Abstract
1. Vision was investigated with behavioural and electrophysiological techniques in three groups of cats: (a) two normally raised kittens in which one eye was rotated at an age of 3 months, (b) three adult cats in which one eye had been rotated and the other closed 6 months prior to recording, (c) two adult cats in which first one eye had been rotated and the other closed and subsequently, after one year, the rotated eye had been closed and the normal eye re-opened. The latter two cats were investigated 6 and 12 months after reverse suture, respectively. All adult cats were at least 2 years old when operated on for the first time.
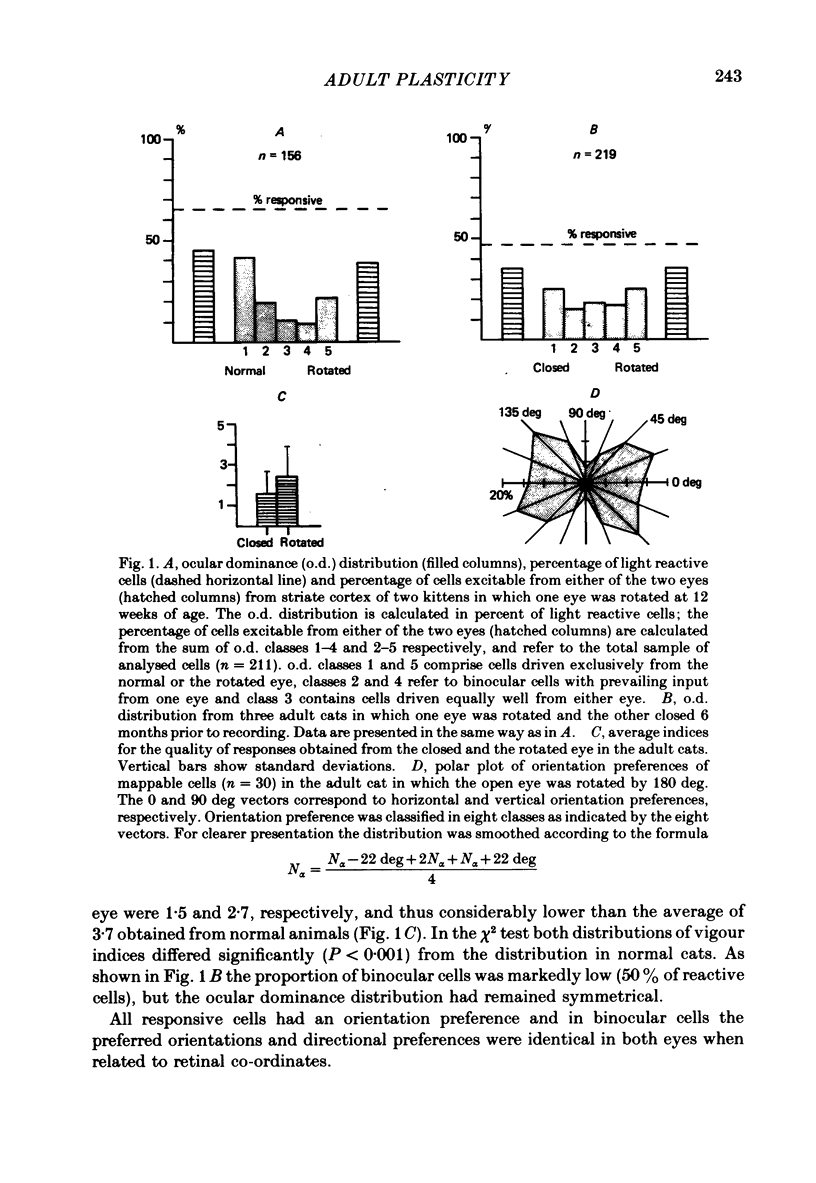
2. Behavioural analysis revealed that the kittens of the first group no longer used the rotated eye for fixation, visuo-motor behaviour being impaired when tested through this eye. Binocularity was found to be disrupted to nearly the same extent as in kittens made strabismic at the beginning of the critical period. In addition, ocular dominance was shifted towards the normal eye.
3. The adult cats in the second group developed a virtually complete neglect of the visual modality subsequent to a period of severely disturbed visuo-motor behaviour.
4. These behavioural abnormalities were associated with clear alterations in the functional state of striate cortex. Only 47% of the recorded cells could be driven with light, the majority of these reactive neurones yielding only sluggish responses to optimally aligned stimuli. The ocular dominance distribution showed a significant reduction of binocular cells but gave no indication of a shift in ocular dominance towards either of the two eyes. Moreover, contrast sensitivity as assessed with pattern-evoked potentials was significantly reduced.
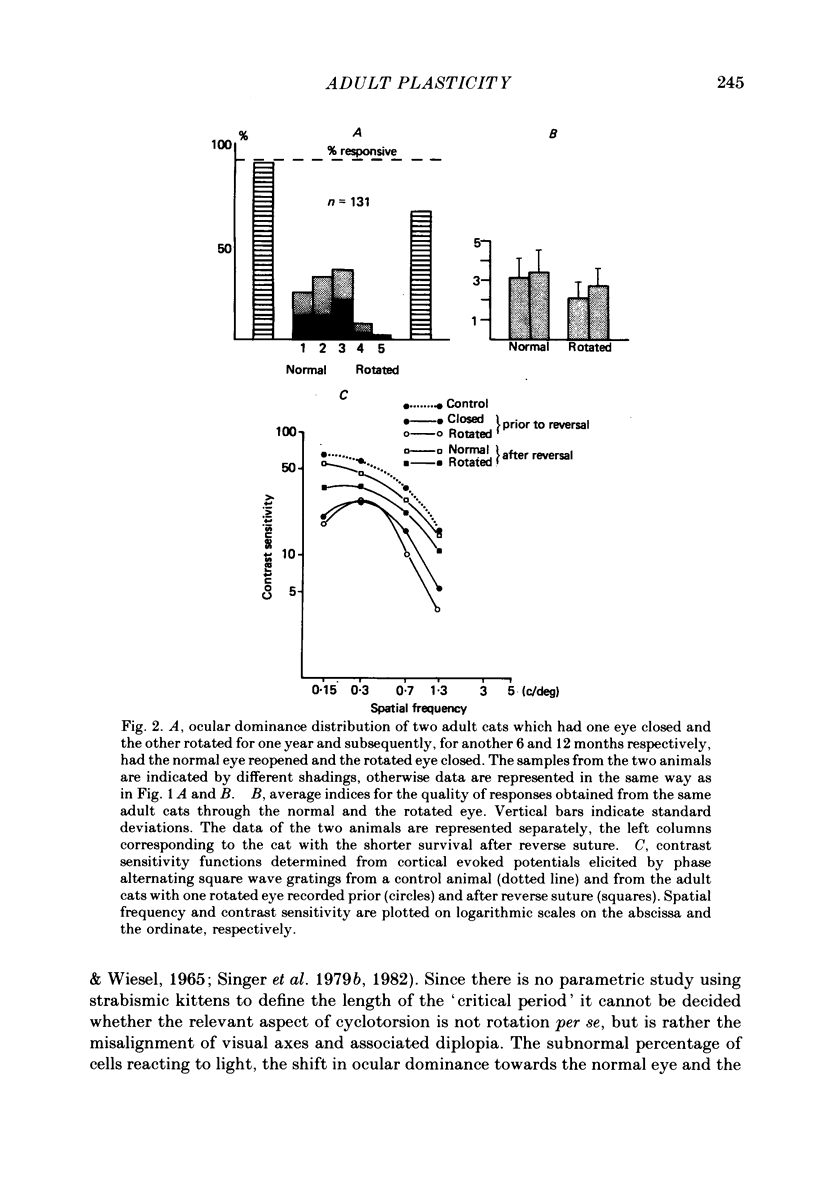
5. The remaining two animals that were reverse sutured after the visual neglect had developed showed complete behavioural recovery when tested through the re-opened normal eye. However, this recovery was not instantaneous and occurred only after the cats had been forced to use their visual sense.
6. Behavioural recovery was paralleled by an increase of cortical reactivity to normal levels and by a marked increase in binocularity. This gain increase of excitatory transmission was, however, selective for neurones dominated by the normal eye, leading to a bias in ocular dominance towards this eye.
7. The observed modifications in the functional state of striate cortex indicate that reversible changes in the gain of excitatory transmission can still occur beyond the end of the classical critical period. These long-lasting changes in synaptic efficiency appear to follow the rules postulated by Hebb for adaptive synaptic connexions.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blakemore C., Garey L. J., Vital-Durand F. The physiological effects of monocular deprivation and their reversal in the monkey's visual cortex. J Physiol. 1978 Oct;283:223–262. doi: 10.1113/jphysiol.1978.sp012498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C. The conditions required for the maintenance of binocularity in the kitten's visual cortex. J Physiol. 1976 Oct;261(2):423–444. doi: 10.1113/jphysiol.1976.sp011566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Van Sluyters R. C. Reversal of the physiological effects of monocular deprivation in kittens: further evidence for a sensitive period. J Physiol. 1974 Feb;237(1):195–216. doi: 10.1113/jphysiol.1974.sp010478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullier J., Henry G. H. Laminar distribution of first-order neurons and afferent terminals in cat striate cortex. J Neurophysiol. 1979 Sep;42(5):1271–1281. doi: 10.1152/jn.1979.42.5.1271. [DOI] [PubMed] [Google Scholar]
- Chow K. L., Stewart D. L. Reversal of structural and functional effects of long-term visual deprivation in cats. Exp Neurol. 1972 Mar;34(3):409–433. doi: 10.1016/0014-4886(72)90038-6. [DOI] [PubMed] [Google Scholar]
- Cynader M., Mitchell D. E. Prolonged sensitivity to monocular deprivation in dark-reared cats. J Neurophysiol. 1980 Apr;43(4):1026–1040. doi: 10.1152/jn.1980.43.4.1026. [DOI] [PubMed] [Google Scholar]
- Ganz L., Hirsch H. V., Tieman S. B. The nature of perceptual deficits in visually deprived cats. Brain Res. 1972 Sep 29;44(2):547–568. doi: 10.1016/0006-8993(72)90318-6. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D. Laminar differences in receptive field properties of cells in cat primary visual cortex. J Physiol. 1977 Jun;268(2):391–421. doi: 10.1113/jphysiol.1977.sp011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry G. H., Harvey A. R., Lund J. S. The afferent connections and laminar distribution of cells in the cat striate cortex. J Comp Neurol. 1979 Oct 15;187(4):725–744. doi: 10.1002/cne.901870406. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Binocular interaction in striate cortex of kittens reared with artificial squint. J Neurophysiol. 1965 Nov;28(6):1041–1059. doi: 10.1152/jn.1965.28.6.1041. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970 Feb;206(2):419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Tremain K. E. Amblyopia occurs in retinal ganglion cells in cats reared with convergent squint without alternating fixation. Exp Brain Res. 1979 May 2;35(3):559–582. doi: 10.1007/BF00236772. [DOI] [PubMed] [Google Scholar]
- Loop M. S., Sherman S. M. The effect of cortical lesions upon visual discriminations in binocularly deprived cats. J Comp Neurol. 1977 Jul 1;174(1):89–94. doi: 10.1002/cne.901740107. [DOI] [PubMed] [Google Scholar]
- Olson C. R., Freeman R. D. Monocular deprivation and recovery during sensitive period in kittens. J Neurophysiol. 1978 Jan;41(1):65–74. doi: 10.1152/jn.1978.41.1.65. [DOI] [PubMed] [Google Scholar]
- Olson C. R., Freeman R. D. Profile of the sensitive period for monocular deprivation in kittens. Exp Brain Res. 1980;39(1):17–21. doi: 10.1007/BF00237065. [DOI] [PubMed] [Google Scholar]
- Rauschecker J. P., Singer W. Changes in the circuitry of the kitten visual cortex are gated by postsynaptic activity. Nature. 1979 Jul 5;280(5717):58–60. doi: 10.1038/280058a0. [DOI] [PubMed] [Google Scholar]
- Rauschecker J. P., Singer W. The effects of early visual experience on the cat's visual cortex and their possible explanation by Hebb synapses. J Physiol. 1981 Jan;310:215–239. doi: 10.1113/jphysiol.1981.sp013545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman S. M. The effect of cortical and tectal lesions on the visual fields of binocularly deprived cats. J Comp Neurol. 1977 Mar 15;172(2):231–245. doi: 10.1002/cne.901720204. [DOI] [PubMed] [Google Scholar]
- Sherman S. M. Visual field defects in monocularly and binocularly deprived cats. Brain Res. 1973 Jan 15;49(1):24–45. [PubMed] [Google Scholar]
- Singer W., Freeman B., Rauschecker J. Restriction of visual experience to a single orientation affects the organization of orientation columns in cat visual cortex. A study with deoxyglucose. Exp Brain Res. 1981;41(3-4):199–215. doi: 10.1007/BF00238877. [DOI] [PubMed] [Google Scholar]
- Singer W., Tretter F., Cynader M. Organization of cat striate cortex: a correlation of receptive-field properties with afferent and efferent connections. J Neurophysiol. 1975 Sep;38(5):1080–1098. doi: 10.1152/jn.1975.38.5.1080. [DOI] [PubMed] [Google Scholar]
- Singer W., Tretter F. Receptive-field properties and neuronal connectivity in striate and parastriate cortex of contour-deprived cats. J Neurophysiol. 1976 May;39(3):613–630. doi: 10.1152/jn.1976.39.3.613. [DOI] [PubMed] [Google Scholar]
- Singer W., Tretter F., Yinon U. Central gating of developmental plasticity in kitten visual cortex. J Physiol. 1982 Mar;324:221–237. doi: 10.1113/jphysiol.1982.sp014108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W., Tretter F., Yinon U. Inverted vision causes selective loss of striate cortex neurons with binocular, vertically oriented receptive fields. Brain Res. 1979 Jul 6;170(1):177–181. doi: 10.1016/0006-8993(79)90950-8. [DOI] [PubMed] [Google Scholar]
- Singer W., Yinon U., Tretter F. Inverted monocular vision prevents ocular dominance shift in kittens and impairs the functional state of visual cortex in adult cats. Brain Res. 1979 Mar 23;164:294–299. doi: 10.1016/0006-8993(79)90024-6. [DOI] [PubMed] [Google Scholar]
- Singer W., von Gruenau M., Rauschecker J. Requirements for the disruption of binocularity in the visual cortex of strabismic kittens. Brain Res. 1979 Aug 10;171(3):536–540. doi: 10.1016/0006-8993(79)91058-8. [DOI] [PubMed] [Google Scholar]
- Singer W., von Grünau M., Rauschecker J. Functional amblyopia in kittens with unilateral exotropia. I. Electrophysiological assessment. Exp Brain Res. 1980;40(3):294–304. doi: 10.1007/BF00237794. [DOI] [PubMed] [Google Scholar]
- Smith D. C., Lorber R., Stanford L. R., Loop M. S. Visual acuity following binocular deprivation in the cat. Brain Res. 1980 Feb 3;183(1):1–11. doi: 10.1016/0006-8993(80)90115-8. [DOI] [PubMed] [Google Scholar]
- Watkins D. W., Wilson J. R., Sherman S. M. Receptive-field properties of neurons in binocular and monocular segments of striate cortex in cats raised with binocular lid suture. J Neurophysiol. 1978 Mar;41(2):322–337. doi: 10.1152/jn.1978.41.2.322. [DOI] [PubMed] [Google Scholar]
- von Grünau M. W., Singer W. Functional amblyopia in kittens with unilateral exotropia. II. Correspondence between behavioural and electrophysiological assessment. Exp Brain Res. 1980;40(3):305–310. doi: 10.1007/BF00237795. [DOI] [PubMed] [Google Scholar]


