Abstract
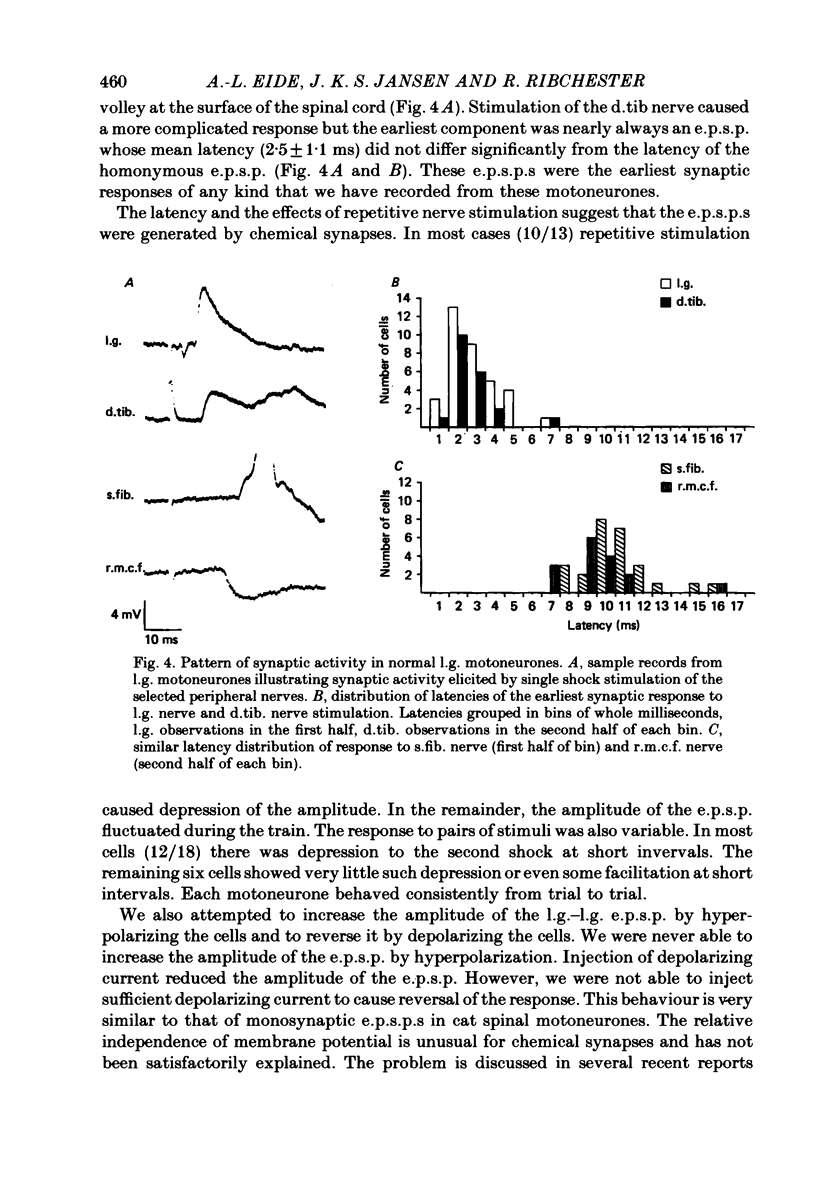
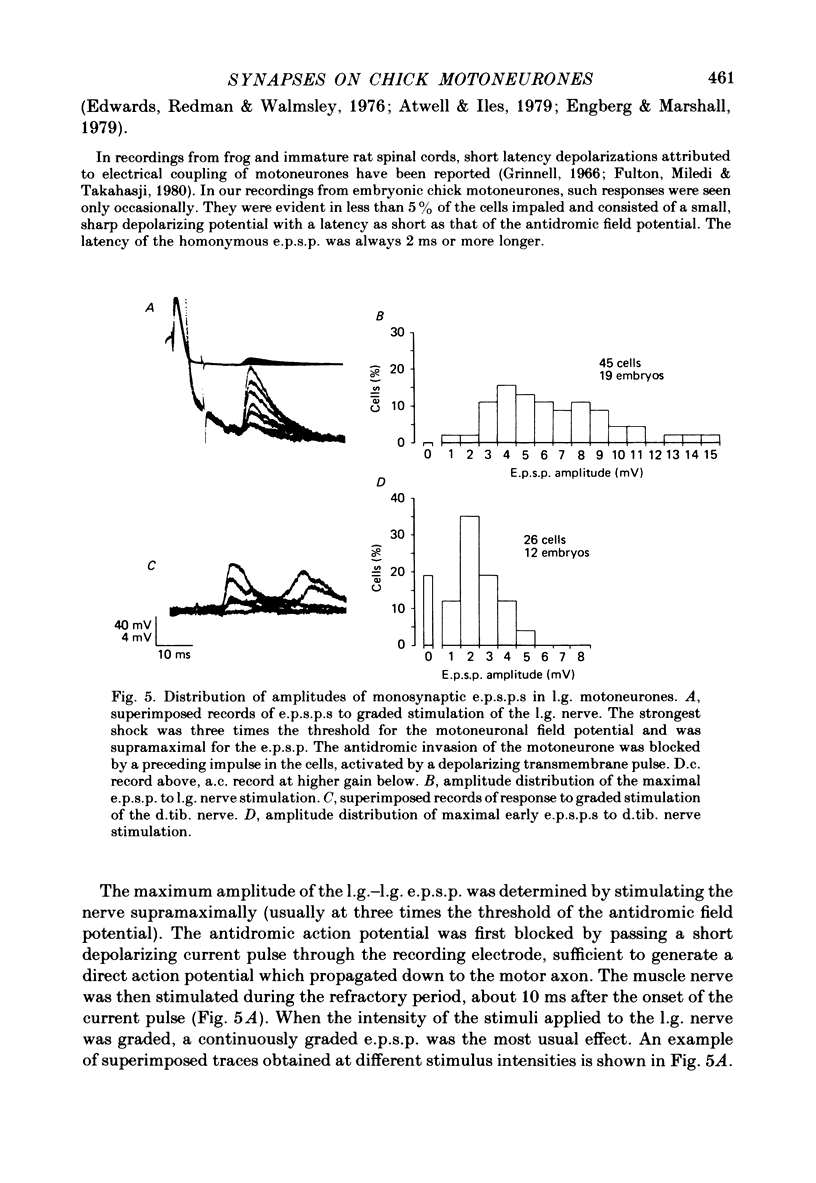
1. The pattern of synaptic activity in lateral gastrocnemius (l.g.) motoneurones in the lumbar spinal cord of chick embryos (Stage 44-45, 19-21 d of incubation) has been examined using intracellular recording. In the motoneurones of normal chick embryos, stimulation of different peripheral, sciatic nerve branches gave rise to characteristic synaptic responses. Stimulation of the lateral gastrocnemius nerve caused a monosynaptic e.p.s.p. which was graded by the intensity of nerve stimulation. Stimulation of synergistic muscle afferents also caused a brief latency e.p.s.p., followed by longer latency excitatory and inhibitory synaptic potentials. Stimulation of antagonistic muscle afferents or cutaneous afferents gave rise to longer latency inhibitory and excitatory synaptic potentials respectively.
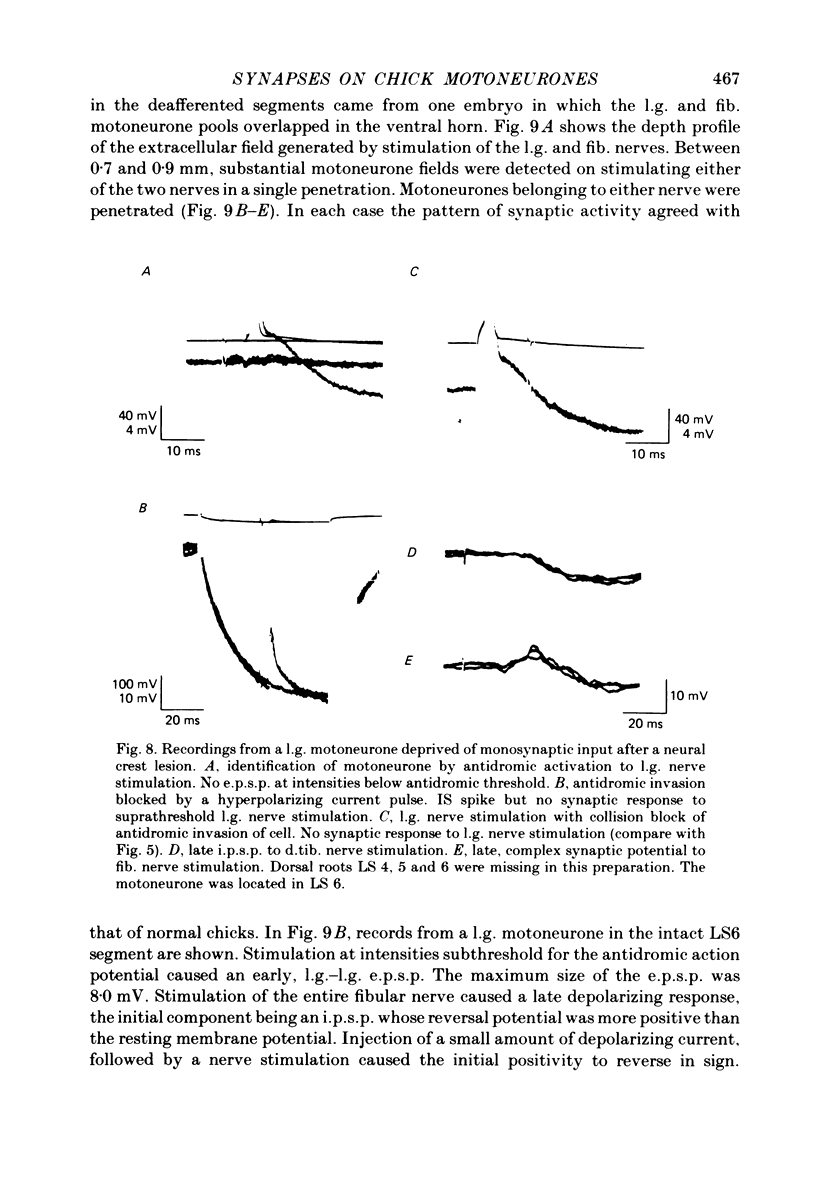
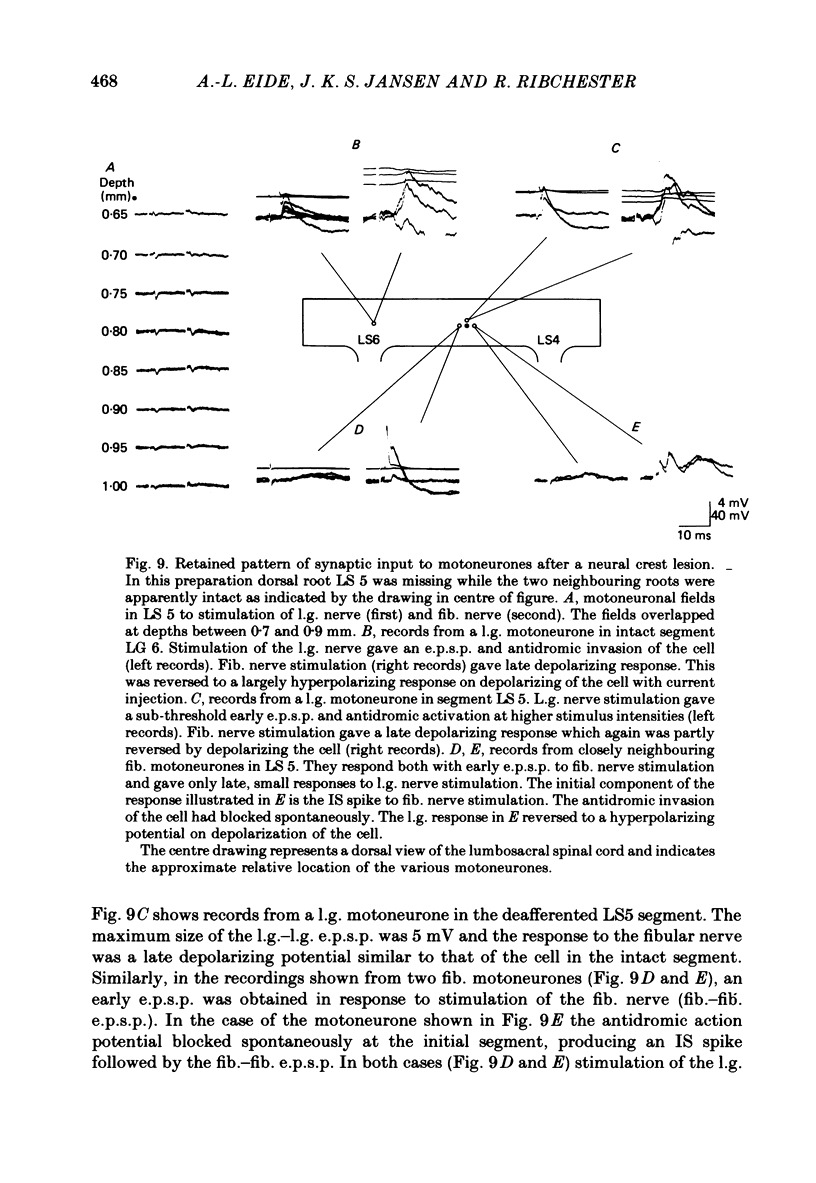
2. The synaptic activity of l.g. motoneurones was also recorded in embryos in which short segments of the lumbar neural crest had been destroyed by microcautery at 3 d of incubation (Stage 18). The embryos developed without sensory ganglia and dorsal roots in the corresponding region.
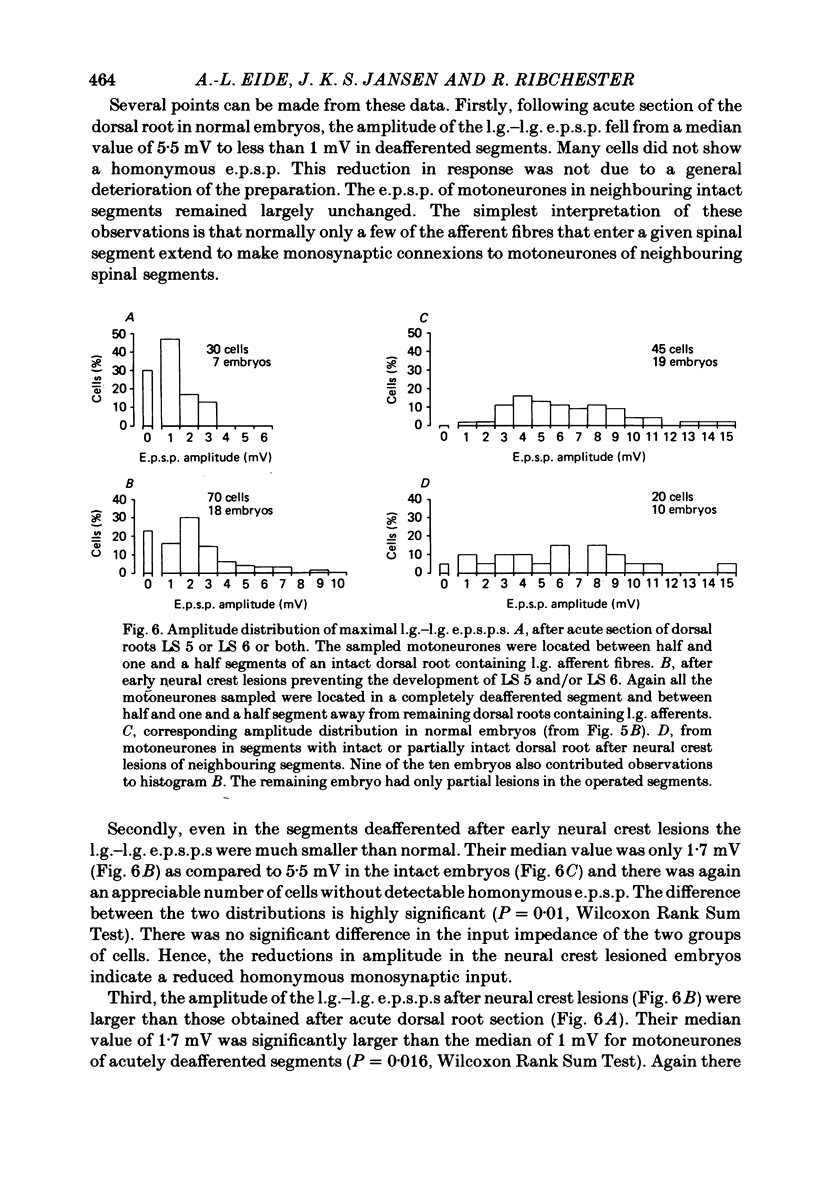
3. At 19-21 d of incubation, the amplitude of the l.g. e.p.s.p. of l.g. motoneurones in deafferented segments was on the average only a half to a third of the amplitude seen in motoneurones of intact spinal segments. However, both the l.g. and synergist e.p.s.p.s were larger than those seen in acutely deafferented segments of normal embryos.
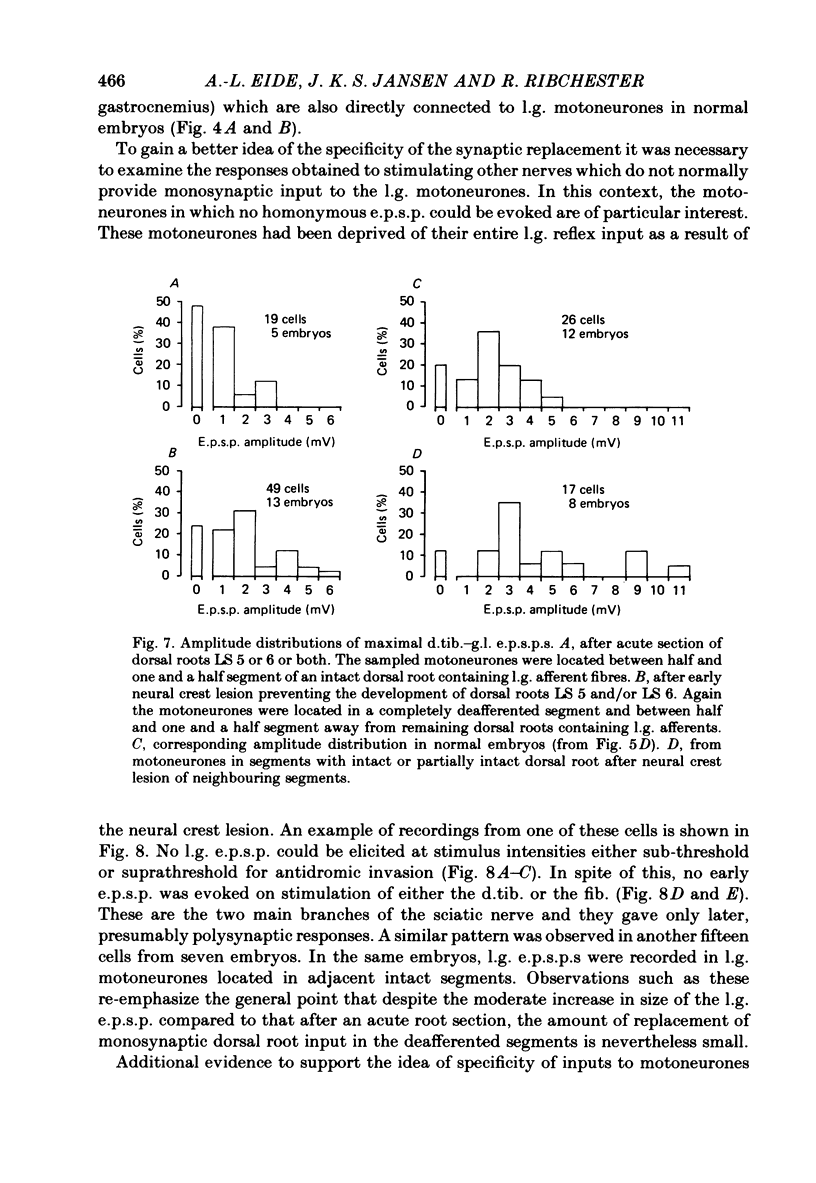
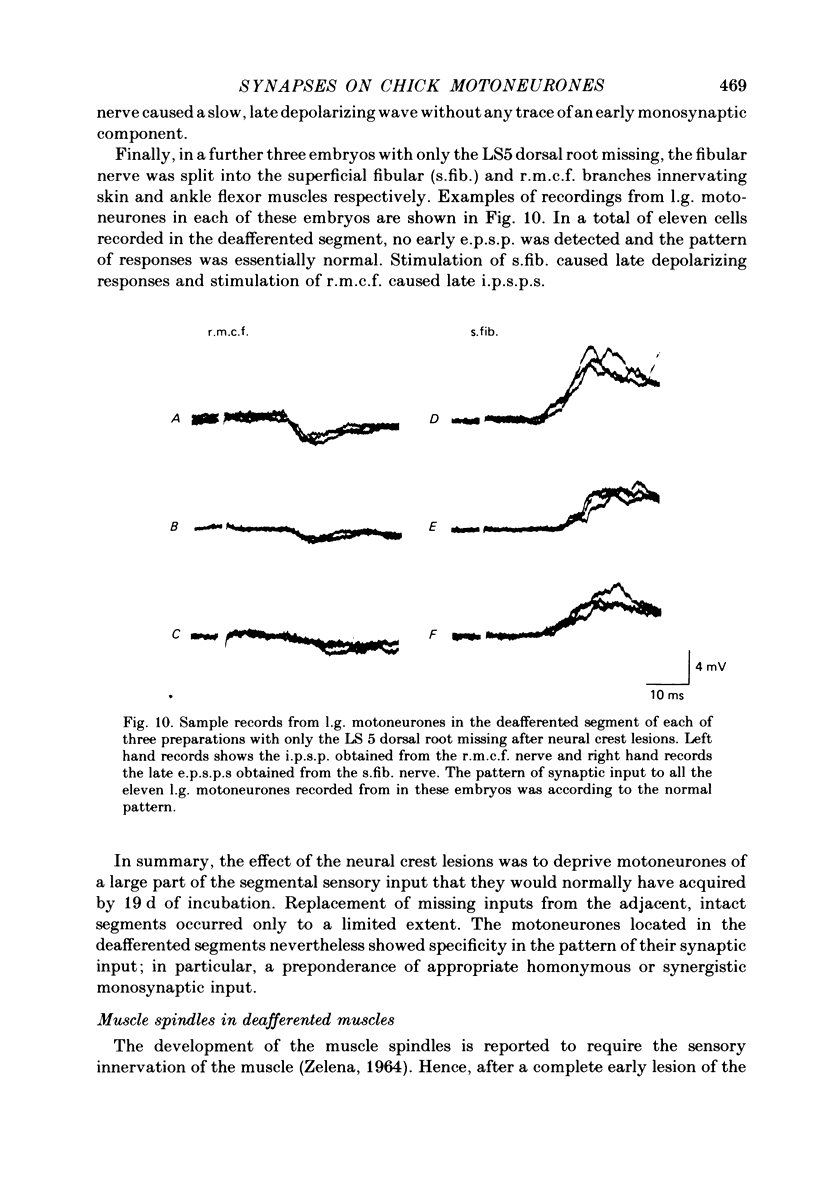
4. In spite of the weak monosynaptic input from l.g. and synergistic afferents, the pattern of synaptic activity evoked by antagonistic muscle afferent or cutaneous afferent stimulation was not different from normal. This was even the case for gastrocnemius motoneurones in which no early e.p.s.p. could be evoked by stimulating the l.g. or synergistic muscle nerves.
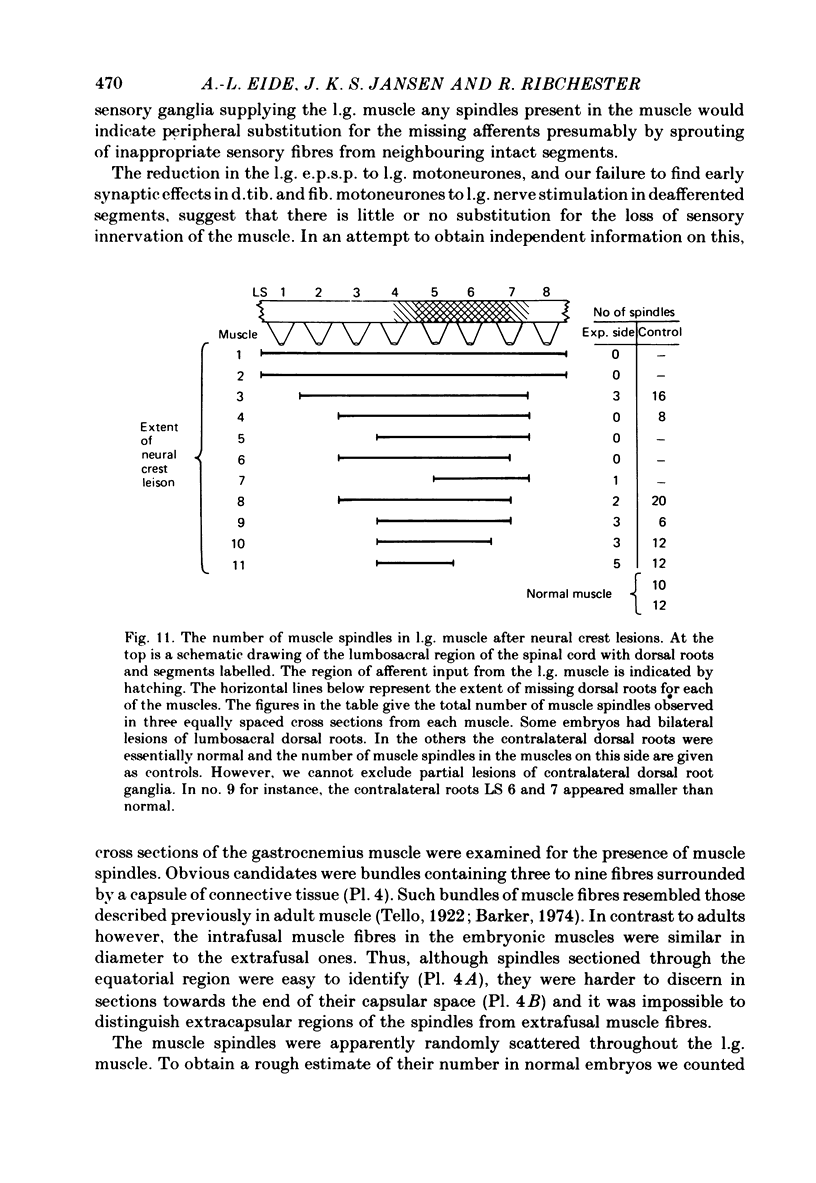
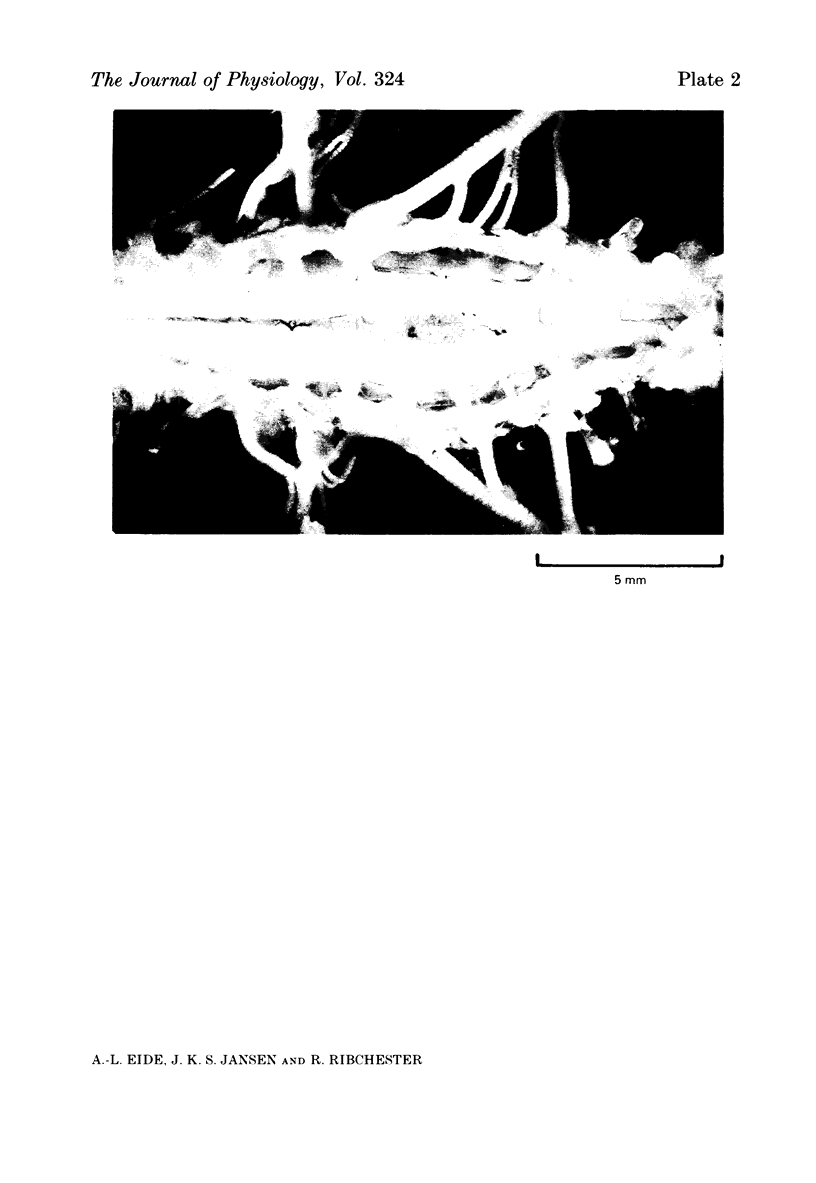
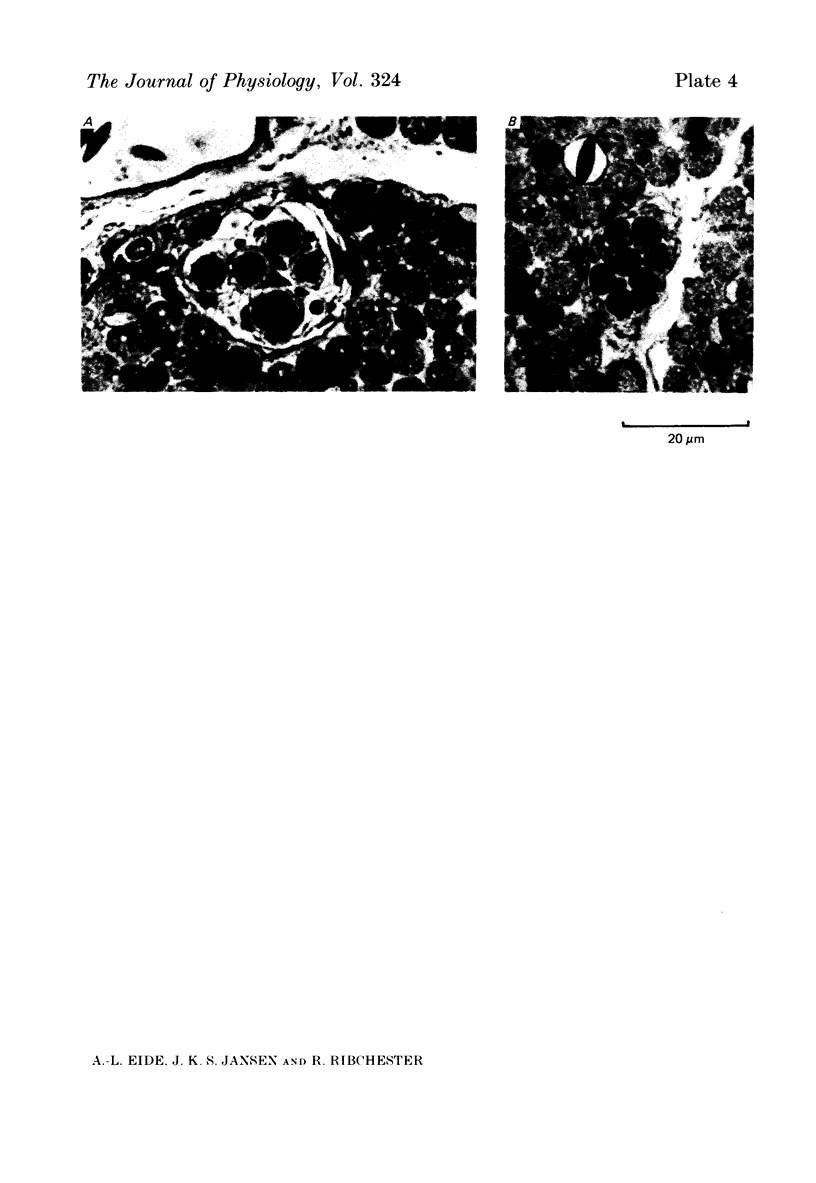
5. No muscle spindles could be seen in sections of l.g. muscles from embryos with extensive lesions of the lumbosacral neural crest. Incomplete lesions of l.g. segments reduced the number of spindles in the muscle.
6. These results suggest that when motoneurones are deprived of part of their normal synaptic input before the formation of peripheral connexions, the identity of the motoneurones (in terms of the origin of their synaptic input) is preserved. Missing synaptic inputs are either replaced by appropriate afferent fibres, if they are available, or not at all. The chick sensory ganglion cells with monosynaptic connexions to motoneurones appear to be unable to compensate significantly for peripheral or central defects in the innervation of the hind limb. They behave as if their developmental possibilities were quite rigidly determined at an early embryonic stage.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez-Leefmans F. J., De Santis A., Miledi R. Effects of some divalent cations on synaptic transmission in frog spinal neurones. J Physiol. 1979 Sep;294:387–406. doi: 10.1113/jphysiol.1979.sp012936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D., Iles J. F. Synaptic transmission: ion concentration changes in the synaptic cleft. Proc R Soc Lond B Biol Sci. 1979 Nov 30;206(1162):115–131. doi: 10.1098/rspb.1979.0095. [DOI] [PubMed] [Google Scholar]
- Betz W. J., Caldwell J. H., Ribchester R. R. The effects of partial denervation at birth on the development of muscle fibres and motor units in rat lumbrical muscle. J Physiol. 1980 Jun;303:265–279. doi: 10.1113/jphysiol.1980.sp013284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Holland R. L., Ironton R. Degenerating nerve products affect innervated muscle fibres. Nature. 1978 Oct 19;275(5681):652–654. doi: 10.1038/275652a0. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Jansen J. K., Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976 Oct;261(2):387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel F., Mariani J., Delhaye-Bouchaud N. Evidence for a multiple innervation of Purkinje cells by climbing fibers in the immature rat cerebellum. J Neurobiol. 1976 Nov;7(6):567–578. doi: 10.1002/neu.480070609. [DOI] [PubMed] [Google Scholar]
- Crossland W. J., Cowan W. M., Rogers L. A., Kelly J. P. The specification of the retino-tectal projection in the chick. J Comp Neurol. 1974 May 15;155(2):127–164. doi: 10.1002/cne.901550202. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. Synaptic actions on motoneurones caused by impulses in Golgi tendon organ afferents. J Physiol. 1957 Sep 30;138(2):227–252. doi: 10.1113/jphysiol.1957.sp005849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. R., Redman S. J., Walmsley B. The effect of polarizing currents on unitary Ia excitatory post-synaptic potentials evoked in spinal motoneurones. J Physiol. 1976 Aug;259(3):705–723. doi: 10.1113/jphysiol.1976.sp011490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg I., Marshall K. C. Reversal potential for Ia excitatory post synaptic potentials in spinal motoneurones of cats. Neuroscience. 1979;4(11):1583–1591. doi: 10.1016/0306-4522(79)90021-6. [DOI] [PubMed] [Google Scholar]
- FRANK K., FUORTES M. G. Stimulation of spinal motoneurones with intracellular electrodes. J Physiol. 1956 Nov 28;134(2):451–470. doi: 10.1113/jphysiol.1956.sp005657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E., Westerfield M. Synaptic organization of sensory and motor neurones innervating triceps brachii muscles in the bullfrog. J Physiol. 1982 Mar;324:479–494. doi: 10.1113/jphysiol.1982.sp014125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E., Westerfield M. The formation of appropriate central and peripheral connexions by foreign sensory neurones of the bullfrog. J Physiol. 1982 Mar;324:495–505. doi: 10.1113/jphysiol.1982.sp014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost D. O., Schneider G. E. Plasticity of retinofugal projections after partial lesions of the retina in newborn Syrian hamsters. J Comp Neurol. 1979 Jun 1;185(3):517–567. doi: 10.1002/cne.901850306. [DOI] [PubMed] [Google Scholar]
- Fulton B. P., Miledi R., Takahashi T. Electrical synapses between motoneurons in the spinal cord of the newborn rat. Proc R Soc Lond B Biol Sci. 1980 Jun 23;208(1170):115–120. doi: 10.1098/rspb.1980.0045. [DOI] [PubMed] [Google Scholar]
- Grinnell A. D. A study of the interaction between motoneurones in the frog spinal cord. J Physiol. 1966 Feb;182(3):612–648. doi: 10.1113/jphysiol.1966.sp007841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollyday M., Hamburger V., Farris J. M. Localization of motor neuron pools supplying identified muscles in normal and supernumerary legs of chick embryo. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3582–3586. doi: 10.1073/pnas.74.8.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N., LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- Lance-Jones C., Landmesser L. Motoneurone projection patterns in embryonic chick limbs following partial deletions of the spinal cord. J Physiol. 1980 May;302:559–580. doi: 10.1113/jphysiol.1980.sp013261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L. T. The generation of neuromuscular specificity. Annu Rev Neurosci. 1980;3:279–302. doi: 10.1146/annurev.ne.03.030180.001431. [DOI] [PubMed] [Google Scholar]
- Landmesser L. The development of motor projection patterns in the chick hind limb. J Physiol. 1978 Nov;284:391–414. doi: 10.1113/jphysiol.1978.sp012546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W. The reorganization of synaptic connexions in the rat submandibular ganglion during post-natal development. J Physiol. 1977 Dec;273(1):155–177. doi: 10.1113/jphysiol.1977.sp012087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G., Gall C., Dunwiddie T. V. Neuroplasticity in the hippocampal formation. Prog Brain Res. 1978;48:113–130. doi: 10.1016/S0079-6123(08)61019-5. [DOI] [PubMed] [Google Scholar]
- MURRAY J. G., THOMPSON J. W. The occurrence and function of collateral sprouting in the sympathetic nervous system of the cat. J Physiol. 1957 Jan 23;135(1):133–162. doi: 10.1113/jphysiol.1957.sp005700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCOUCH G. P., AUSTIN G. M., LIU C. N., LIU C. Y. Sprouting as a cause of spasticity. J Neurophysiol. 1958 May;21(3):205–216. doi: 10.1152/jn.1958.21.3.205. [DOI] [PubMed] [Google Scholar]
- Raisman G. Neuronal plasticity in the septal nuclei of the adult rat. Brain Res. 1969 Jun;14(1):25–48. doi: 10.1016/0006-8993(69)90029-8. [DOI] [PubMed] [Google Scholar]
- Redfern P. A. Neuromuscular transmission in new-born rats. J Physiol. 1970 Aug;209(3):701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades R. W., Chalupa L. M. Effects of neonatal enucleation on receptive-field properties of visual neurons in superior colliculus of the golden hamster. J Neurophysiol. 1980 Mar;43(3):595–611. doi: 10.1152/jn.1980.43.3.595. [DOI] [PubMed] [Google Scholar]
- Ronnevi L. O., Conradi S. Ultrastructural evidence for spontaneous elimination of synaptic terminals on spinal motoneurons in the kitten. Brain Res. 1974 Nov 15;80(2):335–339. doi: 10.1016/0006-8993(74)90696-9. [DOI] [PubMed] [Google Scholar]
- Schimidt J. T., Cicerone C. M., Easter S. S. Expansion of the half retinal projection to the tectum in goldfish: an electrophysiological and anatomical study. J Comp Neurol. 1978 Jan 15;177(2):257–277. doi: 10.1002/cne.901770206. [DOI] [PubMed] [Google Scholar]
- Shapovalov A. I., Shiriaev B. I., Velumian A. A. Mechanisms of post-synaptic excitation in amphibian motoneurones. J Physiol. 1978 Jun;279:437–455. doi: 10.1113/jphysiol.1978.sp012355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So K. F., Schneider G. E. Abnormal recrossing retinotectal projections after early lesions in Syrian hamsters: age-related effects. Brain Res. 1978 May 26;147(2):277–295. doi: 10.1016/0006-8993(78)90840-5. [DOI] [PubMed] [Google Scholar]
- Thompson W., Jansen J. K. The extent of sprouting of remaining motor units in partly denervated immature and adult rat soleus muscle. Neuroscience. 1977;2(4):523–535. doi: 10.1016/0306-4522(77)90049-5. [DOI] [PubMed] [Google Scholar]
- Tsukahara N., Hultborn H., Murakami F., Fujito Y. Electrophysiological study of formation of new synapses and collateral sprouting in red nucleus neurons after partial denervation. J Neurophysiol. 1975 Nov;38(6):1359–1372. doi: 10.1152/jn.1975.38.6.1359. [DOI] [PubMed] [Google Scholar]
- ZELENA J. DEVELOPMENT, DEGENERATION AND REGENERATION OF RECEPTOR ORGANS. Prog Brain Res. 1964;13:175–213. [PubMed] [Google Scholar]