Abstract
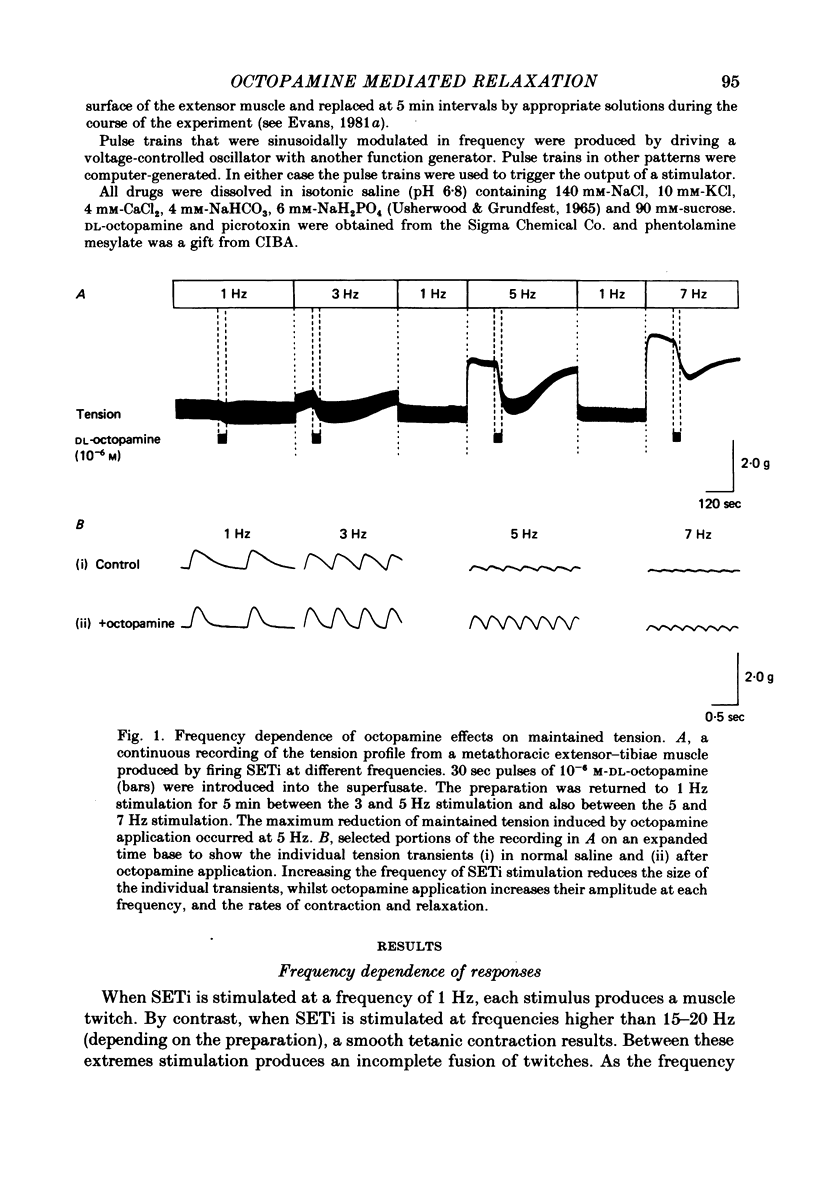
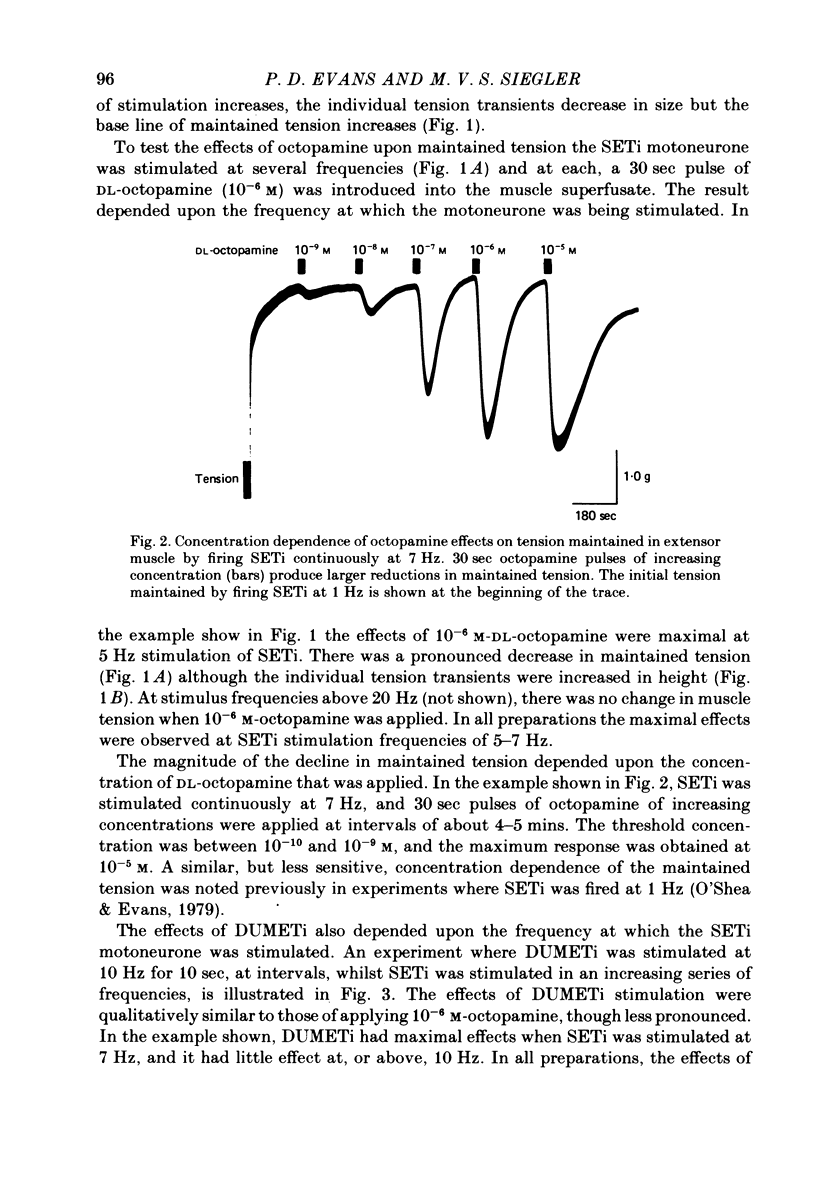
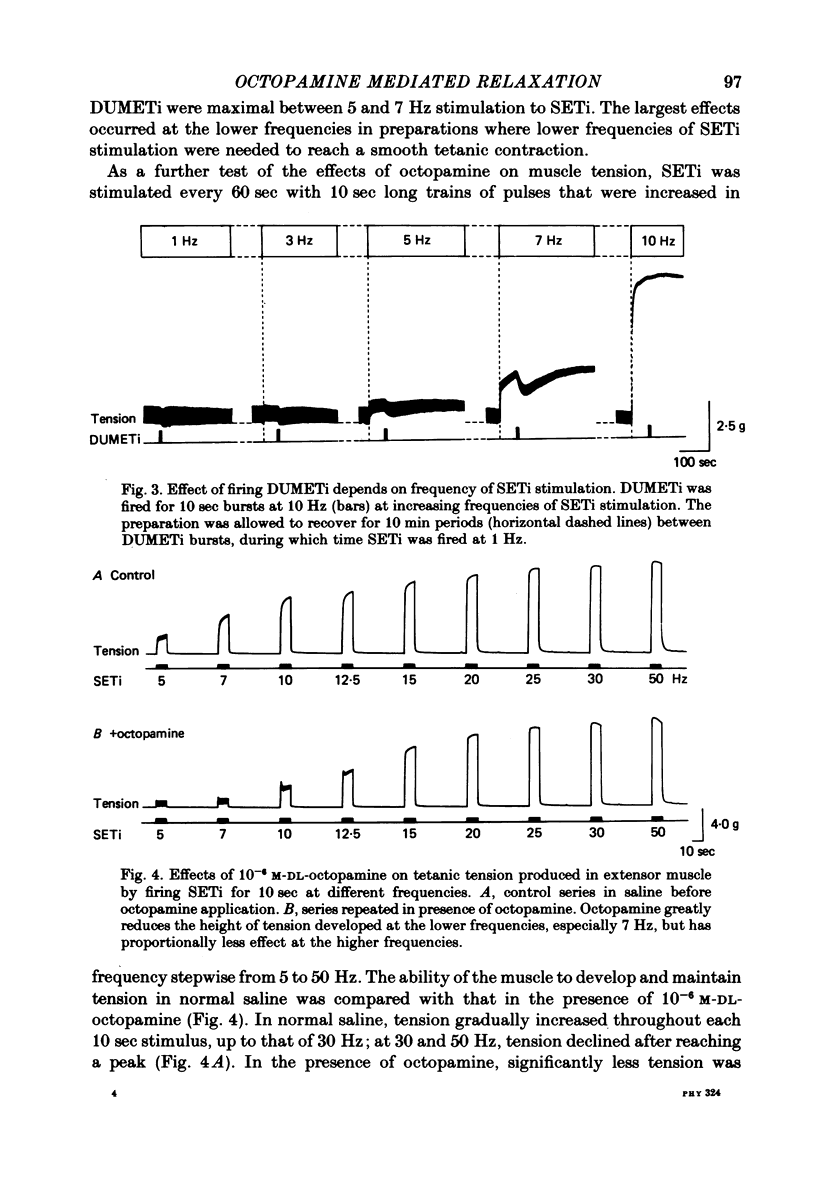
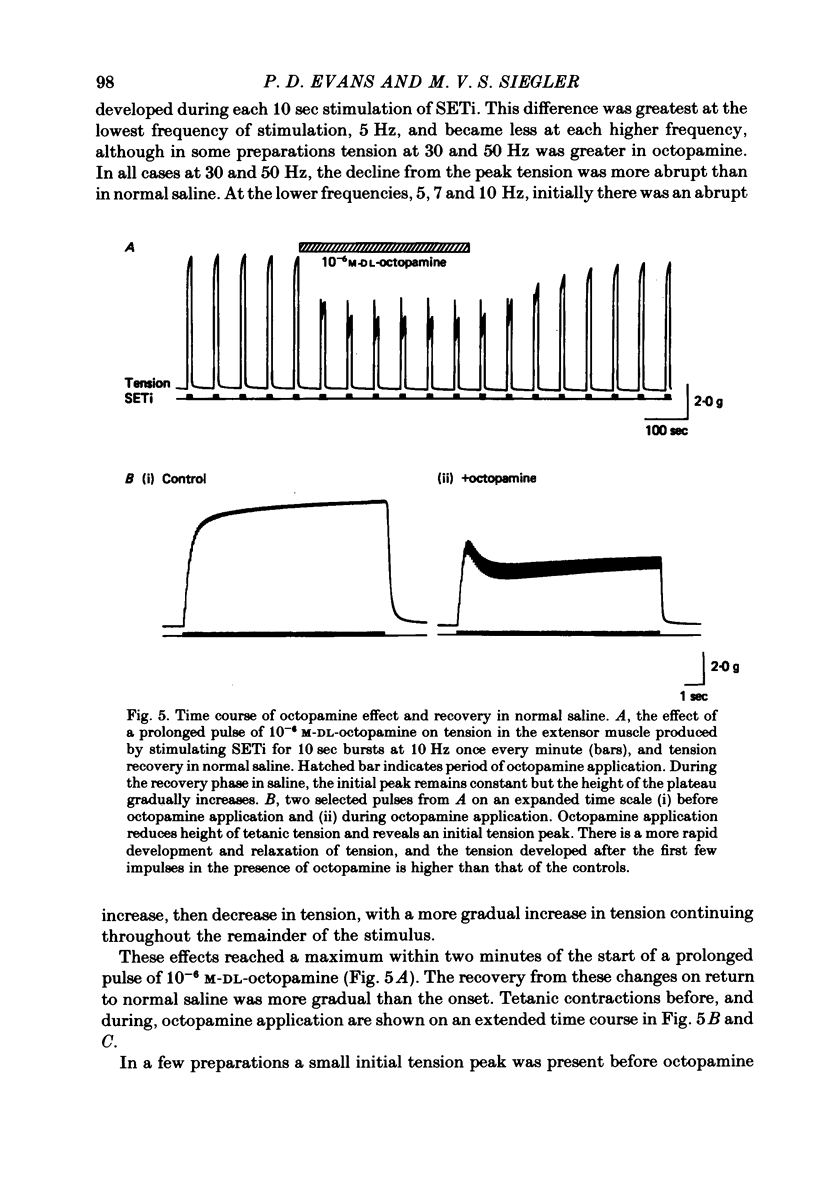
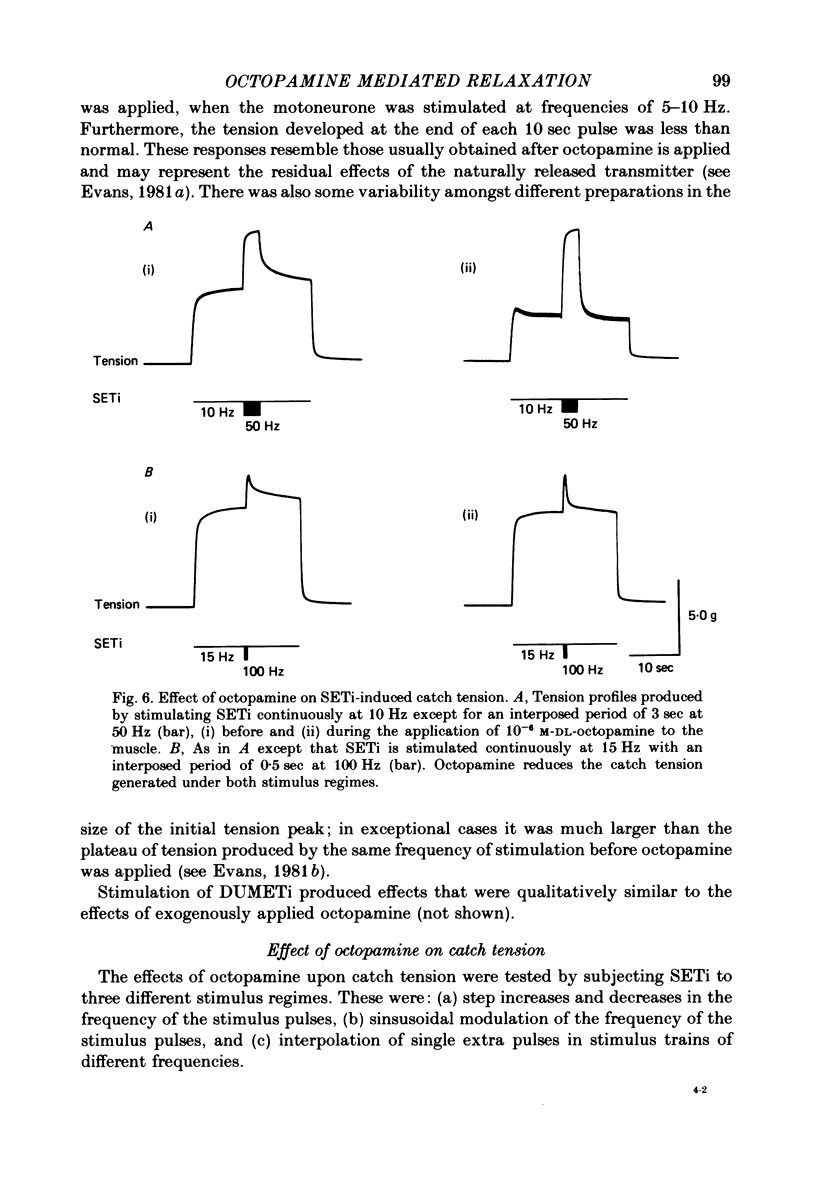
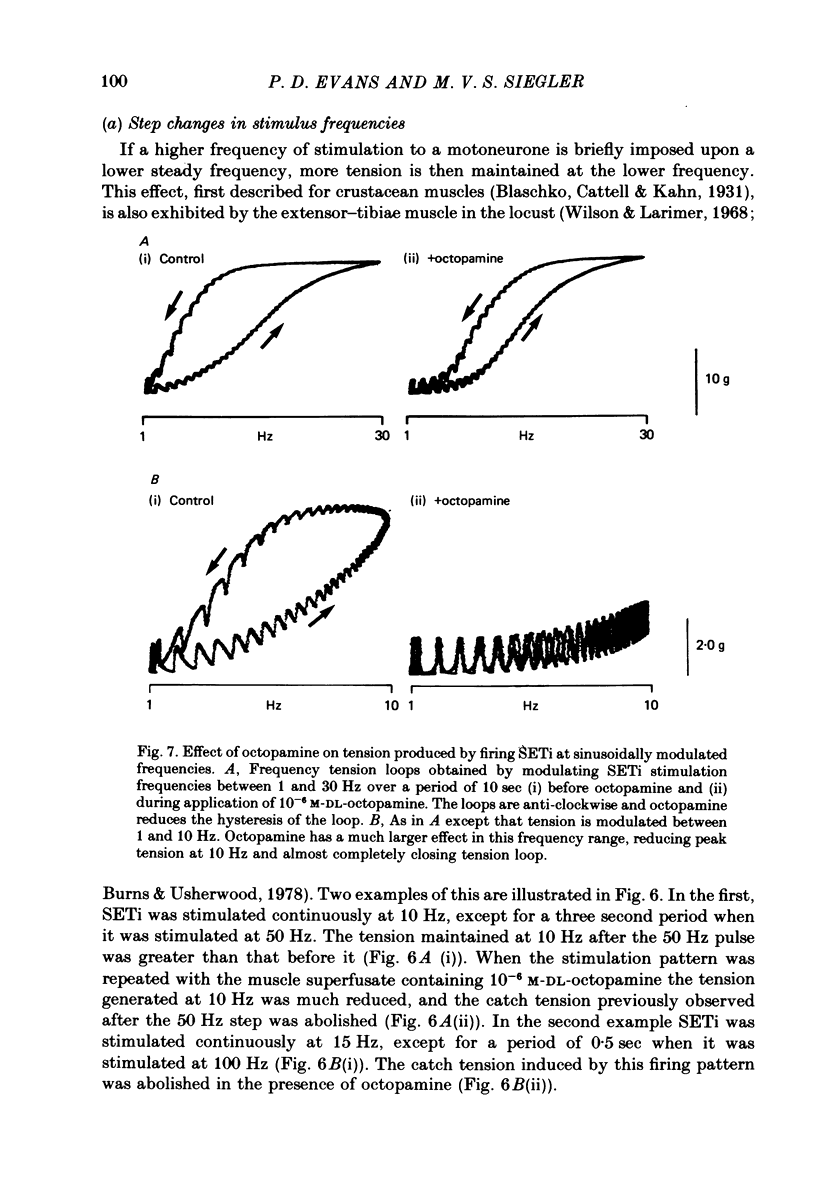
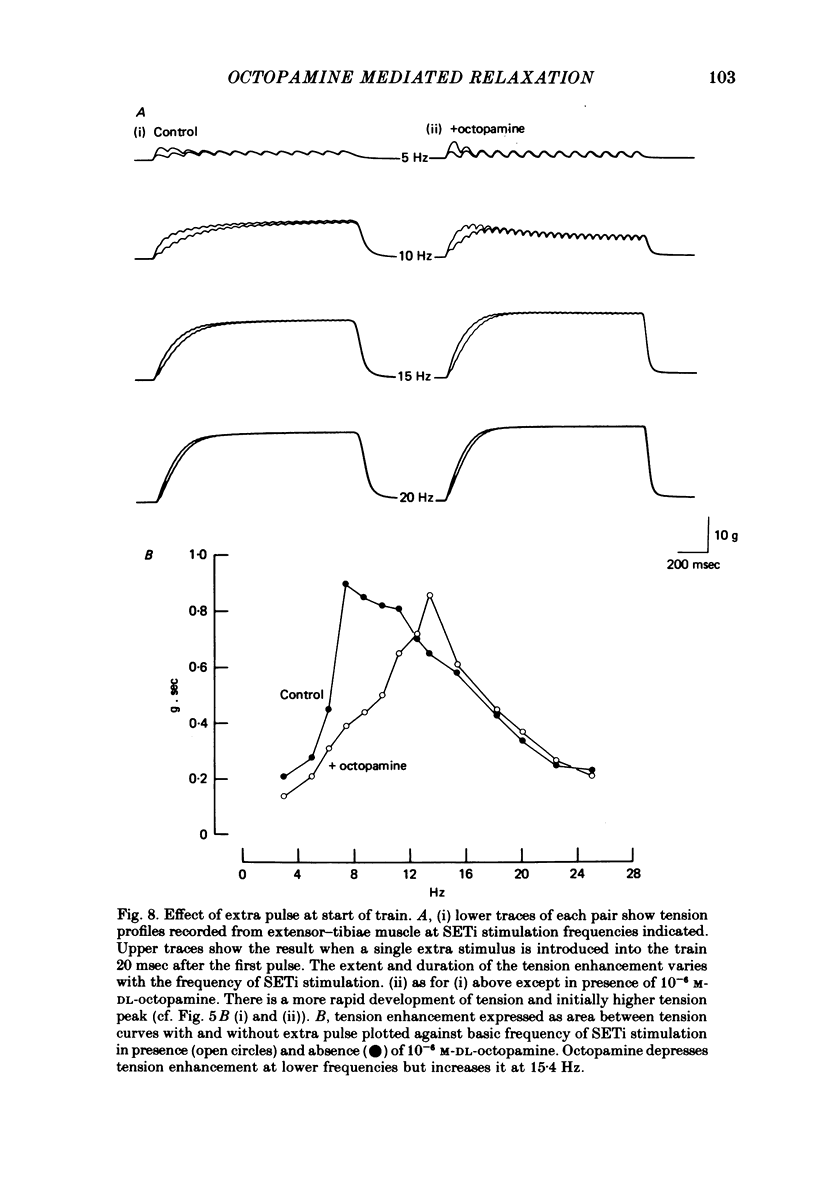
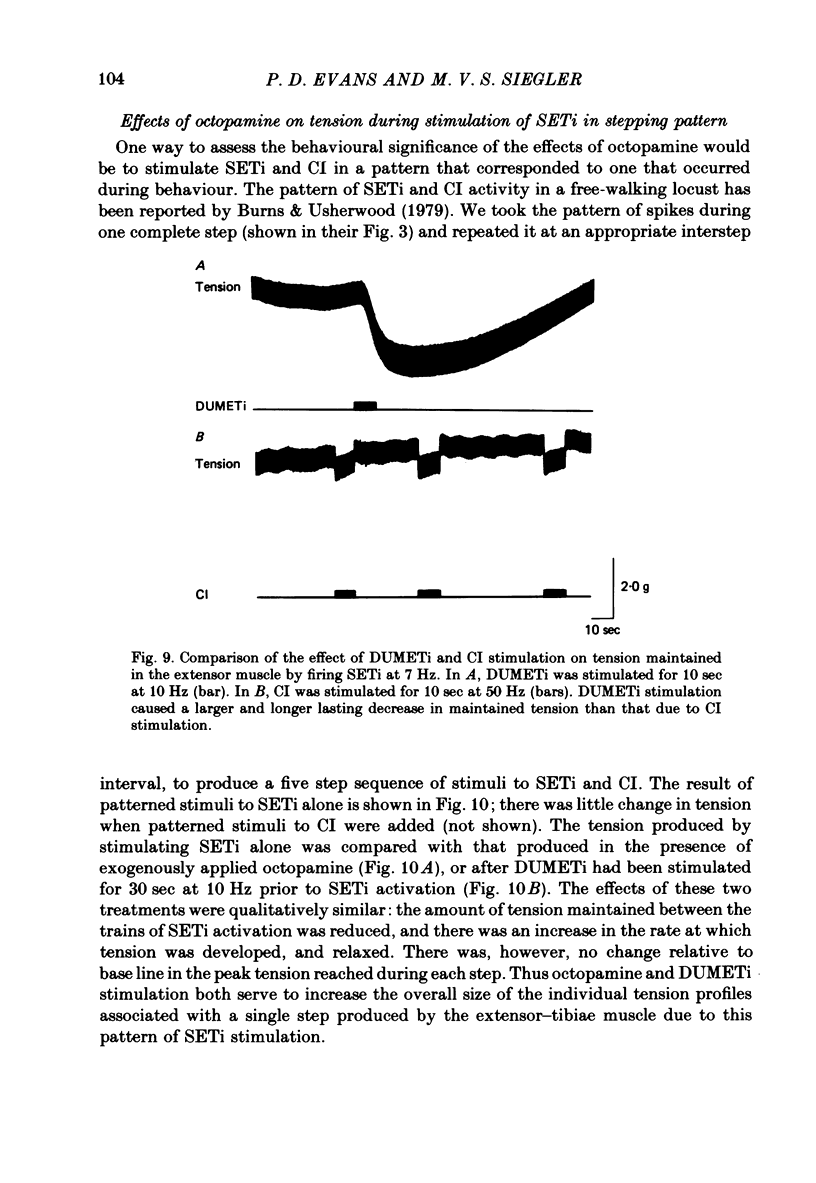
1. The modulatory actions of an identified octopaminergic neurone (DUMETi) that projects to the extensor-tibiae muscle of the locust hind leg depend upon the frequency of stimulation of the slow motoneurone (SETi) to this muscle. 2. At low frequencies of SETi stimulation (1Hz and below) the predominant modulatory effects are increases in the amplitude and relaxation rate of twitch tension. At higher frequencies, where twitches summate but tetanus is incomplete (up to 20 Hz), the reduction of maintained tension becomes considerably more important. 3. Both octopamine application and DUMETi stimulation reduce the amount of catch tension displayed by the extensor muscle when SETi is fired in a variety of different stimulus patterns. The extensor-tibiae muscle is itself 'pattern sensitive' since is shows a 'positive spacing effect' when SETi is stimulated at an average frequency of 1 Hz. 4. It is suggested that a primary function of DUMETi is to change the response of the muscle from one that favours maintenance of posture to one that favours rapid changes in joint position or force, such as might occur during locomotion.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOWMAN W. C., ZAIMIS E. The effects of adrenaline, noradrenaline and isoprenaline on skeletal muscle contractions in the cat. J Physiol. 1958 Nov 10;144(1):92–107. doi: 10.1113/jphysiol.1958.sp006088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman W. C., Nott M. W. Actions of sympathomimetic amines and their antagonists on skeletal muscle. Pharmacol Rev. 1969 Mar;21(1):27–72. [PubMed] [Google Scholar]
- Burke R. E., Rudomin P., Zajac F. E., 3rd Catch property in single mammalian motor units. Science. 1970 Apr 3;168(3927):122–124. doi: 10.1126/science.168.3927.122. [DOI] [PubMed] [Google Scholar]
- Evans P. D., Gee J. D. Action of formamidine pesticides on octopamine receptors. Nature. 1980 Sep 4;287(5777):60–62. doi: 10.1038/287060a0. [DOI] [PubMed] [Google Scholar]
- Evans P. D. Multiple receptor types for octopamine in the locust. J Physiol. 1981 Sep;318:99–122. doi: 10.1113/jphysiol.1981.sp013853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P. D., O'Shea M. An octopaminergic neurone modulates neuromuscular transmission in the locust. Nature. 1977 Nov 17;270(5634):257–259. doi: 10.1038/270257a0. [DOI] [PubMed] [Google Scholar]
- Evans P. D., O'Shea M. The identification of an octopaminergic neurone and the modulation of a myogenic rhythm in the locust. J Exp Biol. 1978 Apr;73:235–260. doi: 10.1242/jeb.73.1.235. [DOI] [PubMed] [Google Scholar]
- Evans P. D., Talamo B. R., Kravitz E. A. Octopamine neurons: morphology, release of octopamine and possible physiological role. Brain Res. 1975 Jun 13;90(2):340–347. doi: 10.1016/0006-8993(75)90317-0. [DOI] [PubMed] [Google Scholar]
- Heitler W. J., Burrows M. The locust jump. I. The motor programme. J Exp Biol. 1977 Feb;66(1):203–219. doi: 10.1242/jeb.66.1.203. [DOI] [PubMed] [Google Scholar]
- Hoyle G., Barker D. L. Synthesis of octopamine by insect dorsal median unpaired neurons. J Exp Zool. 1975 Sep;193(3):433–439. doi: 10.1002/jez.1401930322. [DOI] [PubMed] [Google Scholar]
- Hoyle G., Burrows M. Neural mechanisms underlying behavior in the locust Schistocerca gregaria. I. Physiology of identified motorneurons in the metathoracic ganglion. J Neurobiol. 1973;4(1):3–41. doi: 10.1002/neu.480040104. [DOI] [PubMed] [Google Scholar]
- Hoyle G., Dagan D. Physiological characteristics and reflex activation of DUM (octopaminergic) neurons of locust metathoracic ganglion. J Neurobiol. 1978 Jan;9(1):59–79. doi: 10.1002/neu.480090106. [DOI] [PubMed] [Google Scholar]
- Hoyle G. Distributions of nerve and muscle fibre types in locust jumping muscle. J Exp Biol. 1978 Apr;73:205–233. doi: 10.1242/jeb.73.1.205. [DOI] [PubMed] [Google Scholar]
- Hoyle G. Evidence that insect dorsal unpaired medican (DUM) neurons are octopaminergic. J Exp Zool. 1975 Sep;193(3):425–431. doi: 10.1002/jez.1401930321. [DOI] [PubMed] [Google Scholar]
- Hoyle G. Intrinsic rhythm and basic tonus in insect skeletal muscle. J Exp Biol. 1978 Apr;73:173–203. doi: 10.1242/jeb.73.1.173. [DOI] [PubMed] [Google Scholar]
- Kravitz E. A., Glusman S., Harris-Warrick R. M., Livingstone M. S., Schwarz T., Goy M. F. Amines and a peptide as neurohormones in lobsters: actions on neuromuscular preparations and preliminary behavioural studies. J Exp Biol. 1980 Dec;89:159–175. doi: 10.1242/jeb.89.1.159. [DOI] [PubMed] [Google Scholar]
- Kuba K. Effects of catecholamines on the neuromuscular junction in the rat diaphragm. J Physiol. 1970 Dec;211(3):551–570. doi: 10.1113/jphysiol.1970.sp009293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason A., Sunderland A. J., Leake L. D. Effects of leech Retzius cells on body wall muscles. Comp Biochem Physiol C. 1979;63C(2):359–361. doi: 10.1016/0306-4492(79)90086-8. [DOI] [PubMed] [Google Scholar]
- Partridge L. D. Signal-handling characteristics of load-moving skeletal muscle. Am J Physiol. 1966 May;210(5):1178–1191. doi: 10.1152/ajplegacy.1966.210.5.1178. [DOI] [PubMed] [Google Scholar]
- Pearson K. G., Bergman S. J. Common inhibitory motoneurones in insects. J Exp Biol. 1969 Apr;50(2):445–471. doi: 10.1242/jeb.50.2.445. [DOI] [PubMed] [Google Scholar]
- USHERWOOD P. N., GRUNDFEST H. PERIPHERAL INHIBITION IN SKELETAL MUSCLE OF INSECTS. J Neurophysiol. 1965 May;28:497–518. doi: 10.1152/jn.1965.28.3.497. [DOI] [PubMed] [Google Scholar]
- Weiss K. R., Cohen J. L., Kupfermann I. Modulatory control of buccal musculature by a serotonergic neuron (metacerebral cell) in Aplysia. J Neurophysiol. 1978 Jan;41(1):181–203. doi: 10.1152/jn.1978.41.1.181. [DOI] [PubMed] [Google Scholar]
- Wilson D. M., Larimer J. L. The catch property of ordinary muscle. Proc Natl Acad Sci U S A. 1968 Nov;61(3):909–916. doi: 10.1073/pnas.61.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. M., Smith D. O., Dempster P. Length and tension hysteresis during sinusoidal and step function stimulation of arthropod muscle. Am J Physiol. 1970 Mar;218(3):916–922. doi: 10.1152/ajplegacy.1970.218.3.916. [DOI] [PubMed] [Google Scholar]


