Abstract
1. A voltage clamp study was made of the K+ permeability increases produced in certain identifiable neurones of the cerebral ganglion of Aplysia by any one of three distinct agonists (carbachol, histamine and dopamine).
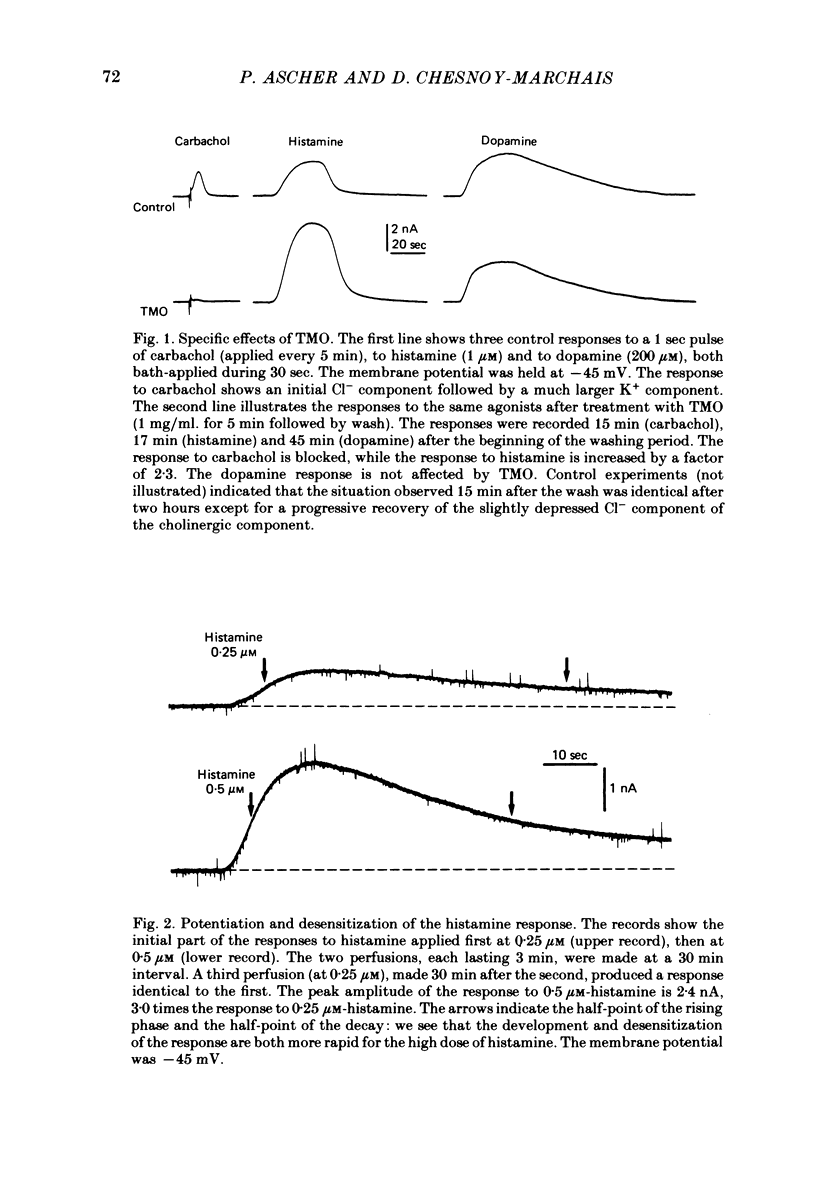
2. The three K+ responses involve three distinct receptors, as shown by the selective effects of reversible antagonists (Gruol & Weinreich, 1979a) as well as by the differential, irreversible effects of trimethyloxonium (TMO) ions.
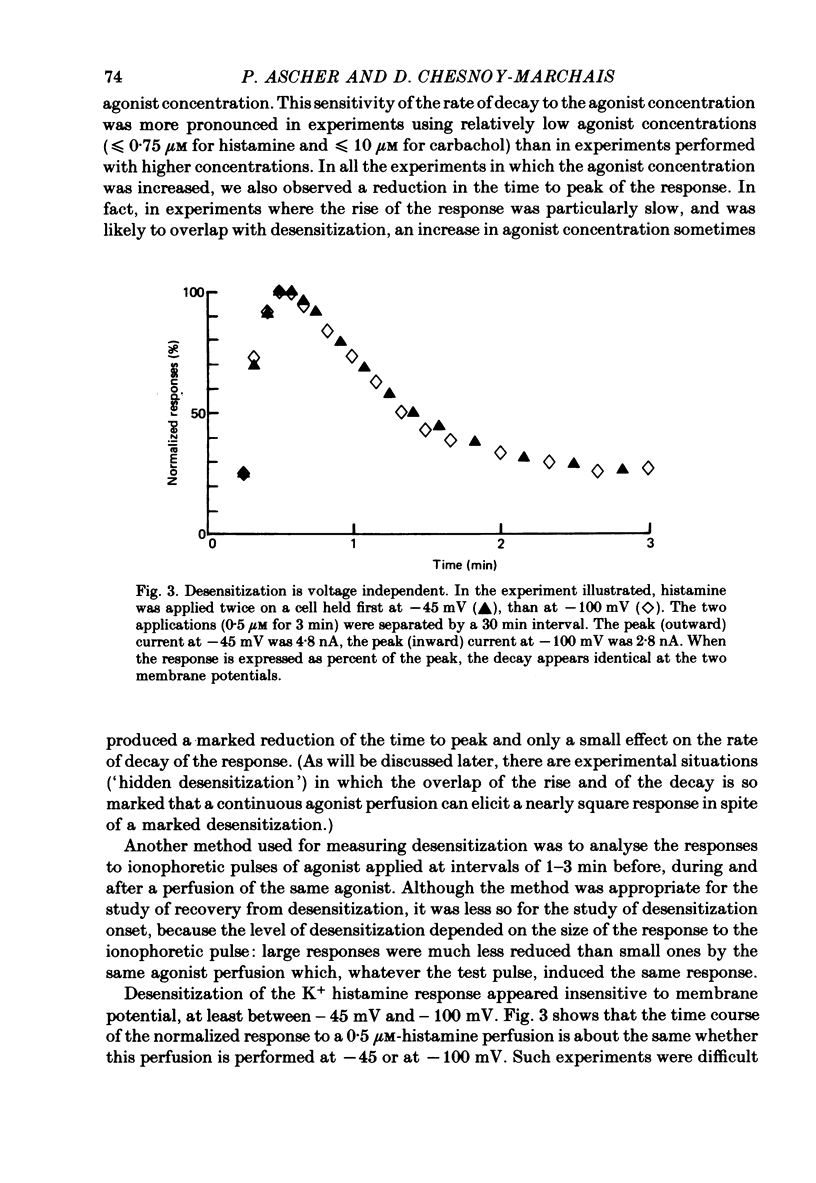
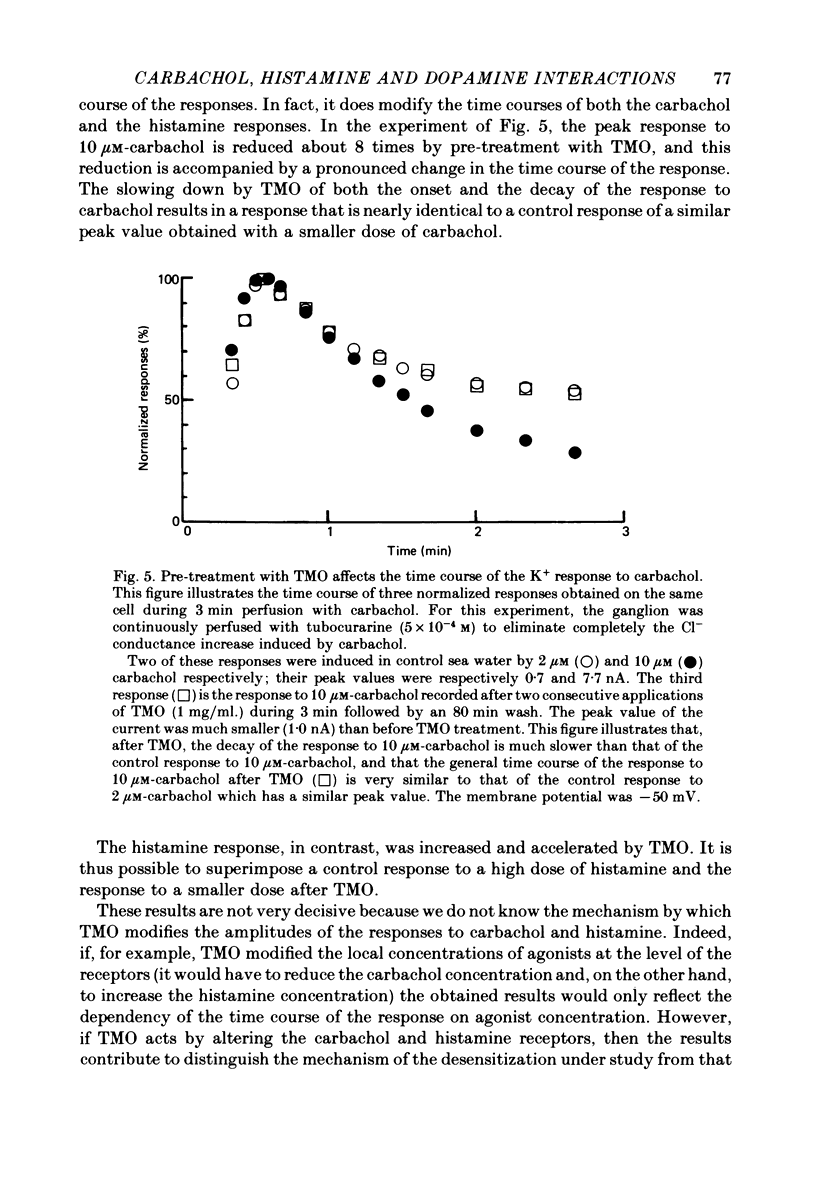
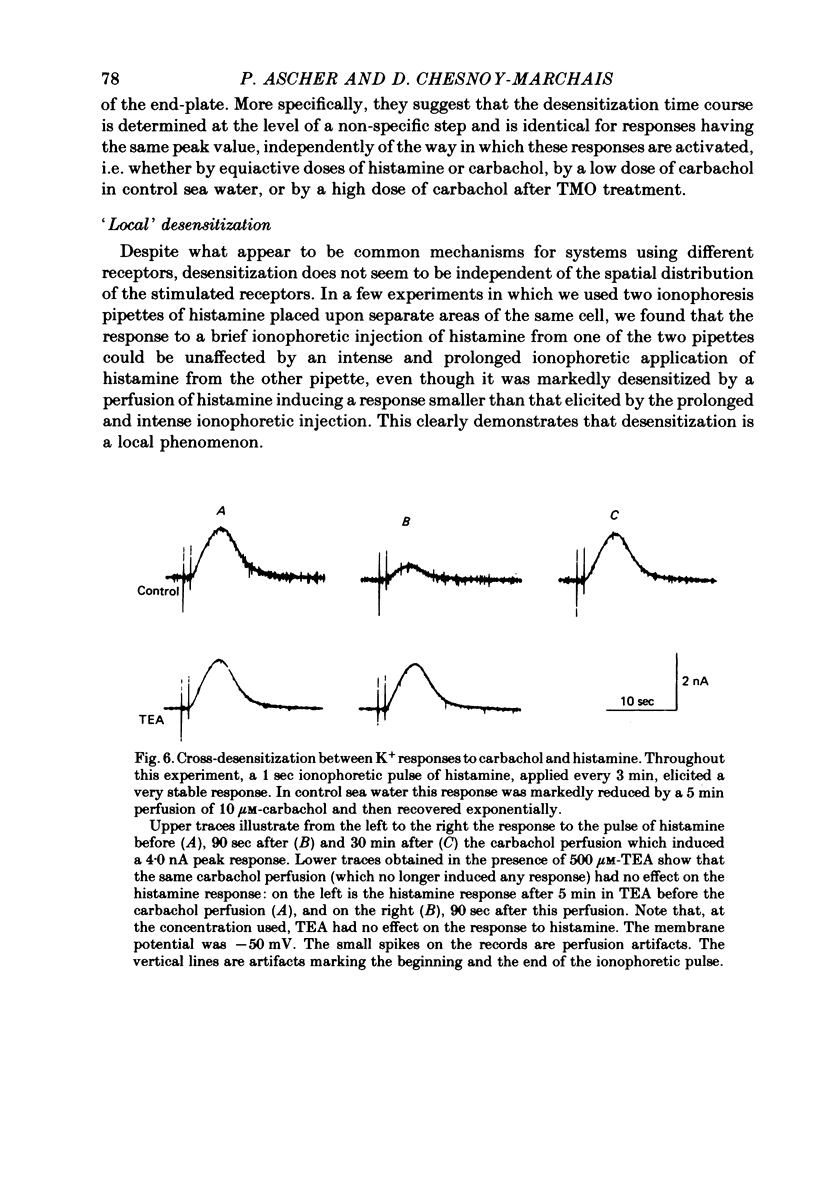
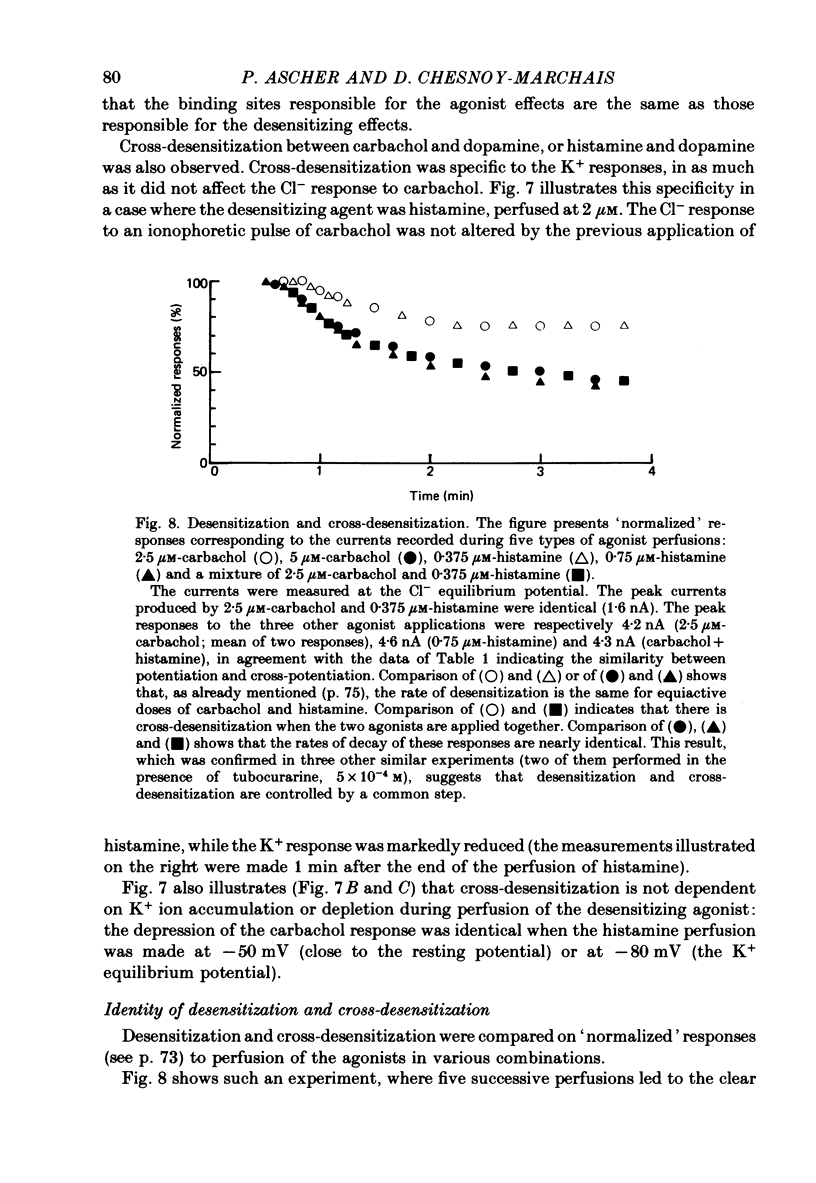
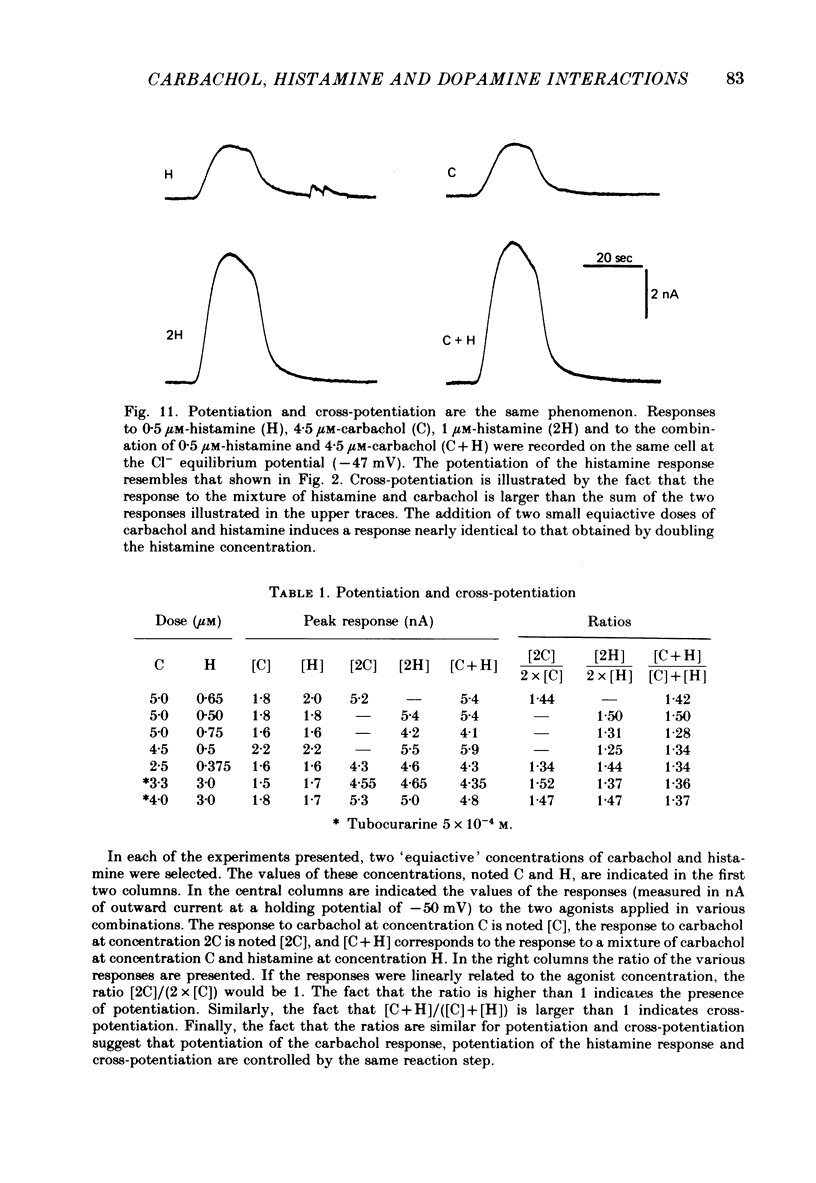
3. Prolonged exposure of the neurones to one of the agonists reduces the response to the same agonist (desensitization) and, over the low concentration range, doubling the concentration of an agonist leads to supra-linear summation (potentiation).
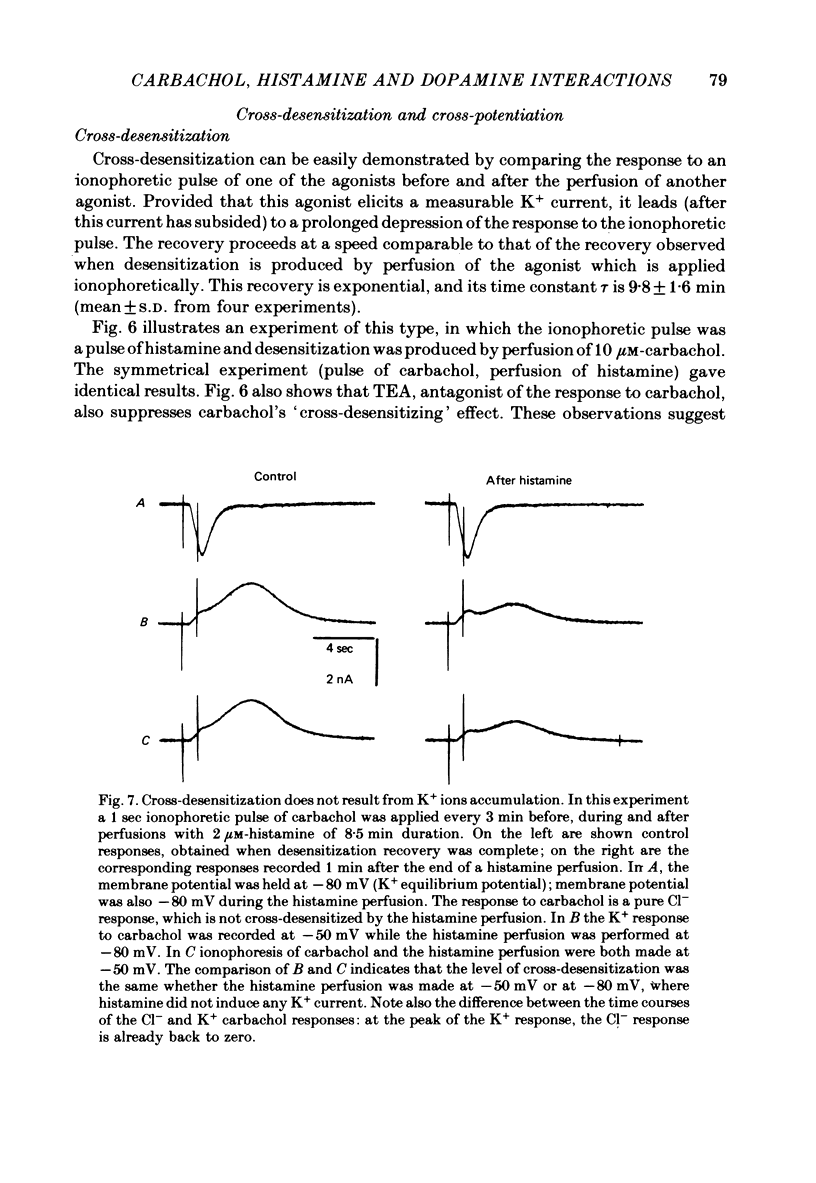
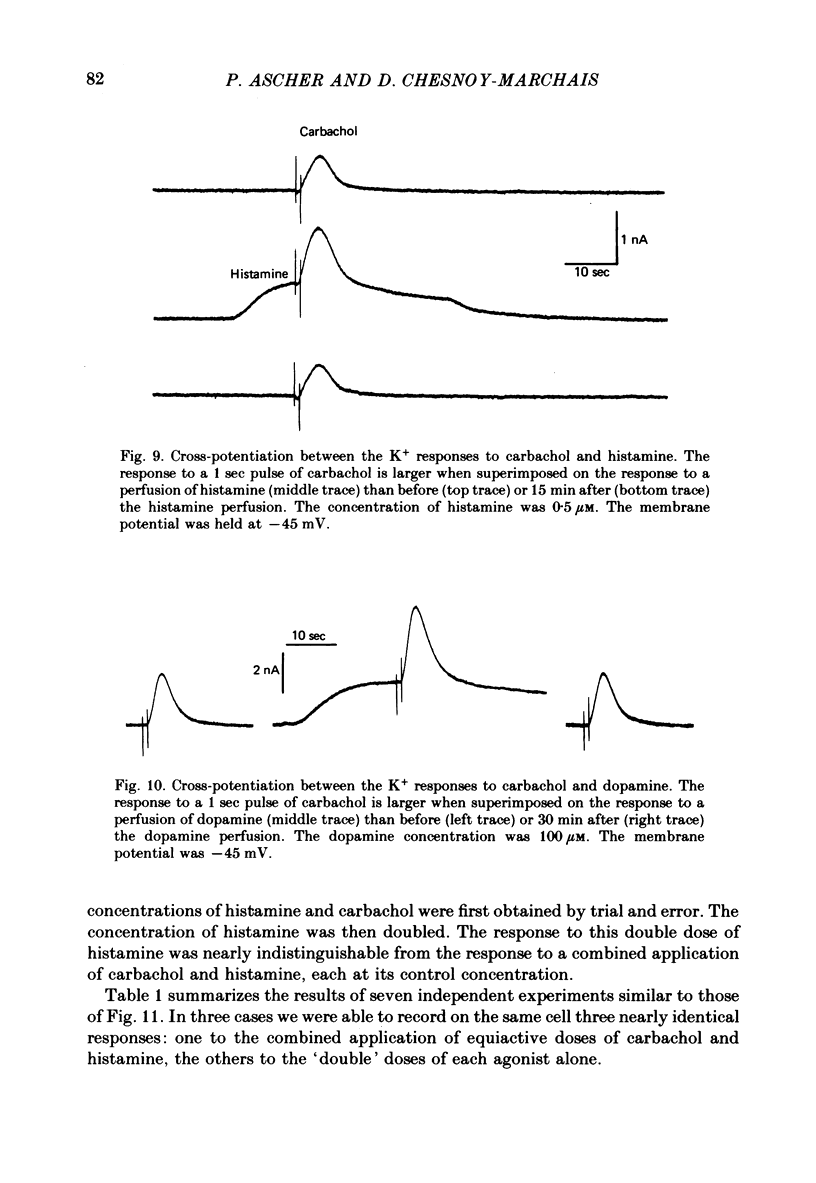
4. Prolonged exposure of the neurones to one of the agonists also reduces the response to the other agonists (cross-desensitization) and combined application of two agonists reveals cross-potentiation.
5. The time course of desensitization (onset and decay) was the same for the histamine and carbachol responses and, except at very high concentration, was indistinguishable from that of cross-desensitization. Likewise, potentiation was similar in the two agonist systems and did not differ significantly from cross-potentiation.
6. The results can be interpreted by assuming that the responses to the three agonists involve specific steps followed by common reaction steps, and that some of the common reaction steps control both potentiation and desensitization.
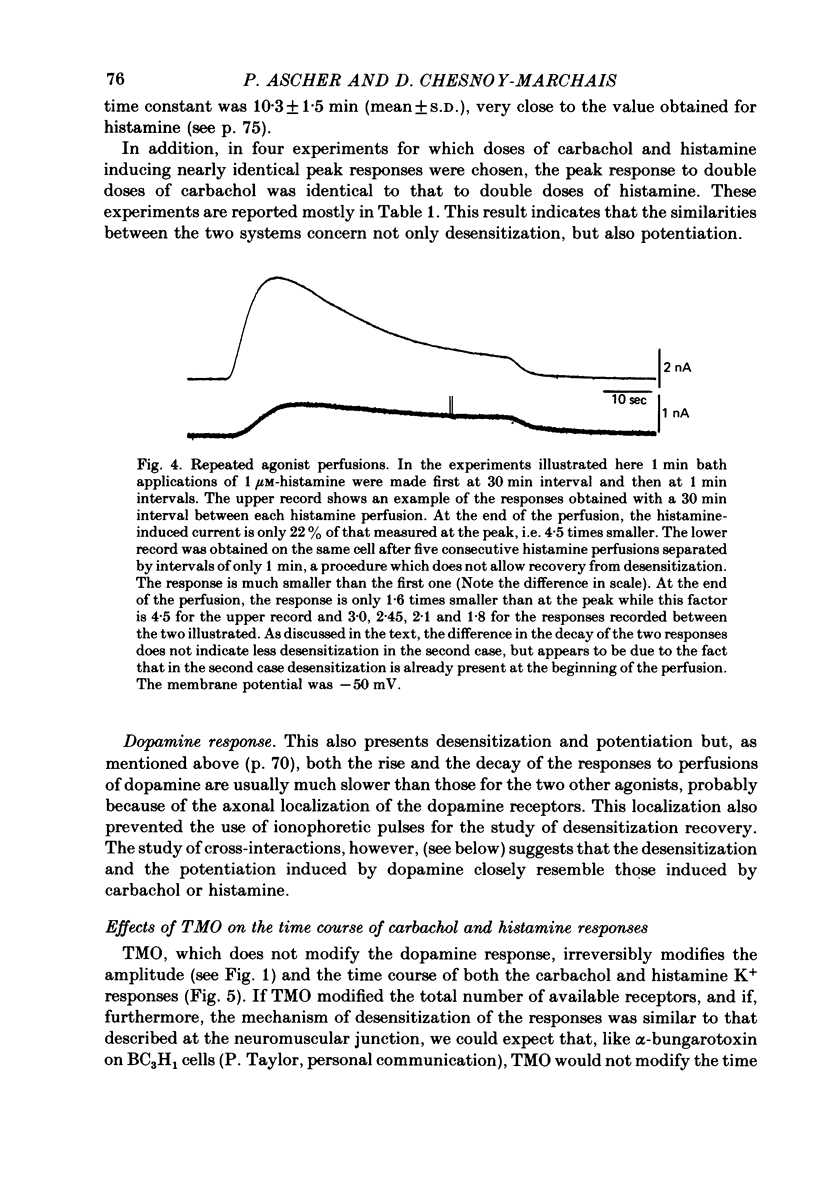
7. The responses to carbachol and histamine differ in their voltage sensitivity. This suggests that one or more of the specific steps are voltage-sensitive.
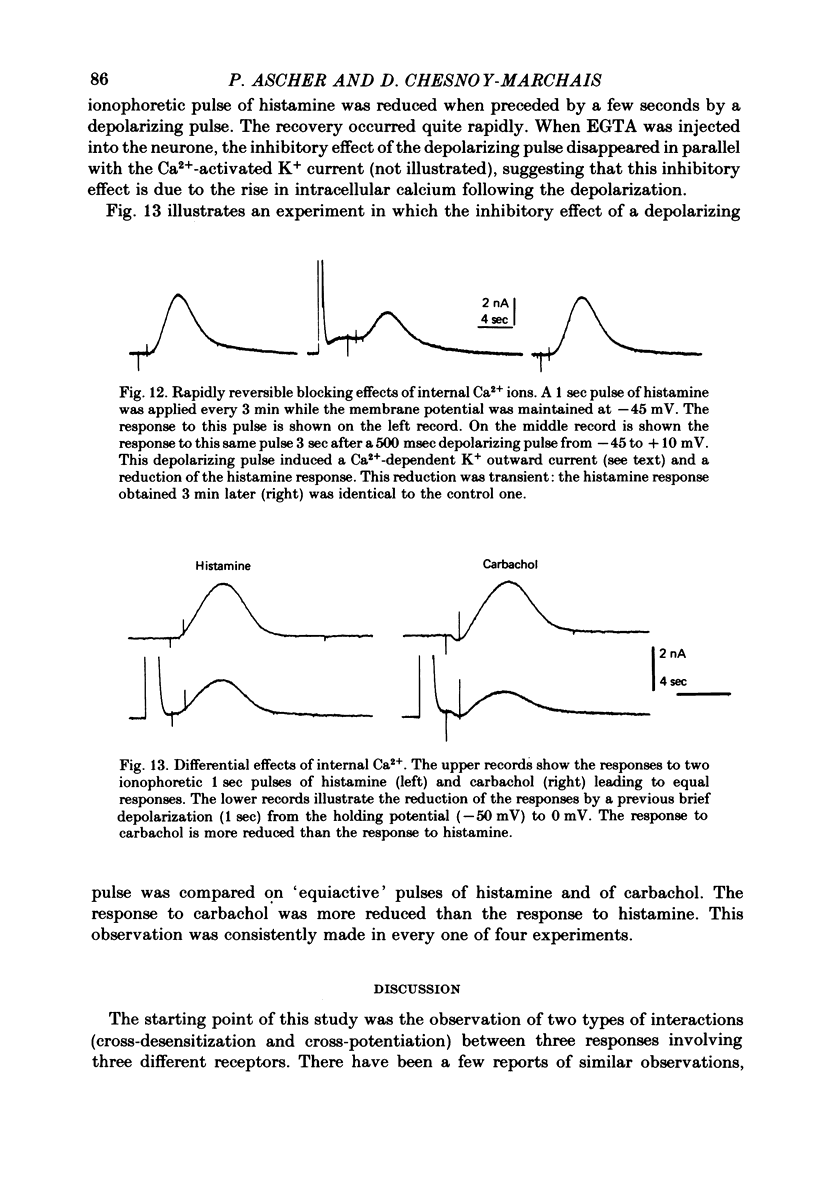
8. Although an increase of the intracellular Ca2+ concentration can itself open K+ channels, and also inhibit the responses to the three agonists, an increase of internal Ca2+ does not appear to play an important role either in the development of the response or in the desensitization process.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascher P. Inhibitory and excitatory effects of dopamine on Aplysia neurones. J Physiol. 1972 Aug;225(1):173–209. doi: 10.1113/jphysiol.1972.sp009933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., McBurney R. N. GABA and glycine may share the same conductance channel on cultured mammalian neurones. Nature. 1979 Jan 18;277(5693):234–236. doi: 10.1038/277234a0. [DOI] [PubMed] [Google Scholar]
- Brown J. H., Rietow M. Muscarinic-dopaminergic synergism on retinal cyclic AMP formation. Brain Res. 1981 Jun 29;215(1-2):388–392. doi: 10.1016/0006-8993(81)90522-9. [DOI] [PubMed] [Google Scholar]
- Chao Y., Vandlen R. L., Raftery M. A. Preferential chemical modification of a binding subsite on the acetylcholine receptor. Biochem Biophys Res Commun. 1975 Mar 3;63(1):300–307. doi: 10.1016/s0006-291x(75)80043-x. [DOI] [PubMed] [Google Scholar]
- Chemeris N. K., Kazachenko V. N., Kislov A. N., Kurchikov A. L. Inhibition of acetylcholine responses by intracellular calcium in Lymnaea stagnalis neurones. J Physiol. 1982 Feb;323:1–19. doi: 10.1113/jphysiol.1982.sp014058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltz A., Trautmann A. Interaction between nerve-related acetylcholine and bath applied agonists at the frog end-plate. J Physiol. 1980 Feb;299:533–552. doi: 10.1113/jphysiol.1980.sp013141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsborg B. L., House C. R., Mitchell M. R. On the role of calcium in the electrical responses of cockroach salivary gland cells to dopamine. J Physiol. 1980 Jun;303:325–335. doi: 10.1113/jphysiol.1980.sp013288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsborg B. L., House C. R. Stimulus-response coupling in gland cells. Annu Rev Biophys Bioeng. 1980;9:55–80. doi: 10.1146/annurev.bb.09.060180.000415. [DOI] [PubMed] [Google Scholar]
- Ginsborg B. L., Kado R. T. Voltage-current relationship of a carbachol-induced potassium-ion pathway in Aplysia neurones. J Physiol. 1975 Mar;245(3):713–725. doi: 10.1113/jphysiol.1975.sp010870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruol D. L., Weinreich D. Cooperative interactions of histamine and competitive antagonism by cimetidine at neuronal histamine receptors in the marine mollusc, Aplysia californica. Neuropharmacology. 1979 Apr;18(4):415–421. doi: 10.1016/0028-3908(79)90151-5. [DOI] [PubMed] [Google Scholar]
- Gruol D. L., Weinreich D. Two pharmacologically distinct histamine receptors mediating membrane hyperpolarization on identified neurons of Aplysia californica. Brain Res. 1979 Feb 23;162(2):281–301. doi: 10.1016/0006-8993(79)90290-7. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C. Mechanisms of slow postsynaptic potentials. Nature. 1981 Jun 18;291(5816):539–544. doi: 10.1038/291539a0. [DOI] [PubMed] [Google Scholar]
- House C. R., Smith R. K. On the receptors involved in the nervous control of salivary secretion by Nauphoeta cinerea Olivier. J Physiol. 1978 Jun;279:457–471. doi: 10.1113/jphysiol.1978.sp012356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan-Parwar B., Fredman S. M. Cerebral ganglion of Aplysia: cellular organization and origin of nerves. Comp Biochem Physiol A Comp Physiol. 1976;54(3):347–357. doi: 10.1016/s0300-9629(76)80124-7. [DOI] [PubMed] [Google Scholar]
- Johnson G. L., Wolfe B. B., Harden T. K., Molinoff P. B., Perkins J. P. Role of beta-adrenergic receptors in catecholamine-induced desensitization of adenylate cyclase in human astrocytoma cells. J Biol Chem. 1978 Mar 10;253(5):1472–1480. [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. S., Marty A. Certain slow synaptic responses: their properties and possible underlying mechanisms. Annu Rev Biophys Bioeng. 1980;9:437–465. doi: 10.1146/annurev.bb.09.060180.002253. [DOI] [PubMed] [Google Scholar]
- Kehoe J. Ionic mechanisms of a two-component cholinergic inhibition in Aplysia neurones. J Physiol. 1972 Aug;225(1):85–114. doi: 10.1113/jphysiol.1972.sp009930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. Pharmacological characteristics and ionic bases of a 2 component postsynaptic inhibition. Nature. 1967 Sep 30;215(5109):1503–1505. doi: 10.1038/2151503b0. [DOI] [PubMed] [Google Scholar]
- Kehoe J. Three acetylcholine receptors in Aplysia neurones. J Physiol. 1972 Aug;225(1):115–146. doi: 10.1113/jphysiol.1972.sp009931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A., Ascher P. Slow relaxations of acetylcholine-induced potassium currents in Aplysia neurones. Nature. 1978 Aug 3;274(5670):494–497. doi: 10.1038/274494a0. [DOI] [PubMed] [Google Scholar]
- Marty A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature. 1981 Jun 11;291(5815):497–500. doi: 10.1038/291497a0. [DOI] [PubMed] [Google Scholar]
- Meech R. W. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng. 1978;7:1–18. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- Nistri A., Constanti A. Pharmacological characterization of different types of GABA and glutamate receptors in vertebrates and invertebrates. Prog Neurobiol. 1979;13(2):117–235. doi: 10.1016/0301-0082(79)90016-9. [DOI] [PubMed] [Google Scholar]
- Osterrieder W., Yang Q. F., Trautwein W. The time course of the muscarinic response to ionophoretic acetylcholine application to the S-A node of the rabbit heart. Pflugers Arch. 1981 Mar;389(3):283–291. doi: 10.1007/BF00584791. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Muscarinic, alpha-adrenergic and peptide receptors regulate the same calcium influx sites in the parotid gland. J Physiol. 1977 Jun;268(1):139–149. doi: 10.1113/jphysiol.1977.sp011851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney J. W., Jr Role of calcium in the fade of the potassium release response in the rat parotid gland. J Physiol. 1978 Aug;281:383–394. doi: 10.1113/jphysiol.1978.sp012429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney J. W., Jr Stimulus-permeability coupling: role of calcium in the receptor regulation of membrane permeability. Pharmacol Rev. 1978 Jun;30(2):209–245. [PubMed] [Google Scholar]
- Soll A. H. The interaction of histamine with gastrin and carbamylcholine on oxygen uptake by isolated mammalian parietal cells. J Clin Invest. 1978 Feb;61(2):381–389. doi: 10.1172/JCI108948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding B. C. Properties of toxin-resistant sodium channels produced by chemical modification in frog skeletal muscle. J Physiol. 1980 Aug;305:485–500. doi: 10.1113/jphysiol.1980.sp013377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y. F., Cubeddu L., Perkins J. P. Regulation of adenosine 3':5'-monophosphate content of human astrocytoma cells: desensitization to catecholamines and prostaglandins. J Cyclic Nucleotide Res. 1976 Jul-Aug;2(4):257–270. [PubMed] [Google Scholar]
- Tougui Z., Do Khac L., Harbon S. Modulation of cyclic AMP content of the rat myometrium: desensitization to isoproterenol, PGE2 and prostacyclin. Mol Cell Endocrinol. 1980 Oct;20(1):17–34. doi: 10.1016/0303-7207(80)90091-x. [DOI] [PubMed] [Google Scholar]
- Weinreich D., McCaman M. W., McCaman R. E., Vaughn J. E. Chemical, enzymatic and ultrastructural characterization of 5-hydroxytryptamine-containing neurons from the ganglia of Aplysia californica and Tritionia diomedia. J Neurochem. 1973 Apr;20(4):969–976. doi: 10.1111/j.1471-4159.1973.tb00067.x. [DOI] [PubMed] [Google Scholar]


