Abstract
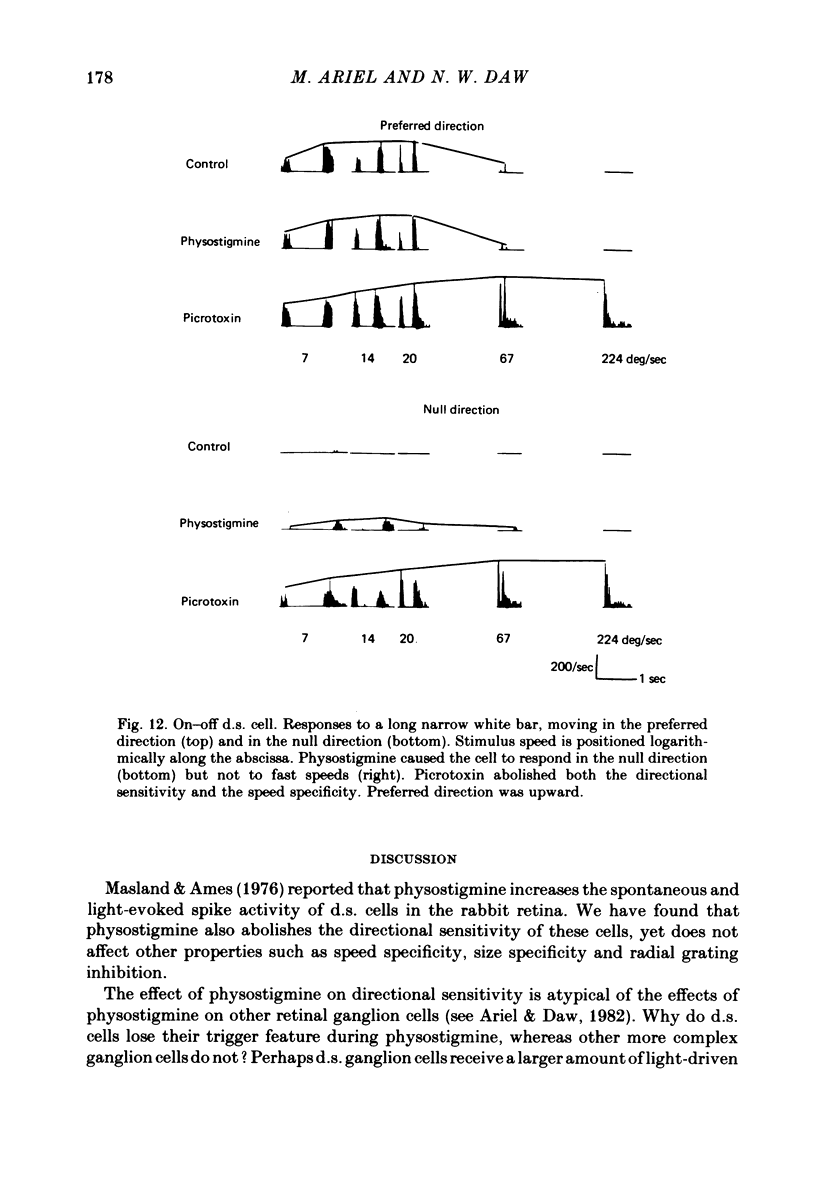
1. Cholinergic drugs were infused into the retinal circulation of the rabbit while we analysed the receptive field properties of directionally sensitive retinal ganglion cells. Physostigmine eliminated the trigger feature, directional specificity, of both types (on-centre and on—off) of these cells. In this respect the action of physostigmine (an ACh potentiator) was very like that of picrotoxin (a GABA antagonist). Therefore, a detailed analysis of the receptive field properties of directionally sensitive ganglion cells was made to analyse the effects of physostigmine and picrotoxin.
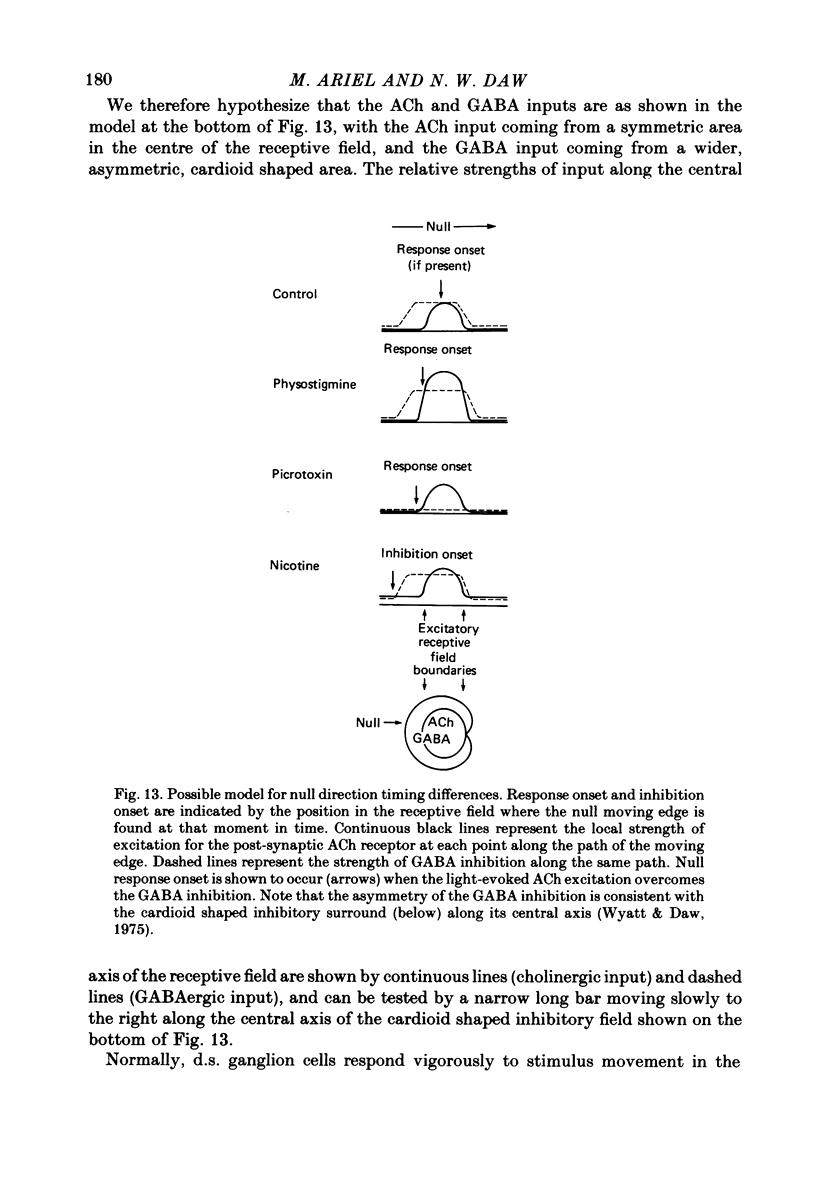
2. Size specificity and radial grating inhibition were not abolished by physostigmine, but were often affected by picrotoxin. The optimal velocity in the preferred direction (as measured by maximum firing frequency) was not much changed by physostigmine, but was higher during infusion of picrotoxin. Infusion of nicotine, a depolarizing ACh agonist which increases the activity of retinal ganglion cells, revealed the presence of inhibition to movement in the null direction. The null direction response during picrotoxin started slightly later than this inhibition. The null direction response during physostigmine was weaker and started later still. Mecamylamine and dihydro-β-erythroidine, nicrotinic receptor antagonists, totally blocked the effect of physostigmine and reduced the control light response by about half.
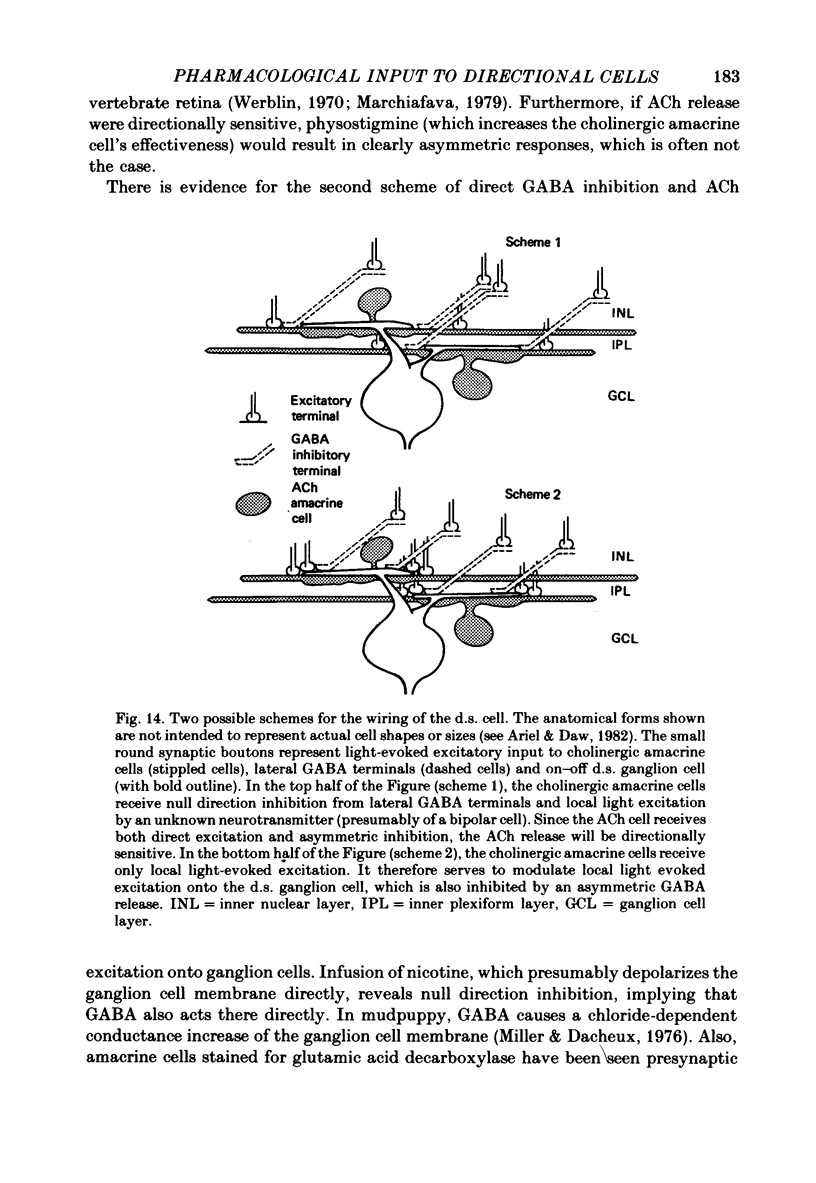
3. From this analysis, it appears that on—off ACh release onto directionally sensitive cells provides a substantial excitation which, when potentiated by physostigmine, overcomes or outlasts the null direction GABA inhibition within the receptive field. The spatial extent of GABA inhibition is asymmetric to and larger than the spatial extent of ACh excitation. Similar pathways appear to be involved in both the on-centre and on—off directionally sensitive ganglion cells, yet the on-centre cell pathway may receive an additional input which suppresses the ACh excitation at light offset. Possible schemes for the cellular mechanism of directional sensitivity are discussed in light of these results and recent anatomical and pharmacological findings.
Full text
PDF




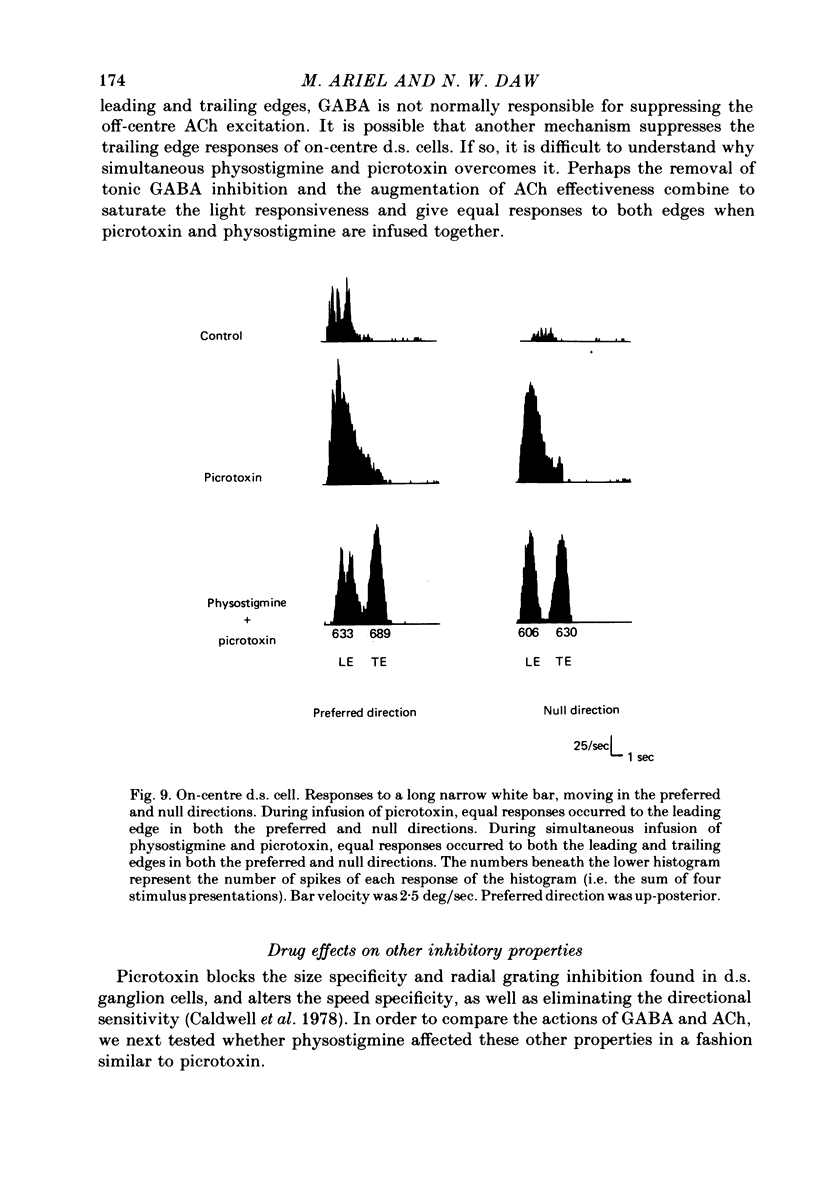
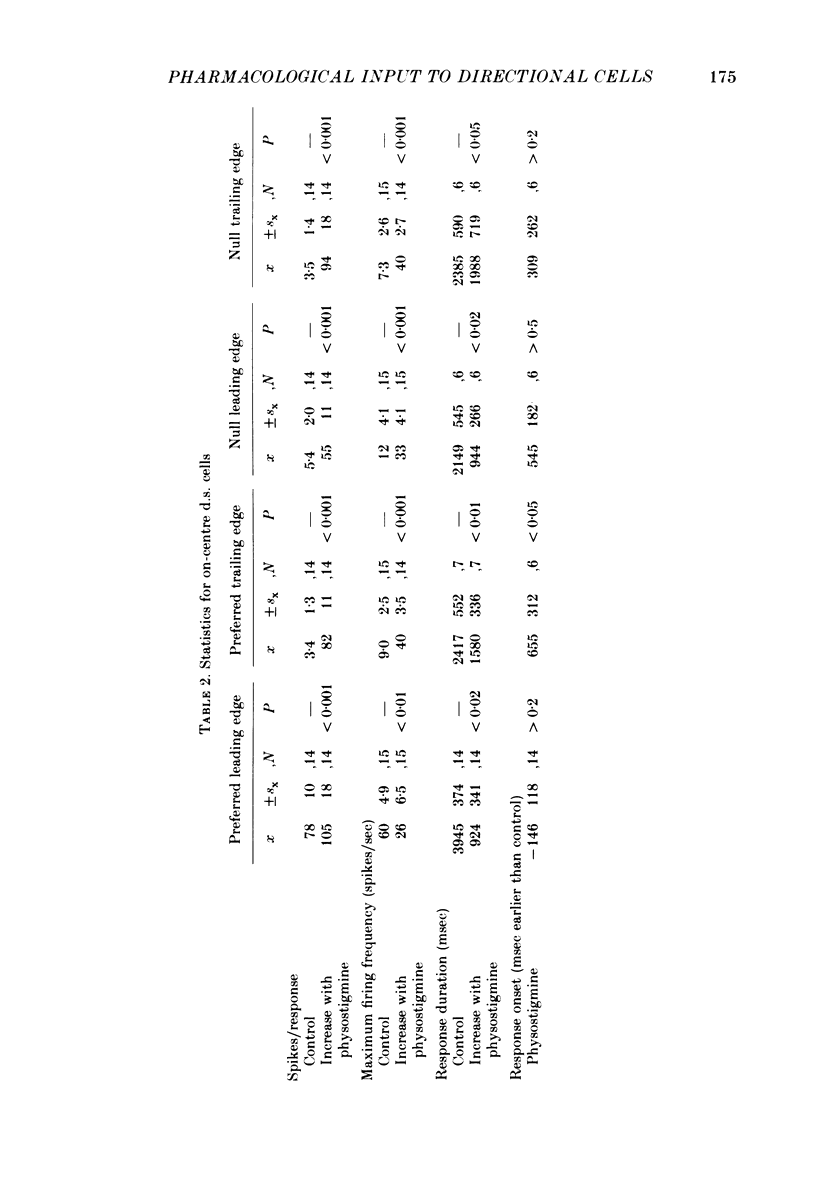
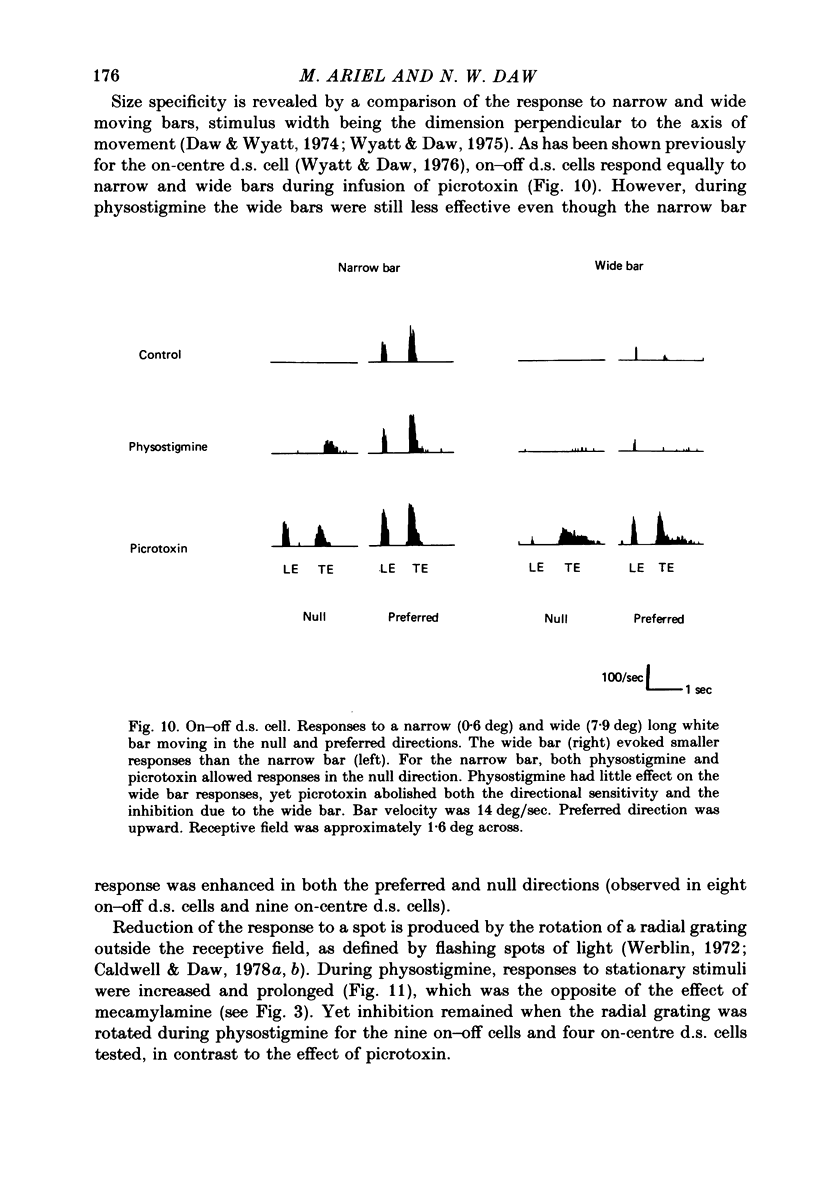
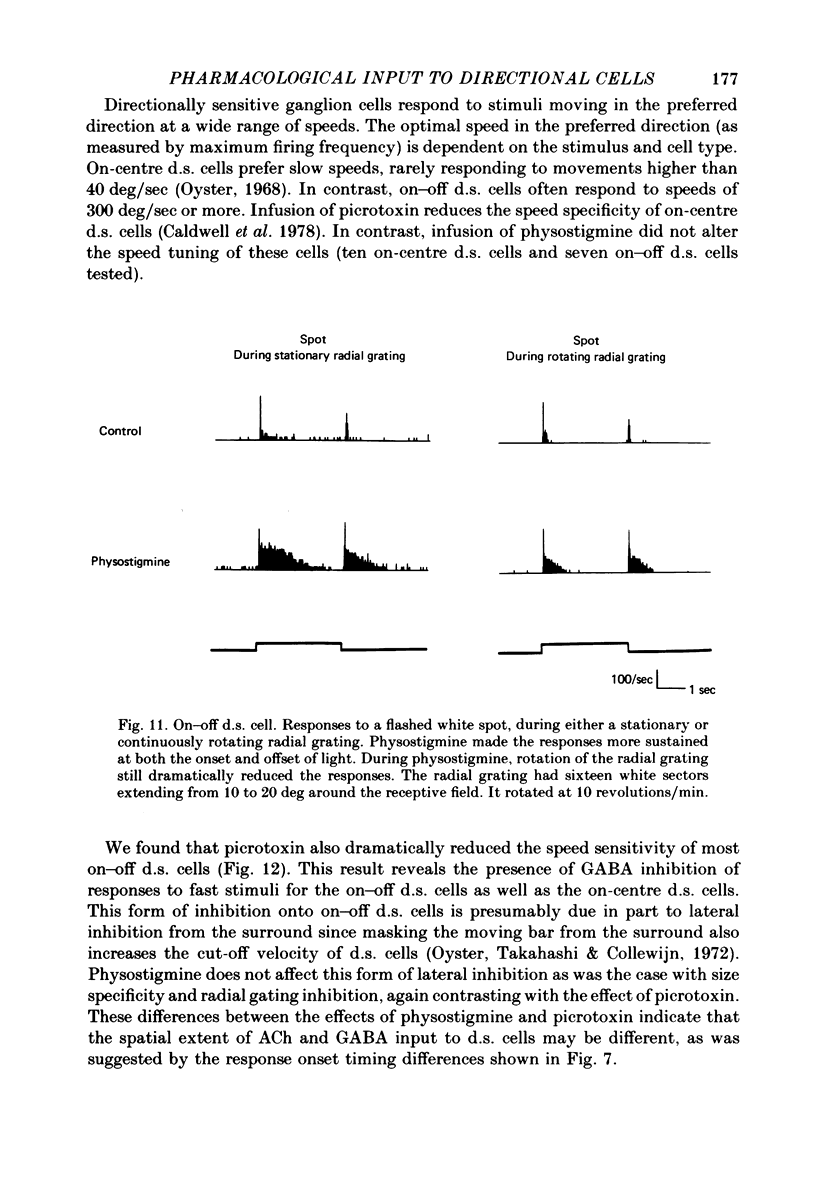





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariel M., Daw N. W. Effects of cholinergic drugs on receptive field properties of rabbit retinal ganglion cells. J Physiol. 1982 Mar;324:135–160. doi: 10.1113/jphysiol.1982.sp014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B., HILL R. M., LEVICK W. R. RETINAL GANGLION CELLS RESPONDING SELECTIVELY TO DIRECTION AND SPEED OF IMAGE MOTION IN THE RABBIT. J Physiol. 1964 Oct;173:377–407. doi: 10.1113/jphysiol.1964.sp007463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B., HILL R. M. Selective sensitivity to direction of movement in ganglion cells of the rabbit retina. Science. 1963 Feb 1;139(3553):412–414. doi: 10.1126/science.139.3553.412. [DOI] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. The mechanism of directionally selective units in rabbit's retina. J Physiol. 1965 Jun;178(3):477–504. doi: 10.1113/jphysiol.1965.sp007638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fettiplace R. Synaptic drive and impulse generation in ganglion cells of turtle retina. J Physiol. 1979 Mar;288:107–127. [PMC free article] [PubMed] [Google Scholar]
- Bourne W. M., Enoch J. M. Some optical principles of the clinical specular microscope. Invest Ophthalmol. 1976 Jan;15(1):29–32. [PubMed] [Google Scholar]
- Caldwell J. H., Daw N. W. Effects of picrotoxin and strychnine on rabbit retinal ganglion cells: changes in centre surround receptive fields. J Physiol. 1978 Mar;276:299–310. doi: 10.1113/jphysiol.1978.sp012234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J. H., Daw N. W. New properties of rabbit retinal ganglion cells. J Physiol. 1978 Mar;276:257–276. doi: 10.1113/jphysiol.1978.sp012232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J. H., Daw N. W., Wyatt H. J. Effects of picrotoxin and strychnine on rabbit retinal ganglion cells: lateral interactions for cells with more complex receptive fields. J Physiol. 1978 Mar;276:277–298. doi: 10.1113/jphysiol.1978.sp012233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw N. W., Wyatt H. J. Raising rabbits in a moving visual environment: an attempt to modify directional sensitivity in the retina. J Physiol. 1974 Jul;240(2):309–330. doi: 10.1113/jphysiol.1974.sp010612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchiafava P. L. The responses of retinal ganglion cells to stationary and moving visual stimuli. Vision Res. 1979;19(11):1203–1211. doi: 10.1016/0042-6989(79)90185-8. [DOI] [PubMed] [Google Scholar]
- Masland R. H., Ames A., 3rd Responses to acetylcholine of ganglion cells in an isolated mammalian retina. J Neurophysiol. 1976 Nov;39(6):1220–1235. doi: 10.1152/jn.1976.39.6.1220. [DOI] [PubMed] [Google Scholar]
- Massey S. C., Neal M. J. The light evoked release of acetylcholine from the rabbit retina iN vivo and its inhibition by gamma-aminobutyric acid. J Neurochem. 1979 Apr;32(4):1327–1329. doi: 10.1111/j.1471-4159.1979.tb11062.x. [DOI] [PubMed] [Google Scholar]
- Oyster C. W., Takahashi E., Collewijn H. Direction-selective retinal ganglion cells and control of optokinetic nystagmus in the rabbit. Vision Res. 1972 Feb;12(2):183–193. doi: 10.1016/0042-6989(72)90110-1. [DOI] [PubMed] [Google Scholar]
- Oyster C. W. The analysis of image motion by the rabbit retina. J Physiol. 1968 Dec;199(3):613–635. doi: 10.1113/jphysiol.1968.sp008671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S. Response of retinal cells to moving spots: intracellular recording in Necturus maculosus. J Neurophysiol. 1970 May;33(3):342–350. doi: 10.1152/jn.1970.33.3.342. [DOI] [PubMed] [Google Scholar]
- Wyatt H. J., Daw N. W. Directionally sensitive ganglion cells in the rabbit retina: specificity for stimulus direction, size, and speed. J Neurophysiol. 1975 May;38(3):613–626. doi: 10.1152/jn.1975.38.3.613. [DOI] [PubMed] [Google Scholar]
- Wyatt H. J., Day N. W. Specific effects of neurotransmitter antagonists on ganglion cells in rabbit retina. Science. 1976 Jan 16;191(4223):204–205. doi: 10.1126/science.1857. [DOI] [PubMed] [Google Scholar]



