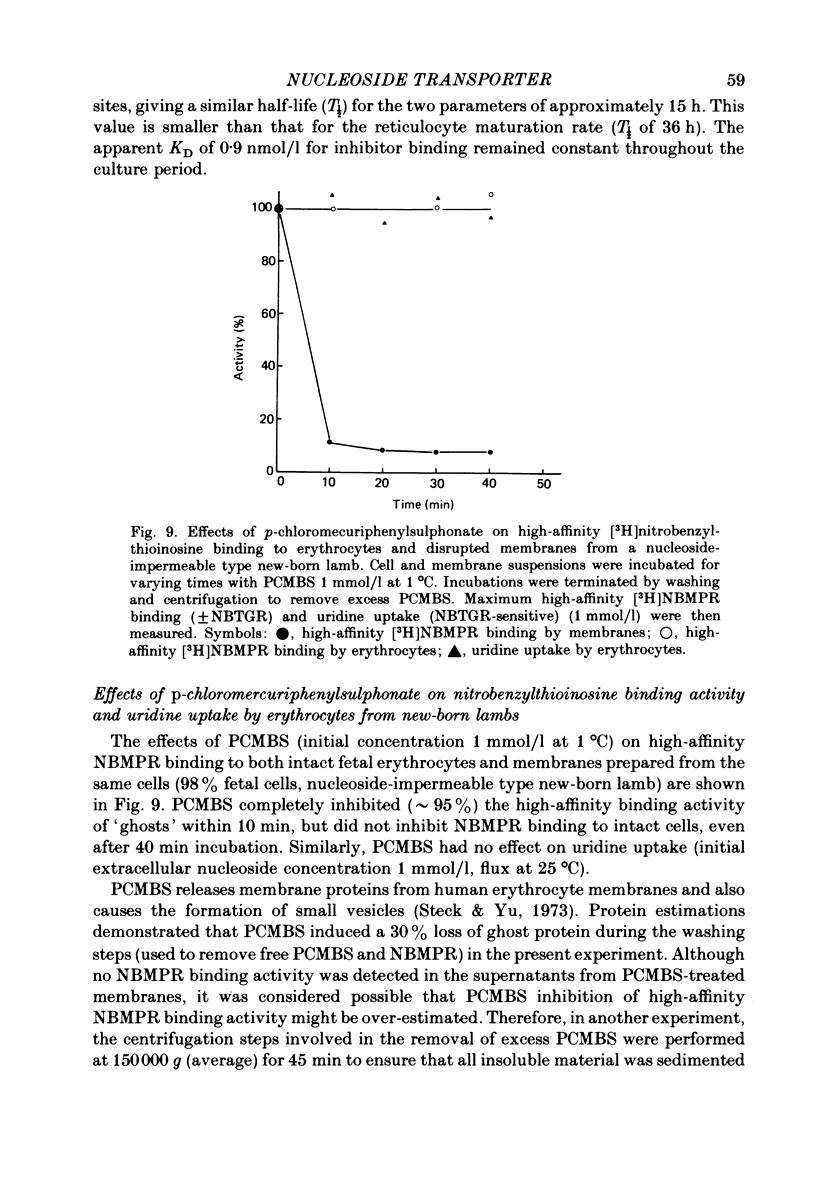
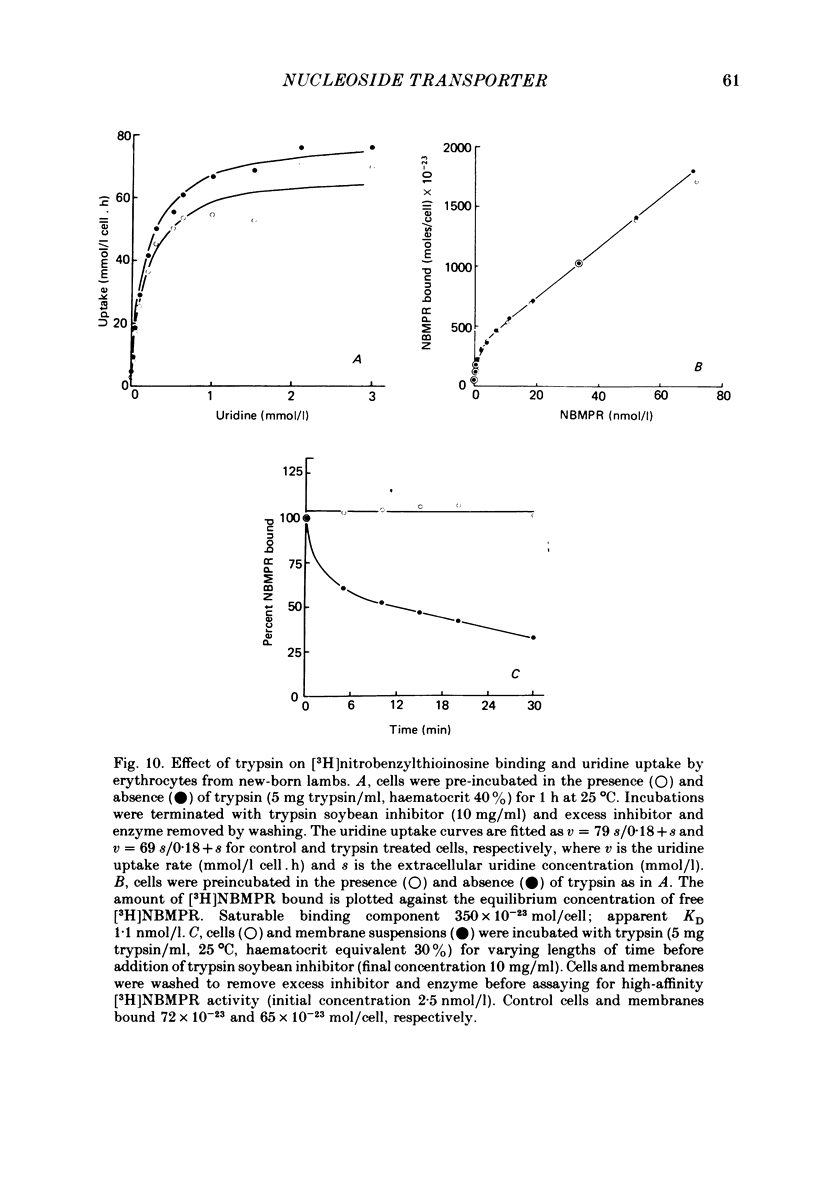
6. p-Chloromercuriphenylsulphonate and trypsin had no effect on [3H]nitrobenzylthioinosine binding to intact fetal cells. In contrast, both agents markedly inhibited binding to isolated `ghosts' where both sides of the cell membrane were accessible to reagent. p-Chloromercuriphenylsulphonate inhibition was markedly reduced in the presence of uridine, and reversed by addition of dithiothreitol.
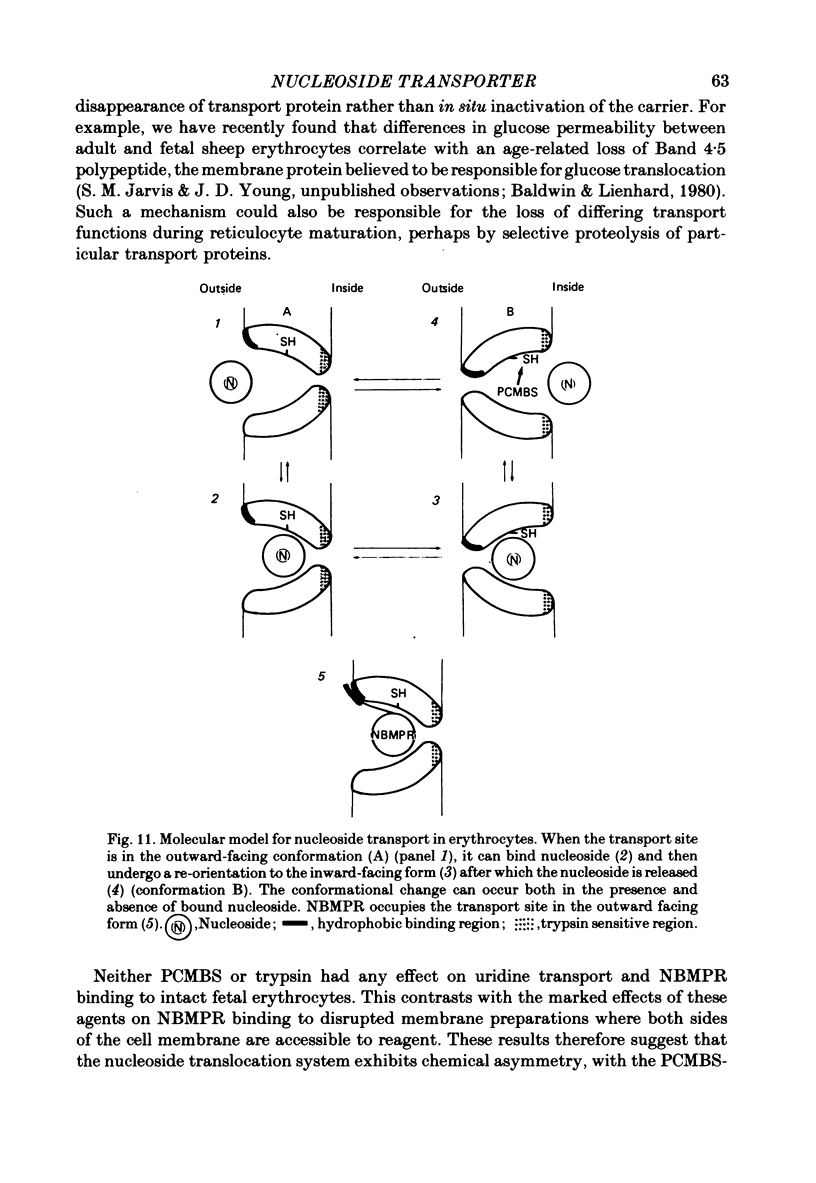
7. We conclude that nucleoside transport changes during ontogeny and reticulocyte maturation in the sheep as well as species differences in nucleoside transport capacity are regulated by variations in the numbers of functional transport sites per cell rather than by changes in the activity of a constant number of sites. It is also likely that the nucleoside carrier exhibits chemical asymmetry.
8. A simple molecular model of the erythrocyte nucleoside transporter consistent with these and other known properties of the carrier is proposed.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin S. A., Lienhard G. E. Immunological identification of the human erythrocyte monosaccharide transporter. Biochem Biophys Res Commun. 1980 Jun 30;94(4):1401–1408. doi: 10.1016/0006-291x(80)90575-6. [DOI] [PubMed] [Google Scholar]
- Cass C. E., Paterson A. R. Mediated transport of nucleosides by human erythrocytes. Specificity toward purine nucleosides as permeants. Biochim Biophys Acta. 1973 Feb 16;291(3):734–746. doi: 10.1016/0005-2736(73)90477-x. [DOI] [PubMed] [Google Scholar]
- Cass C. E., Paterson A. R. Mediated transport of nucleosides in human erythrocytes. Accelerative exchange diffusion of uridine and thymidine and specificity toward pyrimidine nucleosides as permeants. J Biol Chem. 1972 May 25;247(10):3314–3320. [PubMed] [Google Scholar]
- Devés R., Krupka R. M. Cytochalasin B and the kinetics of inhibition of biological transport: a case of asymmetric binding to the glucose carrier. Biochim Biophys Acta. 1978 Jul 4;510(2):339–348. doi: 10.1016/0005-2736(78)90034-2. [DOI] [PubMed] [Google Scholar]
- Ellory J. C., Tucker E. M. Active potassium transport and the development of m antigen on red cells of LK type lambs. J Physiol. 1969 Oct;204(2):101P+–101P+. [PubMed] [Google Scholar]
- Jain S. K., Hochstein P. Membrane alterations in phenylhydrazine-induced reticulocytes. Arch Biochem Biophys. 1980 May;201(2):683–687. doi: 10.1016/0003-9861(80)90560-3. [DOI] [PubMed] [Google Scholar]
- Jain S. K., Subrahmanyam D. Life span of phenylhydrazine-induced reticulocytes in albino rats. Indian J Exp Biol. 1978 Feb;16(2):255–257. [PubMed] [Google Scholar]
- Jarvis S. M., McBride D., Young J. D. Erythrocyte nucleoside transport: asymmetrical binding of nitrobenzylthioinosine to nucleoside permeation sites. J Physiol. 1982 Mar;324:31–46. doi: 10.1113/jphysiol.1982.sp014099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis S. M., Young J. D., Ansay M., Archibald A. L., Harkness R. A., Simmonds R. J. Is inosine the physiological energy source of pig erythrocytes? Biochim Biophys Acta. 1980 Mar 27;597(1):183–188. doi: 10.1016/0005-2736(80)90162-5. [DOI] [PubMed] [Google Scholar]
- Jarvis S. M., Young J. D. Extraction and partial purification of the nucleoside-transport system from human erythrocytes based on the assay of nitrobenzylthioinosine-binding activity. Biochem J. 1981 Jan 15;194(1):331–339. doi: 10.1042/bj1940331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis S. M., Young J. D. Genetic control of nucleoside transport in sheep erythrocytes. Biochem Genet. 1978 Oct;16(9-10):1035–1043. doi: 10.1007/BF00483754. [DOI] [PubMed] [Google Scholar]
- Jarvis S. M., Young J. D. Nucleoside transport in human and sheep erythrocytes. Evidence that nitrobenzylthioinosine binds specifically to functional nucleoside-transport sites. Biochem J. 1980 Aug 15;190(2):377–383. doi: 10.1042/bj1900377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb W. R., Stein W. D. Testing and characterizing the simple carrier. Biochim Biophys Acta. 1974 Dec 10;373(2):178–196. doi: 10.1016/0005-2736(74)90144-8. [DOI] [PubMed] [Google Scholar]
- Mooney N. A., Young J. D. Nucleoside and glucose transport in erythrocytes from new-born lambs. J Physiol. 1978 Nov;284:229–239. doi: 10.1113/jphysiol.1978.sp012538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S. L., Godley W. C., van Vliet G., Lewis J. P., Boyd E., Huisman T. H. The production of hemoglobin C in sheep carrying the gene for hemoglobin A: hematologic aspects. Blood. 1966 Sep;28(3):314–329. [PubMed] [Google Scholar]
- Steck T. L., Yu J. Selective solubilization of proteins from red blood cell membranes by protein perturbants. J Supramol Struct. 1973;1(3):220–232. doi: 10.1002/jss.400010307. [DOI] [PubMed] [Google Scholar]
- Tucker E. M. Genetic variation in the sheep red blood cell. Biol Rev Camb Philos Soc. 1971 Aug;46(3):341–386. doi: 10.1111/j.1469-185x.1971.tb01049.x. [DOI] [PubMed] [Google Scholar]
- Tucker E. M., Kilgour L. An inherited glutathione deficiency and a concomitant reduction in potassium concentration in sheep red cells. Experientia. 1970;26(2):203–204. doi: 10.1007/BF01895584. [DOI] [PubMed] [Google Scholar]
- Tucker E. M., Kilgour L. The effect of anaemia on sheep with inherited differences in red cell reduced glutathione (GSH) concentrations. Res Vet Sci. 1973 May;14(3):306–311. [PubMed] [Google Scholar]
- Tucker E. M., Young J. D. Biochemical changes during reticulocyte maturation in culture. A comparison of genetically different sheep erythrocytes. Biochem J. 1980 Oct 15;192(1):33–39. doi: 10.1042/bj1920033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker E. M., Young J. D., Crowley C. Red cell glutathione deficiency: clinical and biochemical investigations using sheep as an experimental model system. Br J Haematol. 1981 Jul;48(3):403–415. doi: 10.1111/j.1365-2141.1981.tb02732.x. [DOI] [PubMed] [Google Scholar]
- Wood W. G. Haemoglobin synthesis during human fetal development. Br Med Bull. 1976 Sep;32(3):282–287. doi: 10.1093/oxfordjournals.bmb.a071376. [DOI] [PubMed] [Google Scholar]
- Young J. D., Ellory J. C. Substrate specificity of amino acid transport in sheep erythrocytes. Biochem J. 1977 Jan 15;162(1):33–38. doi: 10.1042/bj1620033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D., Ellory J. C., Tucker E. M. Amino acid transport in normal and glutathione-deficient sheep erythrocytes. Biochem J. 1976 Jan 15;154(1):43–48. doi: 10.1042/bj1540043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D. Nucleoside transport in sheep erythrocytes: genetically controlled transport variation and its influence on erythrocyte ATP concentrations. J Physiol. 1978 Apr;277:325–339. doi: 10.1113/jphysiol.1978.sp012274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D., Tucker E. M., Ellory J. C. Amino acid transport properties of erythrocytes from normal newborn lambs and lambs with an inherited defect in amino acid transport. Biochim Biophys Acta. 1978 Aug 17;511(3):513–516. doi: 10.1016/0005-2736(78)90286-9. [DOI] [PubMed] [Google Scholar]



