Abstract
1. In nine 4-week-old, dark-reared kittens we sutured one eye closed and rotated the other surgically. The kittens then grew up in a normally lighted animal colony with adequate room to play.
2. For about two weeks after surgery their visual-motor co-ordination did not differ from that of kittens with conventional monocular deprivation; then severe disturbance of visually guided behaviour became progressively more apparent until, after another two to three weeks, all the kittens stopped responding to most visual stimuli entirely. At that point their behaviour in an unfamiliar environment closely resembled that of binocularly deprived cats exposed to light for the first time.
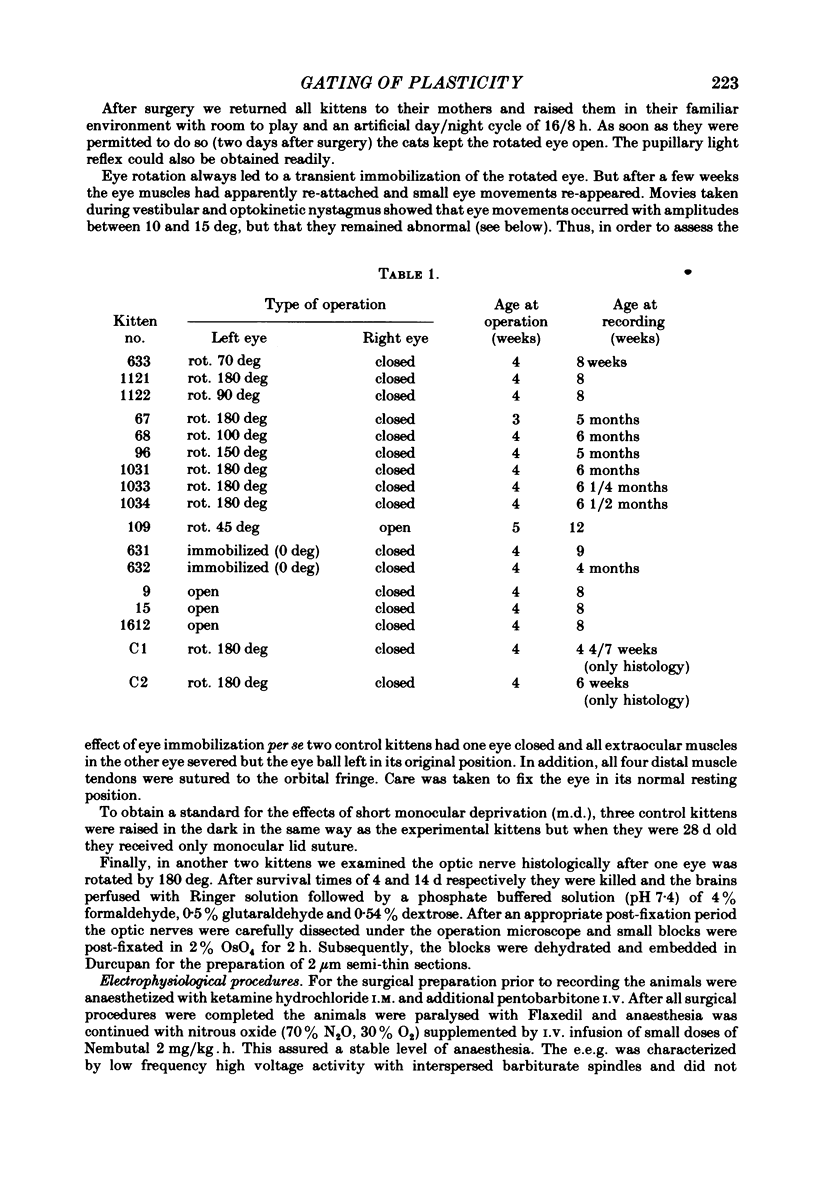
3. Four weeks (n = 3) and 6 months (n = 6) after surgery, we examined the visual cortex with single-unit recordings, and with evoked potentials elicited by electrical stimuli and patterned lights. We obtained the single-unit recordings from 586 neurones of the striate cortex in both hemispheres, both ipsi- and contralateral to the deprived eye.
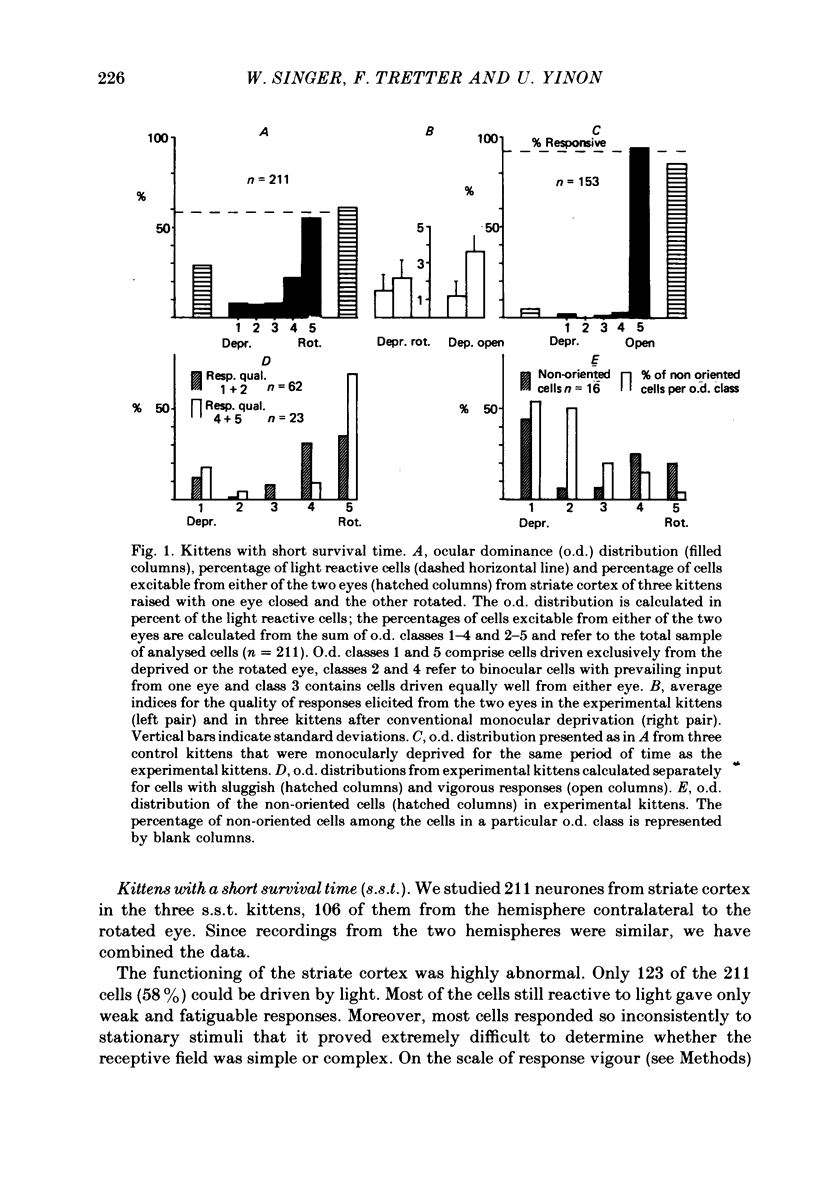
4. The single-unit recordings and the evoked potentials showed a clear relation between the kitten's abnormal visual behaviour and the functioning of the striate cortex. Only about half the normal percentage of cells responded to light, and most of those which did react had abnormal receptive field properties: they responded only sluggishly even when the light stimuli were aligned optimally.
5. We also evoked cortical potentials with phase alternating square wave gratings of variable contrast and spatial frequency. The amplitude of the potentials indicated that contrast-sensitivity was reduced at all spatial frequencies.
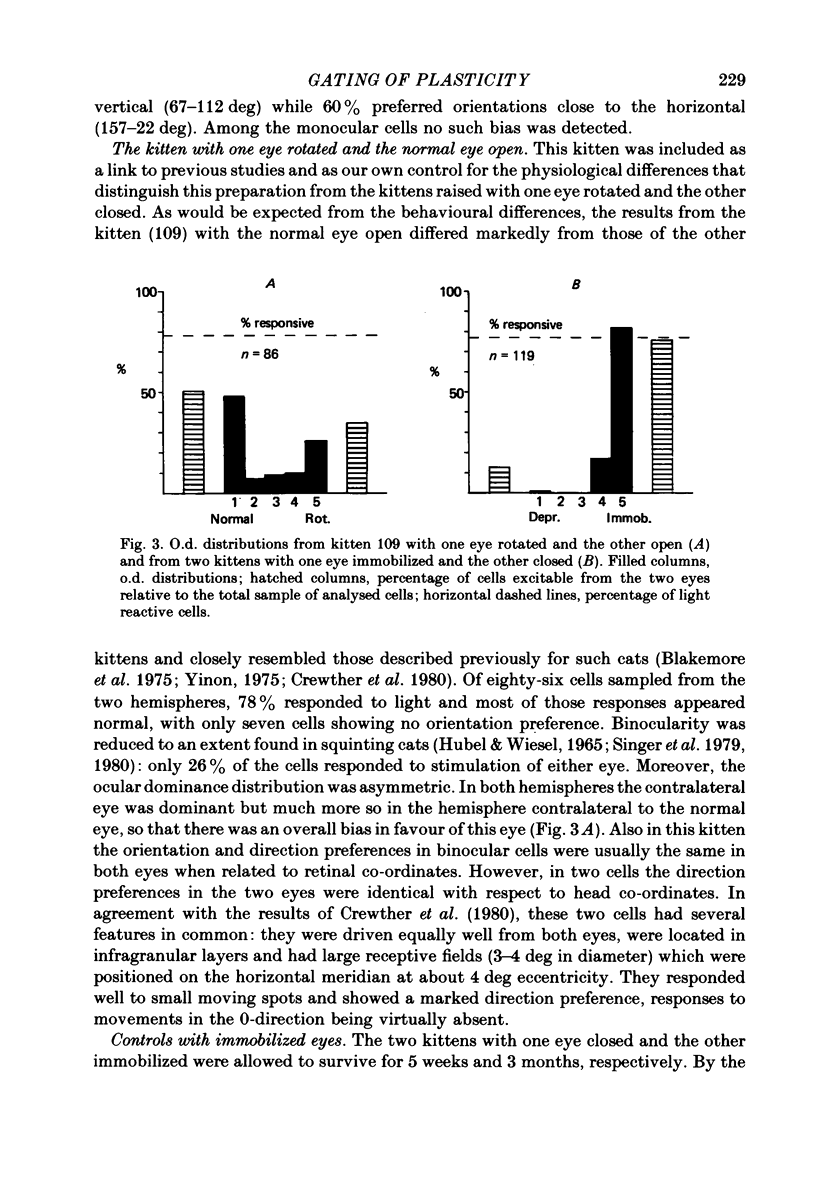
6. In the kittens tested 4 weeks after surgery, ocular dominance had shifted toward the open rotated eye but this shift was considerably less pronounced than in control kittens monocularly deprived for a comparable period of time.
7. In the kittens tested 6 months after surgery fewer cells than normal were binocular; ocular dominance had not shifted towards the open eye.
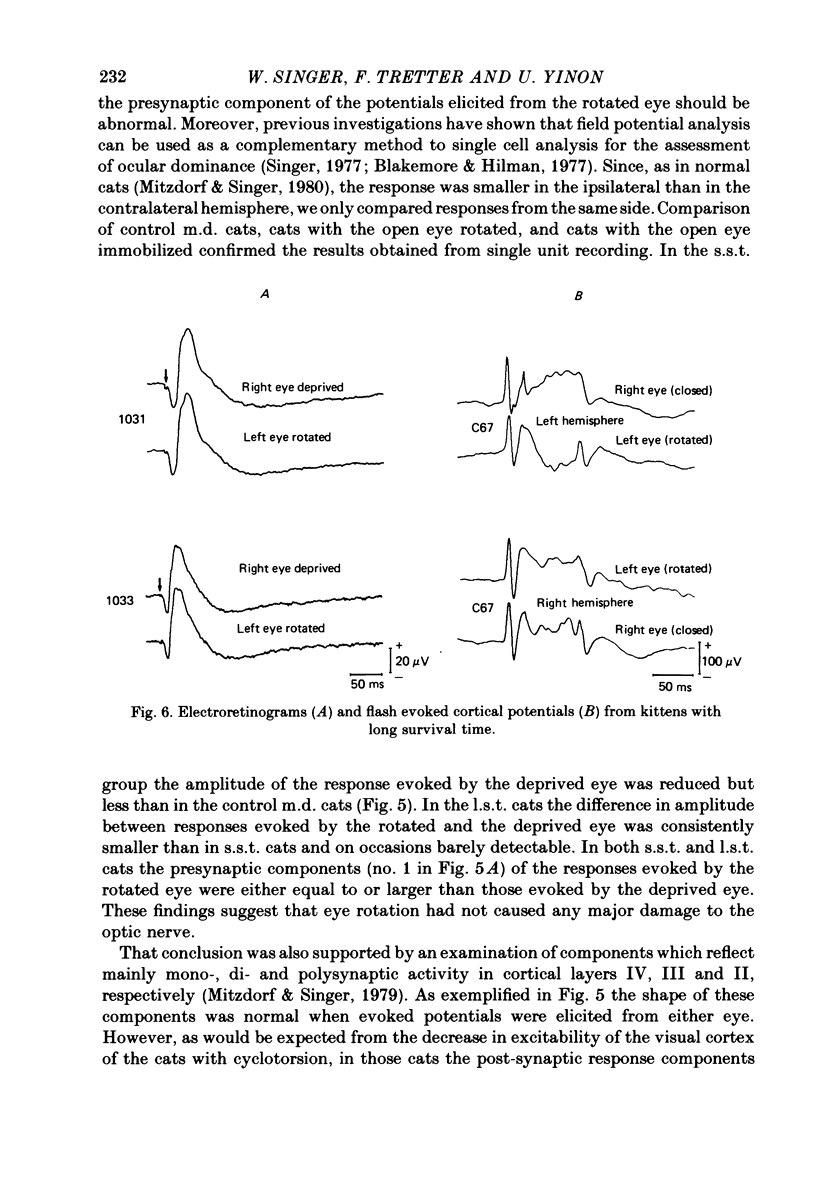
8. Numerous control experiments indicated that these abnormalities did not result from transitory immobilization of the eye alone nor from lesions of the retina or of the optic nerve.
We infer that a central mechanism prevents the inappropriate signals from the rotated eye from influencing the consolidation of central pathways.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow H. B. Visual experience and cortical development. Nature. 1975 Nov 20;258(5532):199–204. doi: 10.1038/258199a0. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Hillman P. An attempt to assess the effects of monocular deprivation and strabismus on synaptic efficiency in the kitten's visual cortex. Exp Brain Res. 1977 Nov 24;30(2-3):187–202. doi: 10.1007/BF00237250. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Van Sluyters R. C. Innate and environmental factors in the development of the kitten's visual cortex. J Physiol. 1975 Jul;248(3):663–716. doi: 10.1113/jphysiol.1975.sp010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Van Sluyters R. C., Peck C. K., Hein A. Development of cat visual cortex following rotation of one eye. Nature. 1975 Oct 16;257(5527):584–586. doi: 10.1038/257584a0. [DOI] [PubMed] [Google Scholar]
- Buisseret P., Imbert M. Visual cortical cells: their developmental properties in normal and dark reared kittens. J Physiol. 1976 Feb;255(2):511–525. doi: 10.1113/jphysiol.1976.sp011293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W., Maffei L., Piccolino M. The contrast sensitivity of the cat. J Physiol. 1973 Mar;229(3):719–731. doi: 10.1113/jphysiol.1973.sp010163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crewther S. G., Crewther D. P., Peck C. K., Pettigrew J. D. Visual cortical effects of rearing cats with monocular or binocular cyclotorsion. J Neurophysiol. 1980 Jul;44(1):97–118. doi: 10.1152/jn.1980.44.1.97. [DOI] [PubMed] [Google Scholar]
- Frégnac Y., Imbert M. Early development of visual cortical cells in normal and dark-reared kittens: relationship between orientation selectivity and ocular dominance. J Physiol. 1978 May;278:27–44. doi: 10.1113/jphysiol.1978.sp012290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein A., Vital-Durand F., Salinger W., Diamond R. Eye movements initiate visual-motor development in the cat. Science. 1979 Jun 22;204(4399):1321–1322. doi: 10.1126/science.313076. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Binocular interaction in striate cortex of kittens reared with artificial squint. J Neurophysiol. 1965 Nov;28(6):1041–1059. doi: 10.1152/jn.1965.28.6.1041. [DOI] [PubMed] [Google Scholar]
- Jacobson S. G., Eames R. A., McDonald W. I. Optic nerve fibre lesions in adult cats: pattern of recovery of spatial vision. Exp Brain Res. 1979 Aug 1;36(3):491–508. doi: 10.1007/BF00238518. [DOI] [PubMed] [Google Scholar]
- Jacobson S. G., Ikeda H. Behavioural studies of spatial vision in cats reared with convergent squint: is amblyopia due to arrest of development? Exp Brain Res. 1979 Jan 2;34(1):11–26. doi: 10.1007/BF00238338. [DOI] [PubMed] [Google Scholar]
- Kalil R. Dark rearing in the cat: effects on visuomotor behavior and cell growth in the dorsal lateral geniculate nucleus. J Comp Neurol. 1978 Apr 1;178(3):451–467. doi: 10.1002/cne.901780304. [DOI] [PubMed] [Google Scholar]
- Mitzdorf U., Singer W. Excitatory synaptic ensemble properties in the visual cortex of the macaque monkey: a current source density analysis of electrically evoked potentials. J Comp Neurol. 1979 Sep 1;187(1):71–83. doi: 10.1002/cne.901870105. [DOI] [PubMed] [Google Scholar]
- Mitzdorf U., Singer W. Monocular activation of visual cortex in normal and monocularly deprived cats: an analysis of evoked potentials. J Physiol. 1980 Jul;304:203–220. doi: 10.1113/jphysiol.1980.sp013320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck C. K., Crewther S. G., Barber G., Johannsen C. J. Pattern discrimination and visuomotor behavior following rotation of one or both eyes in kittens and in adult cats. Exp Brain Res. 1979 Feb 15;34(3):401–408. doi: 10.1007/BF00239139. [DOI] [PubMed] [Google Scholar]
- Peck C. K., Crewther S. G. Perceptual effects of surgical rotation of the eye in kittens. Brain Res. 1975 Nov 28;99(1):213–219. doi: 10.1016/0006-8993(75)90630-7. [DOI] [PubMed] [Google Scholar]
- Rauschecker J. P., Singer W. Changes in the circuitry of the kitten visual cortex are gated by postsynaptic activity. Nature. 1979 Jul 5;280(5717):58–60. doi: 10.1038/280058a0. [DOI] [PubMed] [Google Scholar]
- Rauschecker J. P., Singer W. The effects of early visual experience on the cat's visual cortex and their possible explanation by Hebb synapses. J Physiol. 1981 Jan;310:215–239. doi: 10.1113/jphysiol.1981.sp013545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W. Effects of monocular deprivation on excitatory and inhibitory pathways in cat striate cortex. Exp Brain Res. 1977 Oct 24;30(1):25–41. doi: 10.1007/BF00237856. [DOI] [PubMed] [Google Scholar]
- Singer W., Freeman B., Rauschecker J. Restriction of visual experience to a single orientation affects the organization of orientation columns in cat visual cortex. A study with deoxyglucose. Exp Brain Res. 1981;41(3-4):199–215. doi: 10.1007/BF00238877. [DOI] [PubMed] [Google Scholar]
- Singer W., Tretter F., Cynader M. The effect of reticular stimulation on spontaneous and evoked activity in the cat visual cortex. Brain Res. 1976 Jan 30;102(1):71–90. doi: 10.1016/0006-8993(76)90576-x. [DOI] [PubMed] [Google Scholar]
- Singer W., Tretter F. Receptive-field properties and neuronal connectivity in striate and parastriate cortex of contour-deprived cats. J Neurophysiol. 1976 May;39(3):613–630. doi: 10.1152/jn.1976.39.3.613. [DOI] [PubMed] [Google Scholar]
- Singer W., Tretter F., Yinon U. Evidence for long-term functional plasticity in the visual cortex of adult cats. J Physiol. 1982 Mar;324:239–248. doi: 10.1113/jphysiol.1982.sp014109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W., Tretter F., Yinon U. Inverted vision causes selective loss of striate cortex neurons with binocular, vertically oriented receptive fields. Brain Res. 1979 Jul 6;170(1):177–181. doi: 10.1016/0006-8993(79)90950-8. [DOI] [PubMed] [Google Scholar]
- Singer W., Yinon U., Tretter F. Inverted monocular vision prevents ocular dominance shift in kittens and impairs the functional state of visual cortex in adult cats. Brain Res. 1979 Mar 23;164:294–299. doi: 10.1016/0006-8993(79)90024-6. [DOI] [PubMed] [Google Scholar]
- Singer W., von Gruenau M., Rauschecker J. Requirements for the disruption of binocularity in the visual cortex of strabismic kittens. Brain Res. 1979 Aug 10;171(3):536–540. doi: 10.1016/0006-8993(79)91058-8. [DOI] [PubMed] [Google Scholar]
- Singer W., von Grünau M., Rauschecker J. Functional amblyopia in kittens with unilateral exotropia. I. Electrophysiological assessment. Exp Brain Res. 1980;40(3):294–304. doi: 10.1007/BF00237794. [DOI] [PubMed] [Google Scholar]
- Sireteanu R., Fronius M. Naso-temporal asymmetries in human amblyopia consequence of long-term interocular suppression. Vision Res. 1981;21(7):1055–1063. doi: 10.1016/0042-6989(81)90010-9. [DOI] [PubMed] [Google Scholar]
- Sireteanu R., Fronius M., Singer W. Binocular interaction in the peripheral visual field of humans with strabismic and anisometropic amblyopia. Vision Res. 1981;21(7):1065–1074. doi: 10.1016/0042-6989(81)90011-0. [DOI] [PubMed] [Google Scholar]
- Watkins D. W., Wilson J. R., Sherman S. M. Receptive-field properties of neurons in binocular and monocular segments of striate cortex in cats raised with binocular lid suture. J Neurophysiol. 1978 Mar;41(2):322–337. doi: 10.1152/jn.1978.41.2.322. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol. 1965 Nov;28(6):1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- Yinon U. Eye rotation in developing kittens: the effect on ocular dominance and receptive field organization of cortical cells. Exp Brain Res. 1975 Dec 22;24(2):215–218. doi: 10.1007/BF00234065. [DOI] [PubMed] [Google Scholar]
- van Hof-van Duin J. Visual field measurements in monocularly deprived and normal cats. Exp Brain Res. 1977 Nov 24;30(2-3):353–368. doi: 10.1007/BF00237262. [DOI] [PubMed] [Google Scholar]
- von Grünau M. W., Singer W. Functional amblyopia in kittens with unilateral exotropia. II. Correspondence between behavioural and electrophysiological assessment. Exp Brain Res. 1980;40(3):305–310. doi: 10.1007/BF00237795. [DOI] [PubMed] [Google Scholar]


