Abstract
1. Double barrel potassium-sensitive micro-electrodes were used to measure fluctuations in extracellular potassium ion concentration in large automatic canine Purkinje fibres.
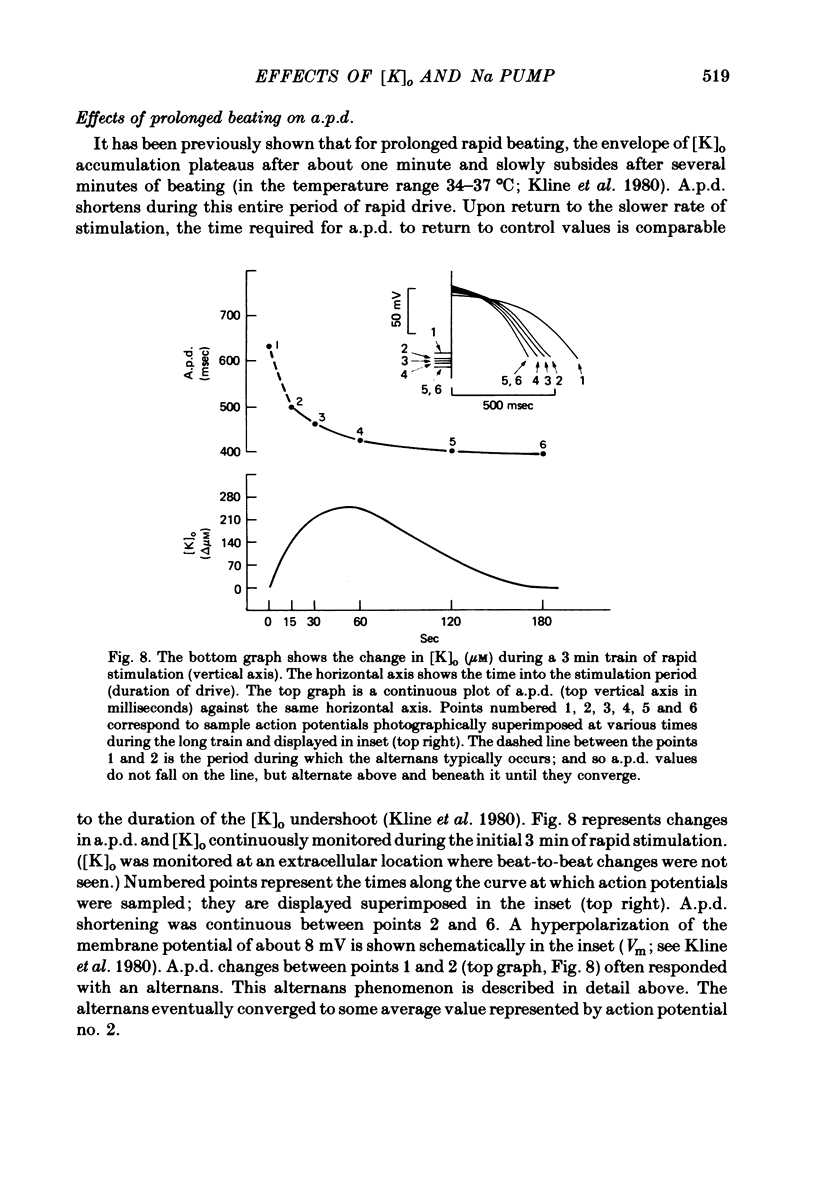
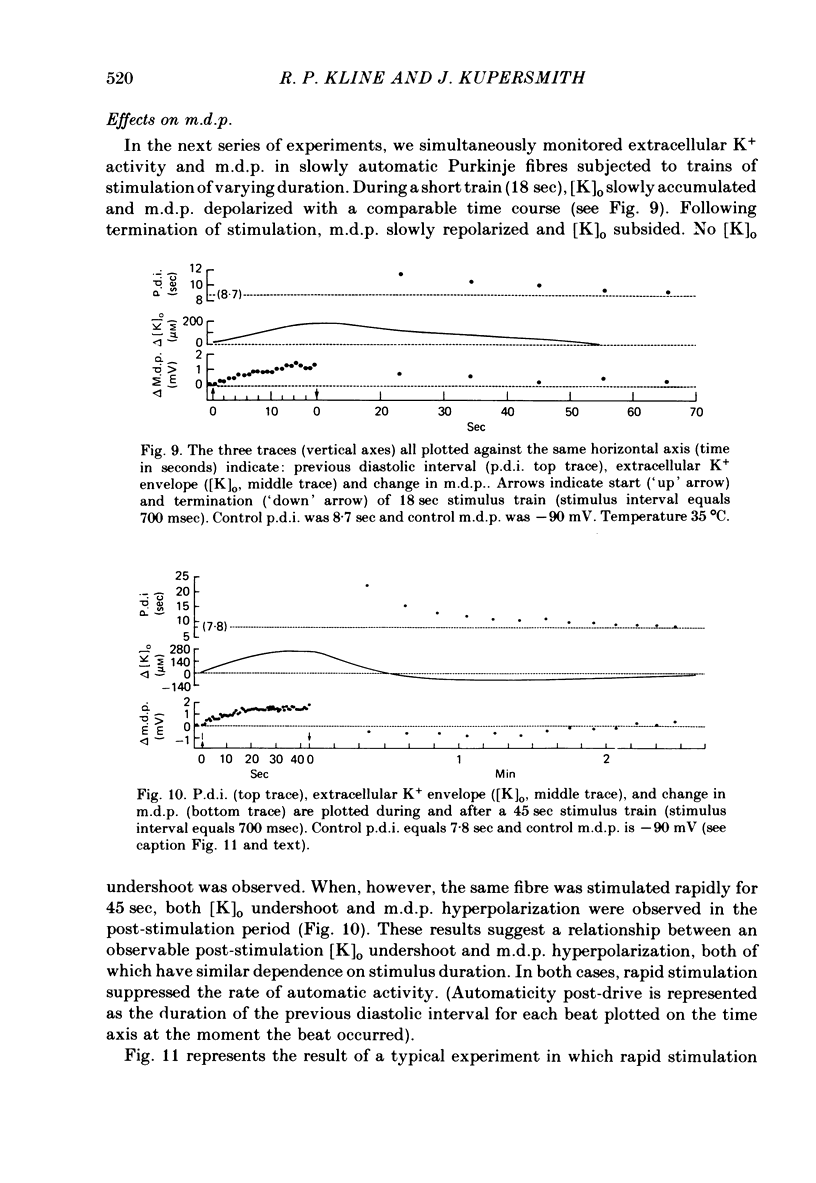
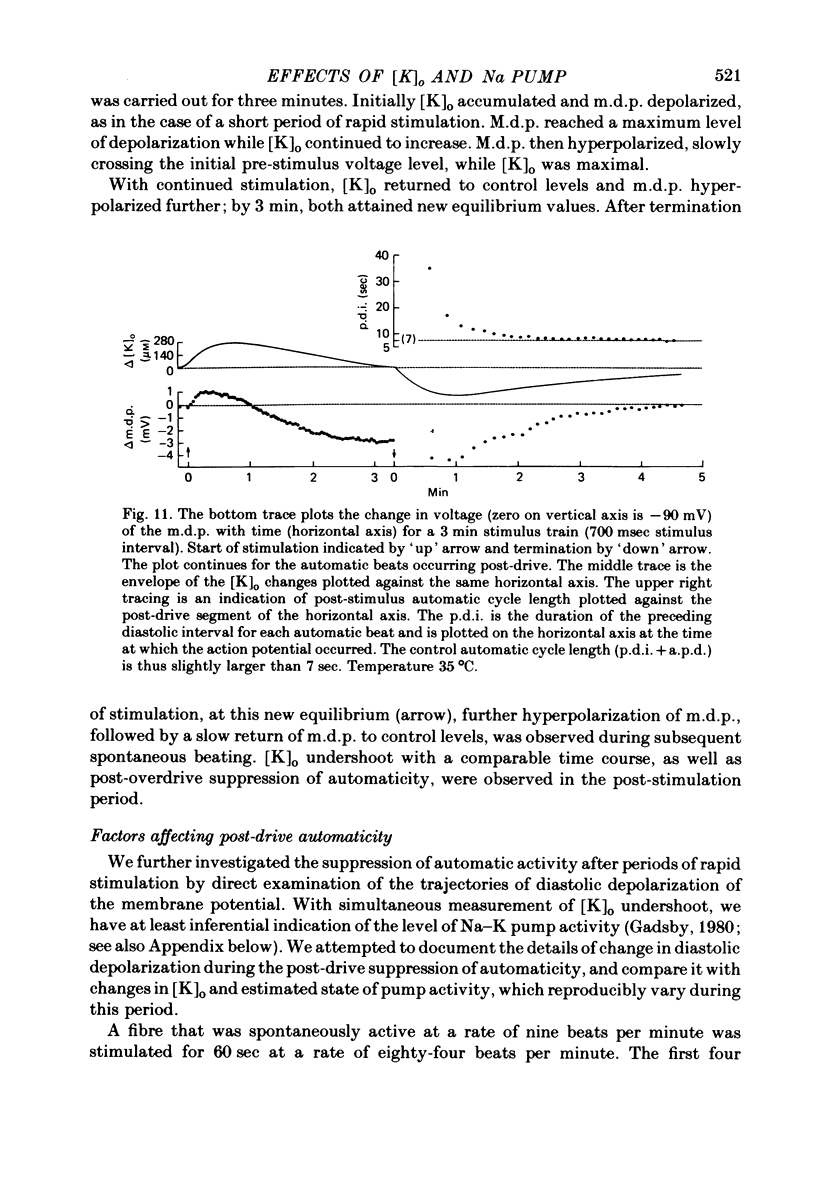
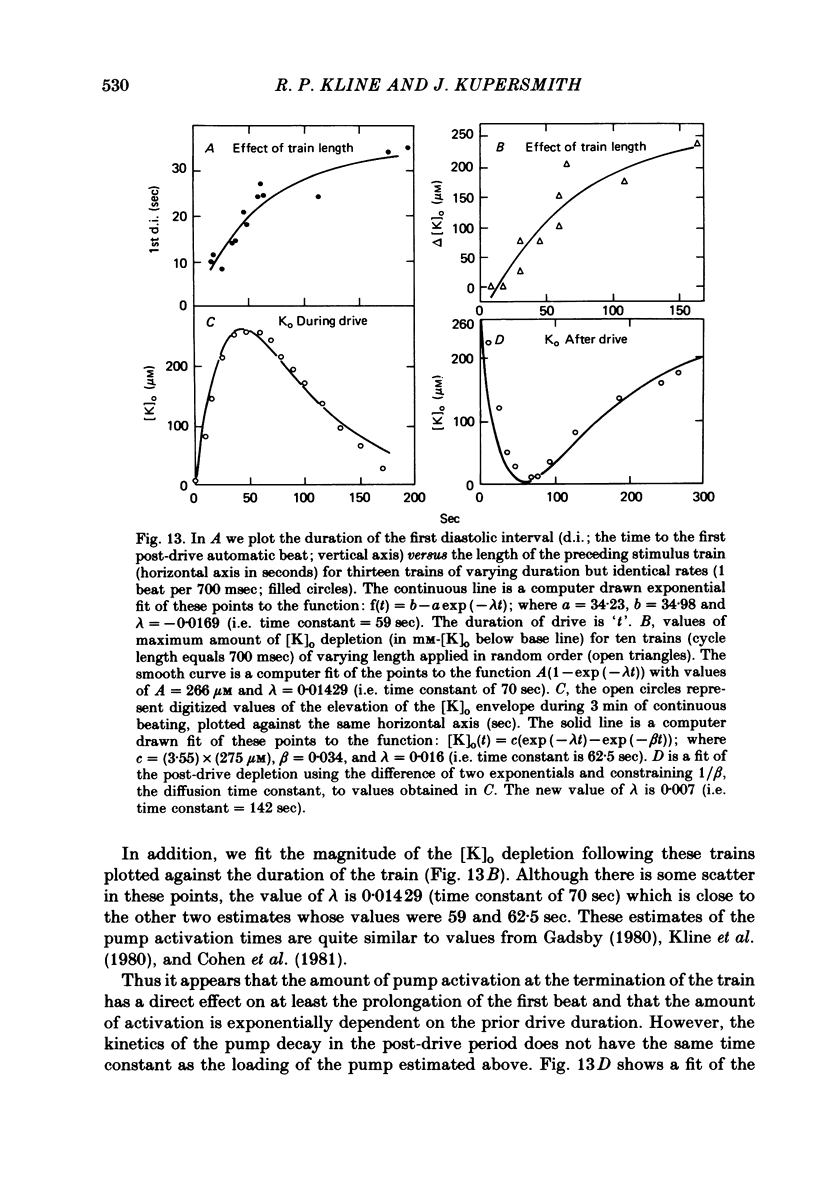
2. Slow accumulations of potassium were seen in the extracellular space during prolonged beating. Following cessation of prolonged beating, a depletion of extracellular potassium ions was noted.
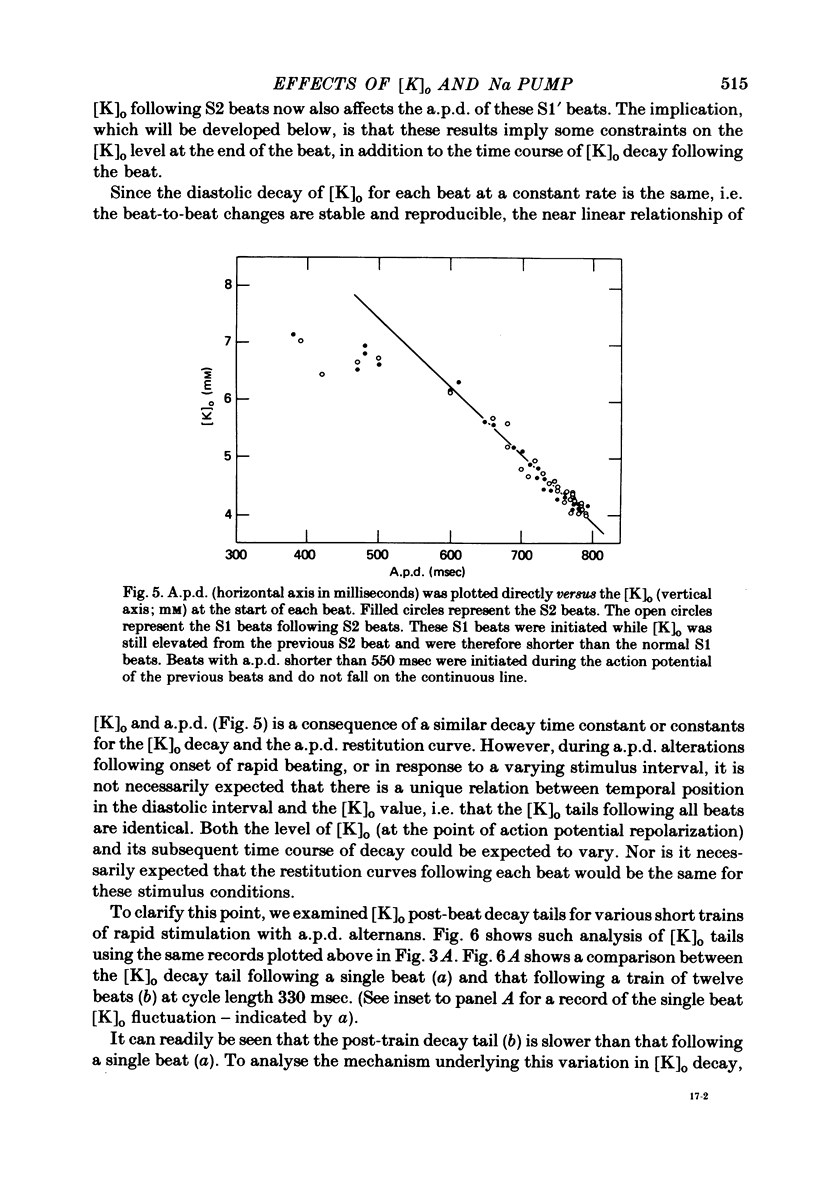
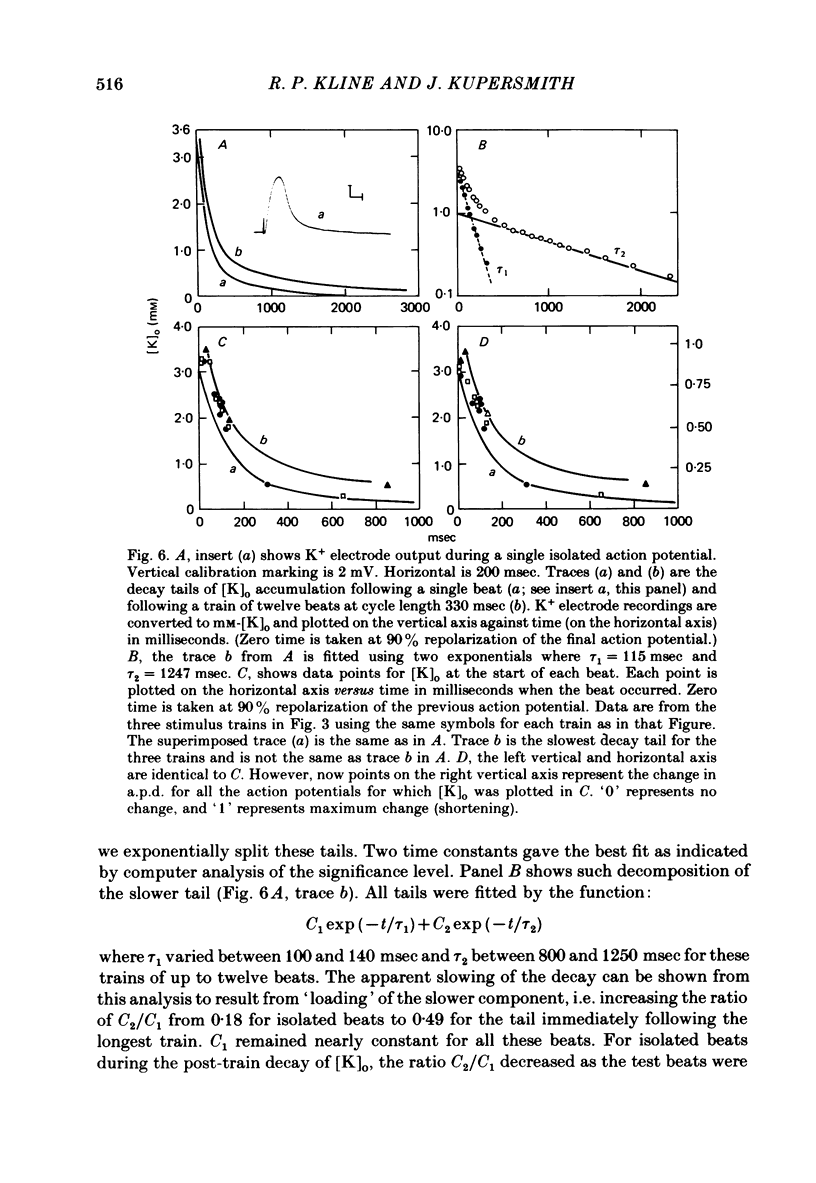
3. The time course of these slow changes in extracellular potassium concentration were shown to be a function of the diffusion properties of the preparation, and the rate and degree of activation of the sodium pump.
4. Action potential duration and maximum diastolic potential were simultaneously monitored during and after these periods of rapid stimulation, using conventional intracellular micro-electrodes.
5. Initial depolarization and later hyperpolarization of the maximum diastolic potential appeared to occur as a direct result of changes in extracellular potassium concentration and level of pump activation induced by the sudden and prolonged alteration in stimulus rate.
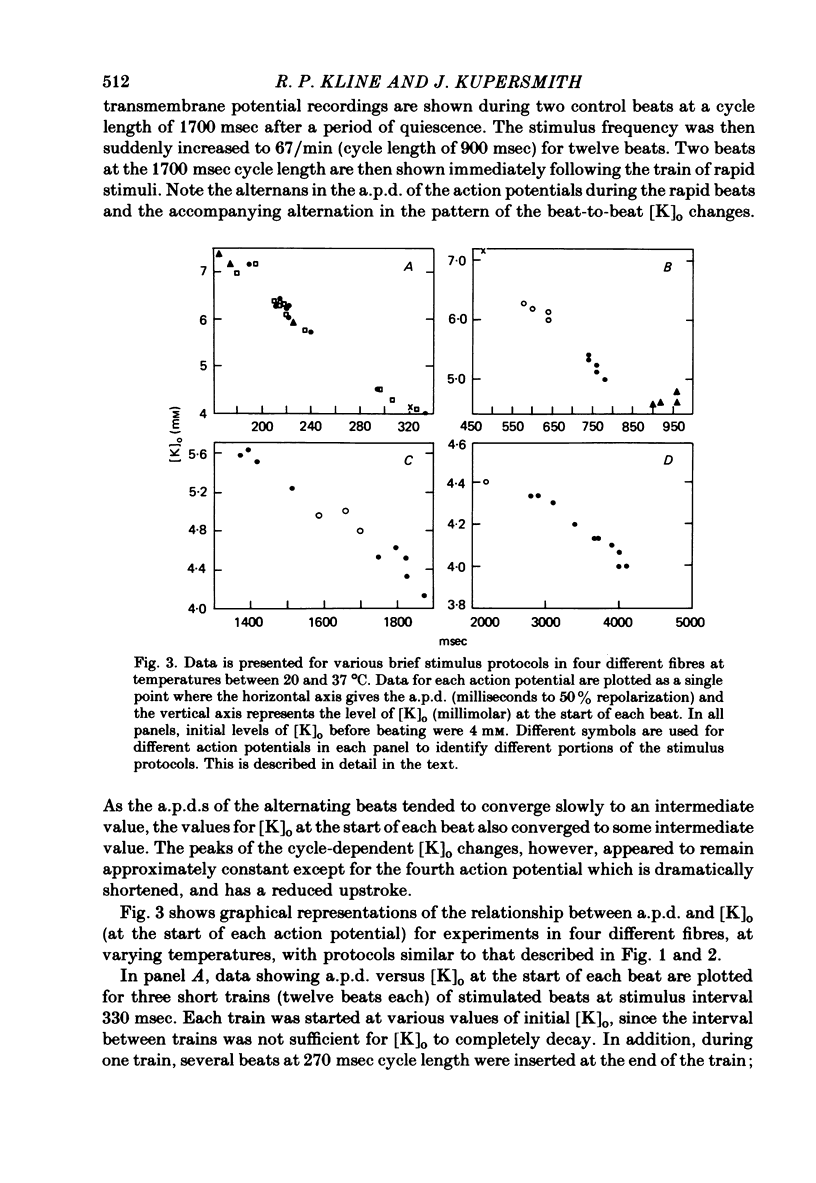
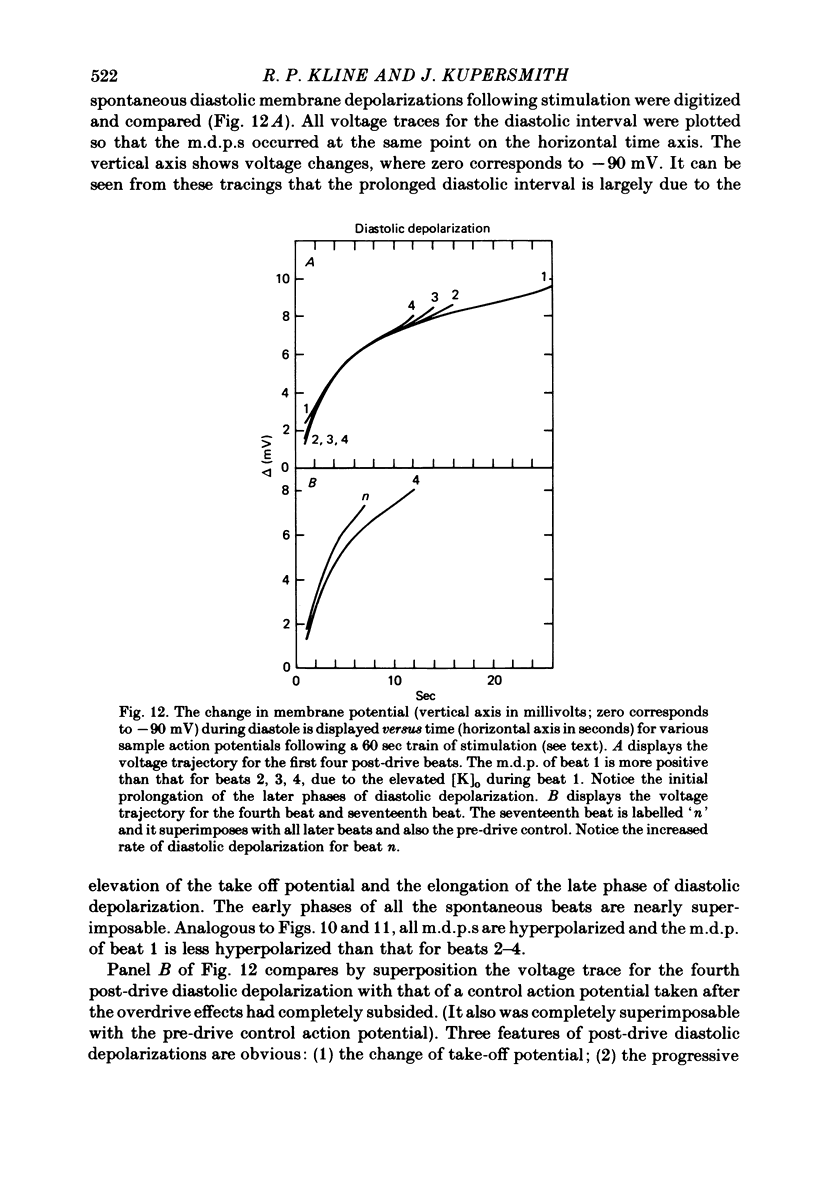
6. Following prolonged stimulation, dramatic changes in the automatic beating rate were correlated with changes in extracellular potassium and pump rate, and their effects on the maximum diastolic potential and other parameters of the diastolic potential depolarization.
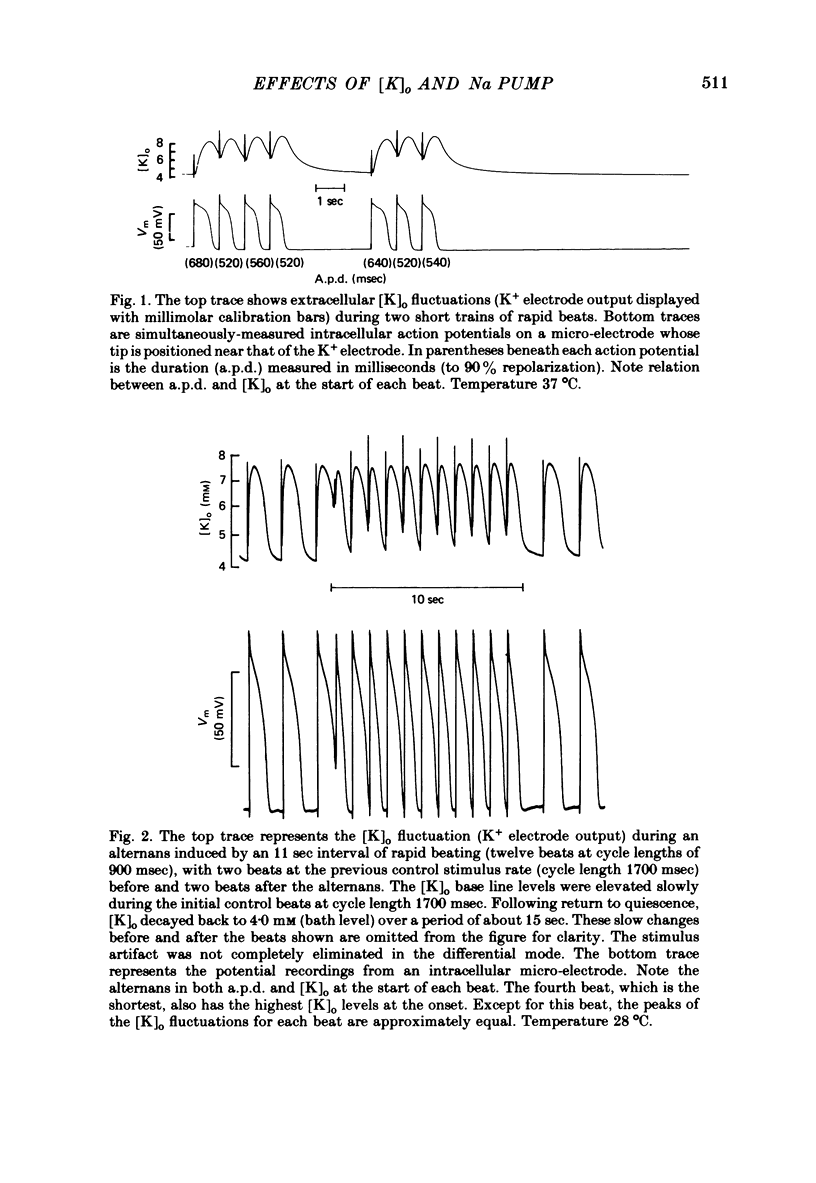
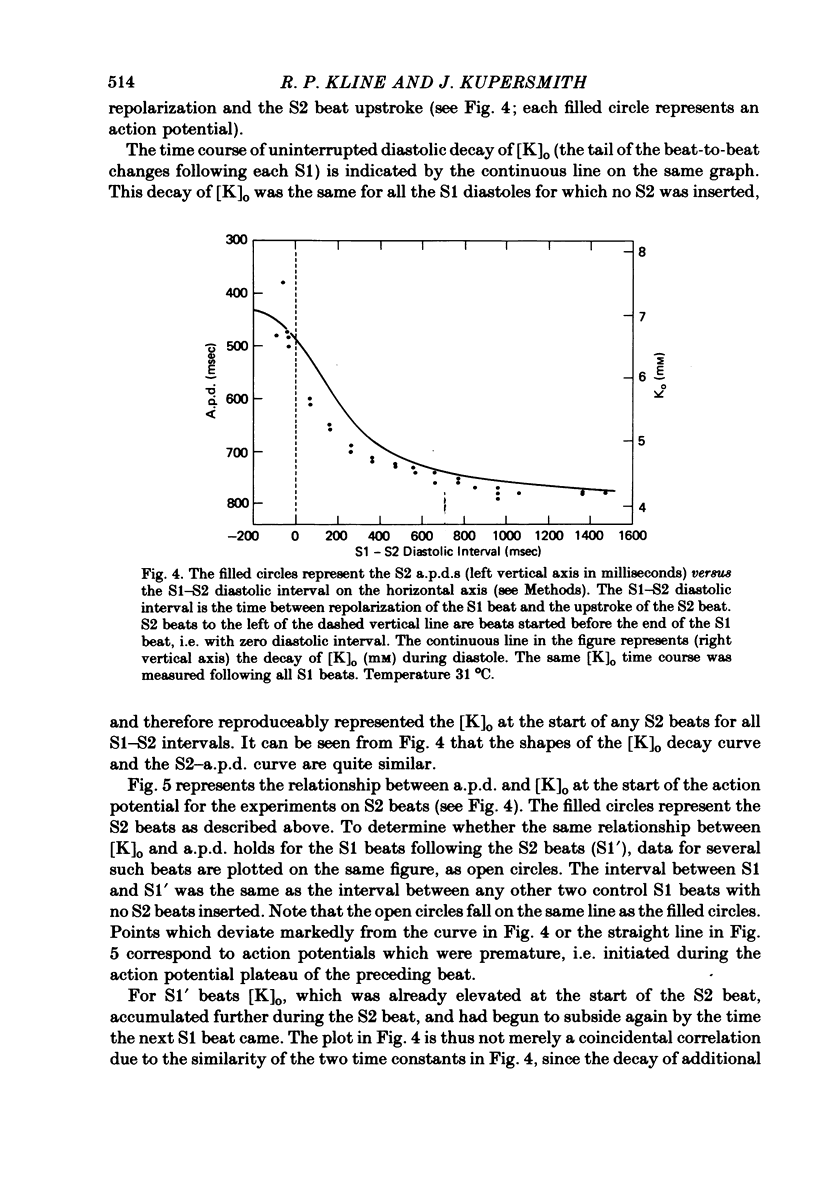
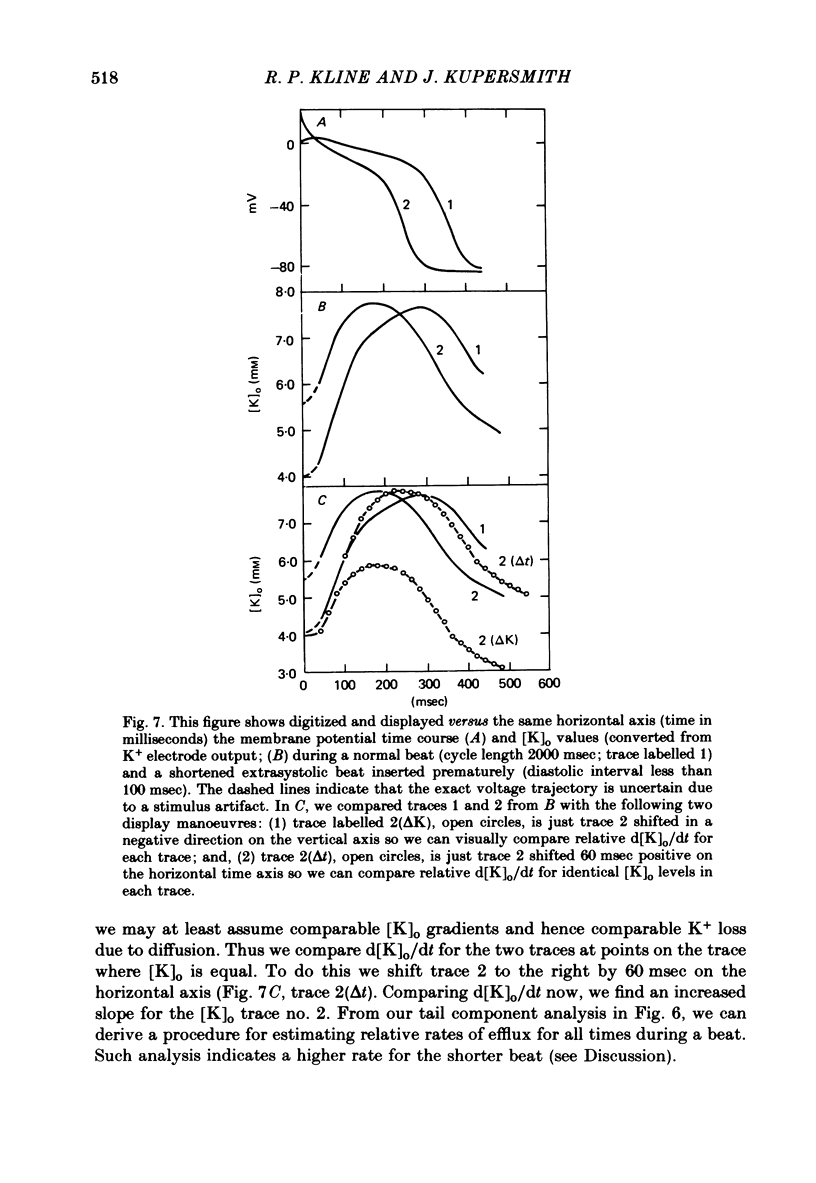
7. For some locations of the potassium-sensitive electrode tip, large fluctuations in extracellular potassium were seen during single beats. Alterations in action potential duration during abrupt rate changes appear to derive in part from modulation by these extracellular potassium concentration fluctuations altering net membrane currents. Slower shifts in action potential duration appear correlated to the degree of sodium pump activation.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attwell D., Eisner D., Cohen I. Voltage clamp and tracer flux data: effects of a restricted extra-cellular space. Q Rev Biophys. 1979 Aug;12(3):213–261. doi: 10.1017/s0033583500005448. [DOI] [PubMed] [Google Scholar]
- Baumgarten C. M., Isenberg G. Depletion and accumulation of potassium in the extracellular clefts of cardiac Purkinje fibers during voltage clamp hyperpolarization and depolarization. Pflugers Arch. 1977 Mar 11;368(1-2):19–31. doi: 10.1007/BF01063450. [DOI] [PubMed] [Google Scholar]
- Boyett M. R., Jewell B. R. Analysis of the effects of changes in rate and rhythm upon electrical activity in the heart. Prog Biophys Mol Biol. 1980;36(1):1–52. doi: 10.1016/0079-6107(81)90003-1. [DOI] [PubMed] [Google Scholar]
- Browning D. J., Tiedeman J. S., Stagg A. L., Benditt D. G., Scheinman M. M., Strauss H. C. Aspects of rate-related hyperpolarization in feline Purkinje fibers. Circ Res. 1979 May;44(5):612–624. doi: 10.1161/01.res.44.5.612. [DOI] [PubMed] [Google Scholar]
- CARMELIET E. Influence du rythme sur la durée du potentiel d'action ventriculaire cardiaque. Arch Int Physiol Biochim. 1955 Jun;63(2):222–232. doi: 10.3109/13813455509150410. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. Repolarisation and frequency in cardiac cells. J Physiol (Paris) 1977;73(7):903–923. [PubMed] [Google Scholar]
- Cleemann L., Morad M. Extracellular potassium accumulation in voltage-clamped frog ventricular muscle. J Physiol. 1979 Jan;286:83–111. doi: 10.1113/jphysiol.1979.sp012608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., Daut J., Noble D. The effects of potassium and temperature on the pace-maker current, iK2, in Purkinje fibres. J Physiol. 1976 Aug;260(1):55–74. doi: 10.1113/jphysiol.1976.sp011504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., Eisner D., Noble D. The action of adrenaline on pace-maker activity in cardiac Purkinje fibres. J Physiol. 1978 Jul;280:155–168. doi: 10.1113/jphysiol.1978.sp012378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., Falk R., Kline R. Membrane currents following activity in canine cardiac Purkinje fibers. Biophys J. 1981 Feb;33(2):281–288. doi: 10.1016/S0006-3495(81)84890-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colatsky T. J., Hogan P. M. Effects of external calcium, calcium channel-blocking agents, and stimulation frequency on cycle length-dependent changes in canine cardiac action potential duration. Circ Res. 1980 Apr;46(4):543–552. doi: 10.1161/01.res.46.4.543. [DOI] [PubMed] [Google Scholar]
- Deitmer J. W., Ellis D. The intracellular sodium activity of cardiac Purkinje fibres during inhibition and re-activation of the Na-K pump. J Physiol. 1978 Nov;284:241–259. doi: 10.1113/jphysiol.1978.sp012539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. A new interpretation of the pace-maker current in calf Purkinje fibres. J Physiol. 1981 May;314:359–376. doi: 10.1113/jphysiol.1981.sp013713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Noble D. On the time course of extracellular ion concentration changes in frog atrium [proceedings]. J Physiol. 1979 Nov;296:80P–80P. [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J. The role of the sodium pump in the effects of potassium-depleted solutions on mammalian cardiac muscle. J Physiol. 1979 Sep;294:279–301. doi: 10.1113/jphysiol.1979.sp012930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREYGANG W. H., Jr, GOLDSTEIN D. A., HELLAM D. C., PEACHEY L. D. THE RELATION BETWEEN THE LATE AFTER-POTENTIAL AND THE SIZE OF THE TRANSVERSE TUBULAR SYSTEM OF FROG MUSCLE. J Gen Physiol. 1964 Nov;48:235–263. doi: 10.1085/jgp.48.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C. Activation of electrogenic Na+/K+ exchange by extracellular K+ in canine cardiac Purkinje fibers. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4035–4039. doi: 10.1073/pnas.77.7.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C., Cranefield P. F. Direct measurement of changes in sodium pump current in canine cardiac Purkinje fibers. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1783–1787. doi: 10.1073/pnas.76.4.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C., Cranefield P. F. Electrogenic sodium extrusion in cardiac Purkinje fibers. J Gen Physiol. 1979 Jun;73(6):819–837. doi: 10.1085/jgp.73.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausworth O., Noble D., Tsien R. W. The dependence of plateau currents in cardiac Purkinje fibres on the interval between action potentials. J Physiol. 1972 Apr;222(1):27–49. doi: 10.1113/jphysiol.1972.sp009786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline R. P., Cohen I., Falk R., Kupersmith J. Activity-dependent extracellular K+ fluctuations in canine Purkinje fibres. Nature. 1980 Jul 3;286(5768):68–71. doi: 10.1038/286068a0. [DOI] [PubMed] [Google Scholar]
- Kline R. P., Morad M. Potassium efflux in heart muscle during activity: extracellular accumulation and its implications. J Physiol. 1978 Jul;280:537–558. doi: 10.1113/jphysiol.1978.sp012400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline R., Morad M. Potassium efflux and accumulation in heart muscle. Evidence from K +/- electrode experiments. Biophys J. 1976 Apr;16(4):367–372. doi: 10.1016/S0006-3495(76)85694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronhaus K. D., Spear J. F., Moore E. N., Kline R. P. Sinus node extracellular potassium transients following vagal stimulation. Nature. 1978 Sep 28;275(5678):322–324. doi: 10.1038/275322a0. [DOI] [PubMed] [Google Scholar]
- Kunze D. L. Rate-dependent changes in extracellular potassium in the rabbit atrium. Circ Res. 1977 Jul;41(1):122–127. doi: 10.1161/01.res.41.1.122. [DOI] [PubMed] [Google Scholar]
- Lee C. O., Fozzard H. A. Activities of potassium and sodium ions in rabbit heart muscle. J Gen Physiol. 1975 Jun;65(6):695–708. doi: 10.1085/jgp.65.6.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux H. D., Neher E. The equilibration time course of (K + ) 0 in cat cortex. Exp Brain Res. 1973 Apr 30;17(2):190–205. doi: 10.1007/BF00235028. [DOI] [PubMed] [Google Scholar]
- McAllister R. E., Noble D. The time and voltage dependence of the slow outward current in cardiac Purkinje fibres. J Physiol. 1966 Oct;186(3):632–662. doi: 10.1113/jphysiol.1966.sp008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister R. E., Noble D., Tsien R. W. Reconstruction of the electrical activity of cardiac Purkinje fibres. J Physiol. 1975 Sep;251(1):1–59. doi: 10.1113/jphysiol.1975.sp011080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura D. S., Hoffman B. F., Rosen M. R. The effect of extracellular potassium on the intracellular potassium ion activity and transmembrane potentials of beating canine cardiac Purkinje fibers. J Gen Physiol. 1977 Apr;69(4):463–474. doi: 10.1085/jgp.69.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Lux H. D. Rapid changes of potassium concentration at the outer surface of exposed single neurons during membrane current flow. J Gen Physiol. 1973 Mar;61(3):385–399. doi: 10.1085/jgp.61.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble S. J. Potassium accumulation and depletion in frog atrial muscle. J Physiol. 1976 Jul;258(3):579–613. doi: 10.1113/jphysiol.1976.sp011436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape L. G., Katzman R. Response of glia in cat sensorimotor cortex to increased extracellular potassium. Brain Res. 1972 Mar 10;38(1):71–92. doi: 10.1016/0006-8993(72)90590-2. [DOI] [PubMed] [Google Scholar]
- Spear J. F., Kronhaus K. D., Moore E. N., Kline R. P. The effect of brief vagal stimulation on the isolated rabbit sinus node. Circ Res. 1979 Jan;44(1):75–88. doi: 10.1161/01.res.44.1.75. [DOI] [PubMed] [Google Scholar]
- Vassalle M. Electrogenic suppression of automaticity in sheep and dog purkinje fibers. Circ Res. 1970 Sep;27(3):361–377. doi: 10.1161/01.res.27.3.361. [DOI] [PubMed] [Google Scholar]
- Vassalle M. The relationship among cardiac pacemakers. Overdrive suppression. Circ Res. 1977 Sep;41(3):269–277. doi: 10.1161/01.res.41.3.269. [DOI] [PubMed] [Google Scholar]
- Vereecke J., Isenberg G., Carmeliet E. K efflux through inward rectifying K channels in voltage clamped Purkinje fibers. Pflugers Arch. 1980 Apr;384(3):207–217. doi: 10.1007/BF00584555. [DOI] [PubMed] [Google Scholar]
- WEIDMANN S. Shortening of the cardiac action potential due to a brief injection of KCl following the onset of activity. J Physiol. 1956 Apr 27;132(1):157–163. doi: 10.1113/jphysiol.1956.sp005510. [DOI] [PMC free article] [PubMed] [Google Scholar]



