Abstract
Many angiosperms, both monocotyledons and dicotyledons, heavily impregnate their vegetative and reproductive organs with solid particles of silicon dioxide (SiO2) known as opaline phytoliths. The underlying mechanisms accounting for the formation of phytoliths in plants are poorly understood, however. Using wild and domesticated species in the genus Cucurbita along with their F1 and F2 progeny, we have demonstrated that the production of large diagnostic phytoliths in fruit rinds exhibits a one-to-one correspondence to the lignification of these structures. We propose that phytolith formation in Cucurbita fruits is primarily determined by a dominant genetic locus, called hard rind (Hr), previously shown to code for lignin deposition. If true, this evidence represents a demonstration of genetic control over phytolith production in a dicotyledon and provides considerable support to hypotheses that silica phytoliths constitute another important system of mechanical defense in plants. Our research also identifies Hr as another single locus controlling more than one important phenotypic difference between wild and domesticated plants, and establishes rind tissue cell structure and hardness under the effects of Hr as an important determinant of phytolith morphology. When recovered from pre-Columbian archaeological sites, Cucurbita phytoliths represent genetically controlled fossil markers of exploitation and domestication in this important economic genus.
The past decade has witnessed considerable growth in the application of plant opal phytolith analysis to archaeological and paleoecological research (1–3). The strengths and limitations of the phytolith record in the study of plant domestication and past climatic and vegetational changes are being clarified in a substantial number of regions of the world (3–10). However, despite accumulating evidence that silicon is necessary for the normal growth and development of some plant species, and provides mechanisms for detoxification, structural support, and protection from animal herbivores in others (1, 11–15), why plants make phytoliths and why they make them in the manifold forms that increasingly are being described from modern plants and ancient sediments are not well understood.
Fruit rinds of the New World genus Cucurbita (squashes, pumpkins, and yellow-flowered gourds), which contributed at least five domesticated species to the roster of American crop plants (16), form distinctive, large spherical phytoliths with surface ornamentations consisting of deep and contiguous scalloped concavities (17, 18) (hereafter, called scalloped phytoliths) (Fig. 1). Large surveys of the Cucurbitaceae indicate that types of scalloped phytoliths are limited to the tribe Cucurbiteae, and that those in Cucurbita are diagnostic at the genus level (9, 17, 18). Studies of phytolith formation in other structures of these plants (e.g., leaves, tendrils, peduncles, stems, fruit pulp) also demonstrate that scalloped phytoliths are rind-specific (9, 17, 18). They survive in archaeological sites long after fruits have decayed, and thus may have significant utility for documenting prehistoric exploitation and domestication in Cucurbita (17, 18).
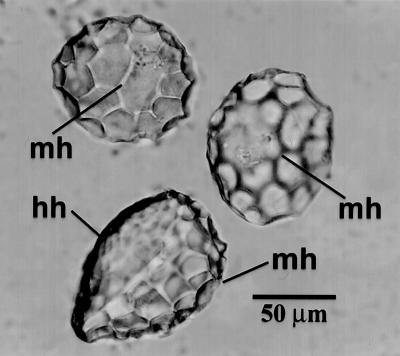
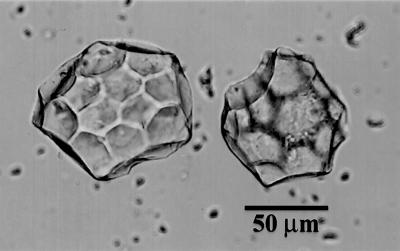
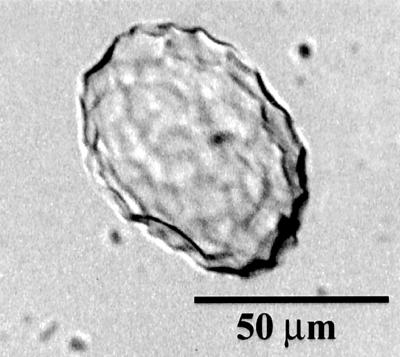
Fig 1.
Spherical, scalloped phytoliths isolated from a rind of C. moschata. The phytolith at the bottom is rotated to show the two distinct hemispheres with scalloped impressions of different sizes created from the hypodermal (hh) and mesocarpal (mh) cells. The two phytoliths above it are oriented with their mesocarpal hemispheres (mh) facing upward. These types of phytoliths are characteristic of many species and varieties of Cucurbita.
During the course of our investigations, we became aware that wild species of Cucurbita, all of which in our possession have a hard, lignified rind, uniformly produce scalloped phytoliths. In domesticated species, however, in which both soft, nonlignified and lignified rinds occur, phytolith production varies considerably. Preliminary examinations of thin sections of these rinds revealed that scalloped phytoliths were formed only in a sharply defined zone located at the interface of the hypodermis and the schlerenchymatized outer mesocarp, that we subsequently refer to as the phytolith formation zone (PFZ) (Fig. 2). Reports also identify cells in the outer mesocarp, the “stone cells,” as the major site of rind lignification (19).
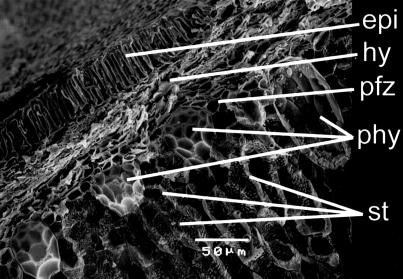
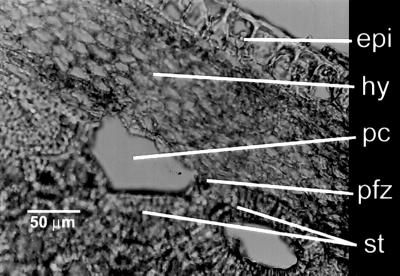
Fig 2.
Scanning electron microscopy (SEM) micrograph of a cross section through a lignified rind from C. moschata showing the hypodermis (hy), stone cells (st), phytoliths (phy), and phytolith forming zone (pfz). epi, epicarp (outer surface) of the rind. The stone cells are elongated, leading to elliptical phytoliths with elongated meoscarp-derived hemispheres and impressions (phytolith on the right) unlike those in Fig. 1.
Lignin is a primary means of defense against herbivory and fungal diseases marshaled by plants. Lignification in Cucurbita has been best studied in the species Cucurbita pepo L., where it is controlled by the dominant genetic locus Hr (hard rind) (20–22). It is likely that a gene homologous to Hr governs lignification in other Cucurbita species (23). Cucurbita evolution under domestication involved a selection for softer, nonlignified rinds (homozygous for hr), although lignified rinds are still common in some domesticated varieties. Because lignin is mostly incorporated into Cucurbita rinds at or close to the time at which fruits reach maturity (ref. 22; also see below), and lignified fruits typically preserve longer than nonlignified varieties, differences in when squash fruits are eaten (mature vs. immature) and storage life considerations can probably account for the persistence of lignified land races today.
In the first research to demonstrate the genetic regulation of phytolith production in a plant, lignification and silicification in the seed bracts and cupules of maize (Zea mays L.) and its wild ancestor, teosinte (Zea mays ssp. parviglumis Iltis and Doebley) were shown to be largely governed by a single Mendelian locus called teosinte glume architecture1 (tga1) (24). Tga1 controls which cells of Zea bracts become silicified and how heavily ornamented the phytoliths are, leading to the production of distinguishable phytolith assemblages in the fruit bracts of teosinte and maize (1, 24). In light of that study, we examined the relationships between rind lignification and phytolith production, location, and morphology in various wild and domesticated species of Cucurbita, and in the progeny of F1 and F2 crosses made from these plants.
Materials and Methods
We studied a total of 148 mature fruits comprising more than 100 different populations, land races, and varieties from nine wild, five domesticated, and one semidomesticated species of Cucurbita as follows. Wild Species:
1. C. pepo ssp. fraterna (L.H. Bailey) AndresM (5 L/S, 0 NL/S). 2. C. pepo ssp. ovifera (L.) var. texana(Scheele) D.S. DeckerUSA(3 L/S, 0 NL/S). 3. Cucurbita argyrosperma Huber ssp. sororia (L.H. Bailey) Merrick and BatesM,P (26 L/S, 0 NL/S). 4. Cucurbita maxima Duchesne ssp. andreana (Naud.) A.I. FilovB (2 L/S, 0 NL/S). 5. Cucurbita lundelliana L.H. BaileyM,G (3 L/S, 0 NL/S). 6. Cucurbita foetidissima HBKUSA,M (4 L/S, 0 NL/S). 7. Cucurbita palmata Watf.USA (3 L/S, 0 NL/S). 8. Cucurbita digitata A. GrayUSA,M (2 L/S, 0 NL/S). 9. Cucurbita pedatifolia L. H. BaileyM (2 L/S, 0 NL/S).
Domesticated Species: 1. C. argyrosperma Huber ssp. argyrospermaM,USA (6 L/S, 1 NL/S). 2. Cucurbita moschata DuchesneC,E,P,M,PR,V (15 L/S, 20 NL/S). 3. Cucurbita ficifolia BouchéE,P,USA (7 L/S, 0 NL/S). 4. C. maxima Duchesne ssp. maximaE,B,USA (5 L/S and 4 NL/S). 5a. C. pepo L. ssp. pepoE,N,USA,Y (5 L/S, 2 NL/S). 5b. C. pepo ssp. ovifera (L.) D.S. Decker var. ovifera (L.) D.S. DeckerUSA (7 L/S , 11 NL/S). Semidomesticated: Cucurbita ecuadorensis Cutler & WhitakerE (15 L/S, 0 NL/S). Superscripts after the species names indicate the locale from where the fruits derived (M, Mexico; N, Nigeria; P, Panama; PR, Puerto Rico; C, Colombia; V, Venezuela; E, Ecuador; B, Bolivia; G, Guatemala; Y, Yugoslavia). Numbers and letters in parentheses indicate the number of fruits studied and their lignification/silicification patterns (e.g., 7 L/S, 11 NL/S = 18 fruits, of which 7 were lignified and silicified and 11 were not lignified and silicified).
We also analyzed 166 fruits of F1 and F2 progeny of crosses made from combinations of lignified and nonlignified parents (Table 1). The crosses were made and grown by L.W.-B. and T.C.A. at the University of Puerto Rico, Agricultural Experiment Station, Isabela, Puerto Rico Substation. The other fruit samples came from three sources: (i) field collections made by various investigators, (ii) specimens grown from seed by L.W.-B. at the Isabela substation, and (iii) commercial sources (for more details on these plants see Table 2, which is published as supporting information on the PNAS web site, www.pnas.org). Each fruit rind was scored for lignification, from plus one (soft = no detectable lignin, rind rolls) to plus three (very hard = heavily lignified, rind does not roll), using a thumbnail penetration test in at least three places at the widest part of the fruit.
Table 1.
Segregation of lignification and silicification in F1 and F2 progenies of crosses between lignified × lignified parents, lignified × nonlignified parents, and nonlignified × nonlignified parents
| Cross
|
No. fruits
|
Observed ratio | Expected ratio in a single, dominant gene model
|
Chi-square/ probability
|
|
|---|---|---|---|---|---|
| Lignified and silicified | Nonlignified, no silicification | ||||
| F1 generation | |||||
| C. sororia 532411M × C. sororia 19P | 16 | 16 | 0 | All lig./sil. | |
| C. sororia 18P × C. argyrosperma 532340M | 8 | 8 | 0 | All lig./sil. | |
| C. argyrosperma 532340M × C. sororia 18P | 22 | 22 | 0 | All lig./sil. | |
| C. sororia 532411M × C. moschata 14-1P | 21 | 21 | 0 | All lig./sil. | |
| C. sororia 532393M × C. moschata 14-1P | 4 | 4 | 0 | All lig./sil. | |
| C. sororia 532411M × C. moschata 162C | 8 | 8 | 0 | All lig./sil. | |
| C. sororia 532411M × C. moschata 166M | 14 | 14 | 0 | All lig./sil. | |
| C. argyrosperma ‘Green Striped Cushaw’USA × C. moschata 166M | 7 | 0 | 7 | All nonlig./no sil. | |
| F2 generation | |||||
| (C. sororia 532411 × C. moschata 162)⊗ | 20 | 15 | 5 | 3:1 | 0.000/P > 0.99 |
| (C. sororia 532411 × C. moschata 166)⊗ | 30 | 23 | 7 | 3:1 | 0.044/P > 0.99 |
| (C. argyrosperma ‘Green Striped Cushaw’ × C. moschata 166)⊗ | 16 | 0 | 16 | All nonlig./no sil. | |
Bold printed parents are lignified and silicified.
One fruit per progeny was utilized.
One rind was completely eaten. Another was too damaged to study completely; however, stone cells were observed. The first was scored as nonlignified because no lignified rinds in the study experienced damage and the second as lignified. Superscripts after species names indicate countries of origin.
We made thin sections of rinds by using a razor blade and studied them with an Olympus BHS microscope at a power of 160× (Olympus, New Hyde Park, NY). We recorded the following features of rind tissue as viewed in cross-section: (i) presence/absence and position of lignified stone cells, which also provided an independent determination of fruit lignification, (ii) presence/absence and position of scalloped phytoliths, (iii) presence/absence of a specialized zone (the PFZ) thought to host phytolith formation, and (iv) overall size and shape attributes of the hypodermal, mesocarpal, and stone cells. Our lignification scores based on fruit penetration criteria were consistent with the stone cell examinations. A domesticated fruit from C. moschata had more puncture resistant rinds than typical of nonlignified specimens. The absence of stone cells in this specimen indicated that the puncture resistance was independent of lignification.
To provide a more precise and independent determination of phytolith quantity in fruits, we isolated phytoliths from a standard unit area (1–2 cm2) of rind by using wet oxidation (9) and mounted them on microscopic slides in Permount. Estimates of phytolith number were made by counting all phytoliths on a slide (++ rinds), or extrapolating to the total number per slide from a count of three slide transects (some ++ and all +++ rinds). Only complete scalloped phytoliths were counted; no complete or incomplete scalloped phytoliths were observed on slides made from + rinds.
Within-fruit variability in phytolith production and location was assessed by thin section analysis and wet digestion of samples taken at six different places from the bottom to the top of four lignified and two nonlignified fruits from two different species (C. ficifolia and C. moschata). We carried out preliminary studies of the timing of phytolith formation in developing fruits of two populations of C. sororia and one population of C. argyrosperma by examining thin sections of fruits (n = 48) sampled from 7 to 50 days after anthesis.
Results
Patterns of Lignification and Silicification in Mature, Nonhybrid Fruits, and Their Influence on Phytolith Morphology.
Our results support a co-control of lignification and silicification in Cucurbita rinds. No nonlignified rind (all from domesticated species) demonstrated the presence of scalloped phytoliths or a PFZ (Fig. 3), whereas all lignified rinds contained phytoliths in a well defined PFZ (Fig. 2; see Materials and Methods). This association was absolute. Moreover, scalloped phytoliths were always deposited in the PFZ, and they were interspersed and often interlocked with the uppermost layer of stone cells, forming a continuous barrier to penetration of the fruit (Fig. 4).
Fig 3.
SEM micrograph of a nonlignified rind of C. moschata showing the absence of phytoliths, phytolith forming zone, and stone cells. Unlike in lignified varieties, this rind shows a steady gradation from the hypodermis to the mesocarp (mes).
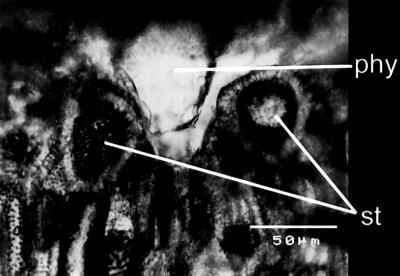
Fig 4.
SEM micrograph of a lignified rind from C. moschata showing how the phytoliths and stone cells are interlocked. The phytolith has been slightly detached from the stone cells during sample preparation.
Lignified rinds from domesticated fruits and hybrids between lignified and nonlignified parents were frequently easier to penetrate than the heaviest lignified rinds, which were characteristic of wild species and some domesticated varieties. Such variation has been attributed to a thinner zone of lignification (22), perhaps resulting from the influence of modifier genes or incomplete dominance of the Hr locus that may produce variations in the degree of lignification. We also observed thinner layers of stone cells in fruits with softer, lignified rinds. All of these rinds (scored as ++) demonstrated the presence of a PFZ, but they often contained considerably fewer phytoliths than did the hardest rinds analyzed [1359 ± 657 phytoliths per cm2 rind (range, 607–2,222) in the +++ rinds vs. 283 ± 497 per cm2 (range, 10–1,167) in ++ rinds]. Multiple thin sections made from some of these ++ fruits showed that although the PFZ was uniformly present, phytoliths were unevenly distributed in this zone, being present in some rind samples but not in others. In contrast, analysis of six different samples taken from the top to the bottom of fruits in each of two nonlignified landraces of C. moschata, two lignified (+++) landraces of C. moschata, and two lignified (+++) landraces of C. ficifolia revealed a regular distribution of phytoliths throughout the lignified fruits with little difference in phytolith number per cm2 of rind, and no variation in the formation of the PFZ. Hence, these characteristics exhibit considerable constancy within a single, nonlignified or well lignified fruit.
It was previously suggested that the placement of scalloped phytoliths at the interface of the hypodermis and upper mesocarp of rinds accounts for their distinctive surface characteristics (18). Our data support this suggestion. Moreover, the effects of Hr are now also seen to contribute significantly to phytolith morphology. For example, the phytoliths have two distinct hemispheres with two different types and sizes of scalloped concavities (Figs. 1 and 2). The deeply impressed scalloped decorations on the upper hemisphere made by the hard edges of the small, isodiametric hypodermal cells, combined with the deep impressions on the bottom hemisphere made by the larger, lignified stone cells, create these features on the phytolith body (Figs. 1 and 2). In many species and varieties of Cucurbita, rind phytoliths are more or less spherical (Fig. 1; also see refs. 17 and 18). This study shows in addition that many land races of C. moschata (7 of 15 silicified varieties studied) have markedly elongated stone cells in the outermost mesocarp, and these plants make phytoliths whose bottom hemispheres extend much deeper into the stone cell layer than is usual in the genus (Fig. 2). The result is distinctive, elliptical phytoliths with markedly elongated stone cell-impressed concavities (Figs. 2 and 5; these were called “thicker than long” phytoliths in ref. 18). They may possibly be an identifier of this species of squash.
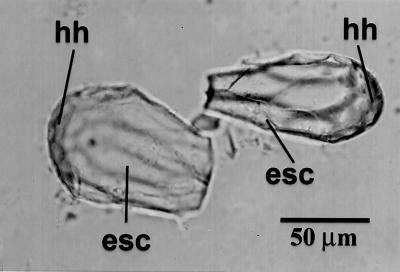
Fig 5.
Phytoliths with markedly elongated stone cell-derived impressions (esc) isolated from a rind of C. moschata. hh, hypodermis-derived part of the phytolith.
Interspecific, together with some apparent subspecific variation in the shape and arrangement of the hypodermis and uppermost layer of stone cells, also results in the production of distinctive phytoliths in C. maxima and its presumed wild progenitor C. andreana (16). In the latter species, scalloped phytoliths are planar, not spherical, with three to five straight edges (Fig. 6). These features largely result from the presence of flattened stone cells with longer and more linear cell walls in the uppermost mesocarp seen only in this species (Fig. 7). In C. maxima, phytoliths are spheres. Further, the hypodermis can be dense and has what appear to be raised, thickened areas, which lead to diagnostic phytoliths in some varieties (Fig. 8). Other varieties of C. maxima contribute scalloped phytoliths standard in the genus, but still distinguishable from the planar phytoliths in its wild ancestor discussed above.
Fig 6.
Phytoliths from C. andreana. They are planar with straight edges.
Fig 7.
A rind of C. andreana showing stone cells with relatively long and straight edges that lead to the formation of phytoliths such as those in Fig. 6. pc, cavity left by a detached phytolith.
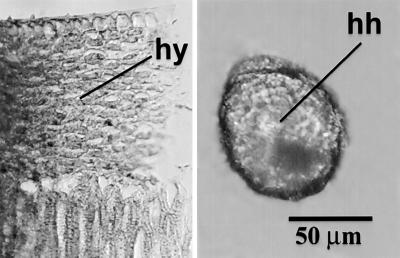
Fig 8.
(Left) A rind of C. maxima with dense and thickened hypodermal tissue. (Right) A phytolith from C. maxima showing how it carries these characteristics on its hypodermis-derived hemisphere (hh). The darkened depression near the bottom of the phytolith caused by the hypodermis is possibly another defining characteristic of phytoliths from this species.
An example of how differences in rind cell structure between different genera of the Cucurbitaceae leads to the formation of genus-specific phytoliths is provided by the bottle gourd [Lagenaria siceraria (Molina) Standl.]. In this species, solid silica is not deposited in the hypodermis, as it is in all species of Cucurbita, and, unlike in Cucurbita, the stone cells have an irregular, loosely organized configuration, with some being large and elongated and others quite small (Fig. 9). These characteristics account for the formation of distinctive, hemi-spherical and hemi-elliptical phytoliths with stone cell-impressed concavities of irregular shapes and sizes in bottle gourd (Fig. 9; ref. 18).
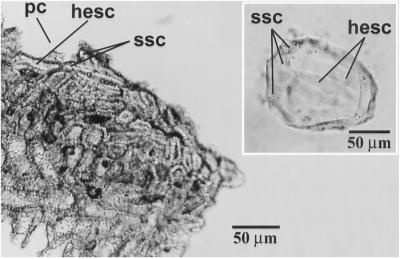
Fig 9.
A rind of Lagenaria siceraria showing how the stone cells have an irregular configuration. The epicarp and hypodermis is missing from this specimen. hesc, horizontally elongated stone cells; ssc, small stone cells; pc, cavity left by a detached phytolith. (Inset) A hemi-elliptical phytolith from bottle gourd demonstrating an irregular configuration of horizontally elongated (hesc) and small (ssc) stone cell-impressed concavities.
The Inheritance of Lignin and Phytolith Formation.
To gain a better understanding of the inheritance of phytolith and lignin formation in Cucurbita, we studied the F1 and F2 progeny of crosses between sets of lignified and nonlignified parents in the closely related species C. sororia, C. argyrosperma, and C. moschata (16). Segregation results for rind lignification and phytolith formation in hybridized fruits are shown in Table 1. Crosses of two lignified parents and of lignified parents with nonlignified parents produced only lignified, phytolith-producing progeny in the F1 generation. Crosses of two nonlignified parents produced no lignified or phytolith-producing F1 progeny. Data on the F2 progeny of some of these crosses showed that, like for the parental generation of fruits and the F1 progeny, lignification and silicification were completely linked. The distribution of progeny in the F2 generation of crosses between lignified and nonlignified fruits gave a good fit (P > 0.99) to the expected ratio of 3:1 for the inheritance of a single, dominant locus. It thus appears from this evidence that lignification and phytolith formation in these species are controlled by a dominant genetic locus, Hr.
Our observations of phytolith morphology in the F1 and F2 progeny of crosses revealed some interesting patterns. For example, crosses between two different populations of C. sororia and a land race of C. moschata (14-1) (Table 1) resulted in C. moschata-type assemblages of elliptical phytoliths with elongated concavities in the F1 generation of fruits from each set of crosses (F2 progeny were not available). This was true even though the C. moschata parents did not have phytoliths, and neither the parental nor other C. sororia specimens produce these kinds of silicified bodies. These data further indicate that some varieties of C. moschata possess a strong, underlying tendency for the formation of markedly elongated stone cells and elliptical phytoliths. Crosses between C. sororia and other land races of C. moschata resulted in spherical, scalloped phytoliths in all of the F1 and F2 progeny, as were present in the C. sororia parents. All progeny of crosses made from two C. sororia parents and between C. sororia and C. argyrosperma, which form an ancestor/domesticated species pair (16), also produced the spherical, scalloped phytoliths characteristic of both these species. None of the crosses produced fruits with the rind cell configurations and phytoliths described above from C. maxima and C. andreana, further indicating that they may be confined to these two species.
In hybrids between wild and domesticated species, many of which were scored as ++, complete, regularly shaped scalloped phytoliths were fewer than in nonhybrid fruits or hybrids between two wild species. In addition, phytoliths were often observed that appeared to lack a definable mesocarp- or hypodermis-derived hemisphere, and they also had fainter scalloped impressions (Fig. 10). In some hybrid fruits, they substantially outnumbered standard, scalloped phytoliths. The lack of deeply impressed scalloped decorations and formation of incompletely developed mesocarpal or hypodermal hemispheres appears to mirror the reduced degree of lignification and cell hardness in ++ fruits, further illustrating the significance of the expression of the Hr locus in determining phytolith characteristics.
Fig 10.
A phytolith from a cross between C. sororia and C. moschata. It lacks the mesocarpal hemisphere and has less deeply impressed hypodermal concavities than in heavily lignified fruits.
Patterns of Lignification and Silicification in Developing Fruits.
Our thin section and rind penetration studies of developing fruits in C. sororia and C. argyrosperma showed that a well defined PFZ containing a large number of phytoliths (1,384 and 703 per cm2 rind, respectively) was present in both species by seven days after anthesis, whereas lignification leading to rinds scored as ++ or +++ does not become evident until 17 to 19 days after anthesis. At seven days after anthesis some minimal stone cell development in the uppermost mesocarp is visible. It is possible that a major deposition of phytoliths takes place well before that of lignin because phytoliths are placed in such a way in the rind (e.g., in a noninterlocked arrangement along the hypodermis/mesocarp interface) such that they do not impede the ability of cells to expand. Thus, young fruits can summon some mechanical support and protection and still continue to grow by silicifying interspersed areas of their rinds, and then defend themselves more heavily through major stone cell development and interlinkage with phytoliths close to maturity.
Discussion
Our data indicate that a simple, monogenic system can explain the inheritance of lignin and phytoliths in Cucurbita, and they provide additional evidence that a dominant gene analogous to the Hr locus demonstrated for C. pepo primarily controls lignification in other Cucurbita species. The dominant gene known to govern the inheritance of flesh bitterness in Cucurbita (25) is not implicated in the process because lignified fruits with nonbitter flesh in our study (e.g., domesticated fruits plus a specimen of the wild species C. fraterna that has probably hybridized with a domesticated species) produced phytoliths, and phytoliths were absent in a bitter-fruited, nonlignified specimen of C. moschata. Thus, the Hr locus, with pleiotropic effects, appears to be another single gene controlling the evolution of more than one important phenotypic trait during plant domestication (24).
The mechanisms underlying the formation of solid silica in plants were originally proposed for the Poaceae, and involved a passive uptake of soluble silica from ground water by the root system followed by polymerization of silica in areas of the plant where evapotranspiration (water loss) was highest and a supersaturated solution of silica was likely to occur (26). Demonstration of active uptake of silica by some grasses [e.g., rice (Oryza sativa L.) and wheat (Triticum aestivum L.)], however, coupled with a heavy deposition of solid silica in areas of grasses and numerous other plants not associated with significant water loss, suggested that the passive model of solid silica formation in plants was inadequate (9, 14). The functional significance of phytoliths in plants has been debated, despite persuasive evidence that plants accrue mechanical support, decreased damage from toxic substances in soils, and increased resistance to herbivory and pathogenic fungi from phytolith presence (9, 13–15, 27). Moreover, leaf silicification in African grass species is an inducible defense against herbivores (12).
To our knowledge, our data represent the first evidence for genetic control of phytolith formation in a dicotyledon, and they provide considerable support for the idea that protection from herbivory is an important function of phytoliths (1, 12). Several characteristics of phytoliths would enhance their effectiveness as structural barriers to herbivory. They are the only substance hard enough to grind and abrade the tooth enamel of large grazers (28). The evolution of hypsodont dentition during the late Miocene, when open woodlands and savannas expanded globally at the expense of forests and C4 grasses appeared, has long been adaptively linked to the high phytolith content of grass leaves (12, 29).
It was previously believed that phytolith production was limited to the Poaceae and a few other monocotyledons. However, numerous species of monocotyledons and dicotyledons, including many tropical trees, are now understood to faithfully deposit phytoliths in specific tissues and cells of their vegetative and reproductive organs irrespective of local environmental variability in growing conditions (1–10, 17, 18). Many silicification sites are in places likely to cause considerable discomfort to phytophagous insects and vertebrate herbivores alike (e.g., leaf hairs, seed bracts, fruit pericarps, and seed and leaf epidermes), potentially inhibiting penetration and detachment of plant matter, and digestibility. It thus appears likely that genetic factors underpin phytolith formation in other plants.
Terrestrial communities have been significantly shaped by the strong ecological and evolutionary interactions between plants and their vertebrate and insect herbivores (30). The role of phytoliths in these processes has barely been explored. Questions that deserve study include the timing of phytolith, lignin, and chemical responses for defense during plant growth (e.g., defenses that toughen or harden plant structures may be a less viable option when they are young and need to expand, possibly necessitating heavier investments in chemicals at that stage), and the tradeoffs involved in the production of different kinds of mechanical and chemical defenses. There is evidence, for example, that it is cheaper in energetic terms for grasses to incorporate silica in their cell walls than it is to form lignin (Si = 3.7% the cost of lignin), but because lignin is a lighter material to carry than solid silica, plants ultimately derive the most benefit by using both substances (11). Phytoliths are probably less costly for plants to produce than many secondary metabolites, and once solid silica is deposited it can be carried unaltered until the leaf and fruit drops, potentially lessening the need for the expensive turnover of chemical compounds that might be particularly troublesome for long-lived (3–14 years) tropical leaves (30).
Our results bear considerable importance for investigations of agricultural origins and dispersals in the New World. The formation of phytoliths in two genera that yielded important crop plants, Zea (24) and Cucurbita, is now shown to be under the control of genetic loci that accounted for significant phenotypic changes during the evolution of the domesticated species from their wild progenitors. At least five different species of Cucurbita ranging from North America to southern South America were brought under cultivation and domesticated during the pre-Columbian era, and some species were members of the earliest crop plant complexes known to the New World (16, 18, 31). Presently available evidence on the subject is slim, however, because in many regions of the Americas archaeological preservation of fruits, seeds, and peduncles (fruit stalks) is poor. Archaeologists can employ the durable and diagnostic rind phytoliths to follow prehistoric exploitation of Cucurbita knowing that phytolith representation in ancient sites has not been biased by past environmental variability (e.g., rainfall, soil conditions) affecting plant production, but rather hinges on whether people used lignified or nonlignified fruits. Hence, an absence of scalloped Cucurbita phytoliths in archaeological sediments does not necessarily mean that the genus was not exploited. Rather, depending on the age and context of the site, the possibility would exist that nonlignified, and therefore, domesticated fruits, were used.
Those characteristics of Cucurbita rind phytoliths that impart genus- and, possibly at times, species-level attributes to them (e.g., differences in the location, shapes, and sizes of the surface concavities, and overall phytolith shape) were demonstrated to be strongly influenced by the arrangements and cell structures of the hypodermal and upper mesocarpal (stone cells) tissues. These tissue and cell arrangements provide a taxonomic basis for the identification of archaeological rind remains of Cucurbita and other genera (Lagenaria) (31), underscoring the taxonomic value of scalloped phytoliths when isolated as discrete bodies from archaeological sediments into which rinds had decayed.
Our data suggest that intermediate levels of lignification and silicification occur in Cucurbita fruits, and such levels were characteristic of domesticated plants and hybrids made between wild and domesticated species. These patterns seem to indicate an incomplete dominance of the Hr locus, perhaps resulting from the influence of modifier genes. Reduced degrees of lignification often resulted in the production of considerably fewer scalloped phytoliths, together with the formation of many atypical phytoliths with incompletely developed mesocarp- and hypodermis-derived hemispheres and less deeply impressed surface decorations. The potential appears to exist for using these phytoliths in archaeological contexts to follow human selection under cultivation for reduced rind lignification.
The facts that scalloped phytoliths from wild fruits are significantly smaller than those formed in many larger-fruited, domesticated specimens (ref. 18; D.R.P., unpublished data), and human selection for increased seed and fruit size may have preceded selection for more desirable fruit characteristics (e.g., edible flesh and softer, nonlignified rinds; refs. 18 and 31) further illustrate how the phytolith record may inform our understanding of early cultural uses of Cucurbita. Finally, small, abraded fragments of Cucurbita rinds typically recovered from archaeological sites, which because of their size and poor condition often cannot demonstrate possible effects of selection under cultivation, may now yield indications as to whether they originated from wild or domesticated plants because an absence of phytoliths or the PFZ in rinds would suggest that selection for nonlignified, and thus domesticated fruits, had already occurred. This should also be true of fruit varieties that were eaten when immature.
Supplementary Material
Acknowledgments
We thank Mr. Obed Román and Ms. Luisa Flores for field work in Puerto Rico. We are indebted to Dr. Ira Rubinoff, Director of Smithsonian Tropical Research Institute (STRI), for his support of phytolith research over many years, and we fondly remember Martin Moynihan who always thought that phytoliths must have important functions in plants. This research was supported by STRI, a grant to STRI from the Andrew W. Mellon Foundation, and the University of Puerto Rico, Agricultural Experiment Station.
Abbreviations
PFZ, phytolith formation zone
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Piperno D. R. (1998) J. World Prehist. 12, 393-449. [Google Scholar]
- 2.Fredlund G. G. & Tiezen, L. T. (1997) Quat. Res. 136, 206-217. [Google Scholar]
- 3.Runge F. (1999) Rev. Paleobot. Palynol. 107, 23-53. [Google Scholar]
- 4.Piperno D. R. & Pearsall, D. M., (1998) The Origins of Agriculture in the Lowland Neotropics (Academic, San Diego).
- 5.Bozarth S. R. (1992) in Phytolith Systematics: Emerging Issues, eds. Rapp, G., Jr. & Mulholland, S. C. (Plenum, New York), pp. 193–214.
- 6.Pearsall D. M., (2000) Paleoethnobotany: A Handbook of Techniques (Academic, San Diego).
- 7.Zhao Z. (1998) Antiquity 72, 885-897. [Google Scholar]
- 8.Kealhofer, L. & Piperno, D. R. (1998) Smithson. Contrib. Bot. 88.
- 9.Piperno D. R., (1988) Phytolith Analysis: An Archaeological and Geological Perspective (Academic, San Diego). [DOI] [PubMed]
- 10.Piperno D. R. (1989) Rev. Paleobot. Palynol. 61, 147-173. [Google Scholar]
- 11.Raven J. A. (1983) Biol. Rev. Cambridge Philos. Soc. 58, 179-207. [Google Scholar]
- 12.McNaughton S. J. & Tarrants, J. L. (1983) Proc. Nat. Acad. Sci. USA 80, 70-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodson M. J. & Evans, D. E. (1995) J. Exp. Bot. 46, 161-171. [Google Scholar]
- 14.Sangster A. G. & Hodson, M. J. (1997) in The State-of-the-Art of Phytoliths in Soils and Plants, eds. Pinilla, A., Juan-Tresserras, J. & Machado, M. J. (Centro de Ciencias Medioambientales, Madrid), pp. 113–121.
- 15.Epstein E. (1994) Proc. Natl. Acad. Sci. USA 91, 11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanjur O., Piperno, D. R., Andres, T. C. & Wessel-Beaver, L. (2002) Proc. Natl. Acad. Sci. USA 99, 535-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bozarth S. R. (1987) Am. Antiq. 52, 607-615. [Google Scholar]
- 18.Piperno D. R., Andres, T. C. & Stothert, K. E. (2000) J. Archeol. Sci. 27, 193-208. [Google Scholar]
- 19.Barber K. G. (1909) Bot. Gaz. (Chicago) 47, 263-310. [Google Scholar]
- 20.Mains E. B. (1950) Pap. Mich. Acad. Sci. Arts Lett. 36, 27-30. [Google Scholar]
- 21.Robinson R. W., Munger, H. M., Whitaker, T. W. & Bohn, G. W. (1976) Hortic. Sci. 11, 554-568. [Google Scholar]
- 22.Schaffer A. A., Boyer, Ch. D. & Paris, H. S. (1986) Z. Pflanzenzücht. 96, 147-153. [Google Scholar]
- 23.Weeden N. F., Graham, J. D. & Robinson, R. W. (1986) Hortic. Sci. 21, 1431-1433. [Google Scholar]
- 24.Dorweiler J. E. & Doebley, J. (1997) Am. J. Bot. 84, 1313-1322. [PubMed] [Google Scholar]
- 25.Dane F., Hunter, A. G & Chambliss, O. L. (1987) Cucurbit Genet. Coop. Rep. 10, 76-77. [Google Scholar]
- 26.Jones L. H. P. & Handreck, K. A. (1967) Adv. Agron. 19, 107-149. [Google Scholar]
- 27.Iler R., (1979) The Chemistry of Silica: Solubility, Polymerization, Colloid and Surface Properties, and Biochemisty (Wiley, New York).
- 28.Baker G., Jones, L. H. P. & Wardrop, D. (1959) Nature (London) 18, 1583-1584. [DOI] [PubMed] [Google Scholar]
- 29.Cerling T. E., Ehleringer, J. R. & Harris, J. M. (1998) Philos. Trans. R. Soc. London B 353, 159-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coley P. & Barone, J. A. (1996) Annu. Rev. Ecol. Syst. 27, 303-335. [Google Scholar]
- 31.Smith B. D. (1997) Science 276, 932-934. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.