Abstract
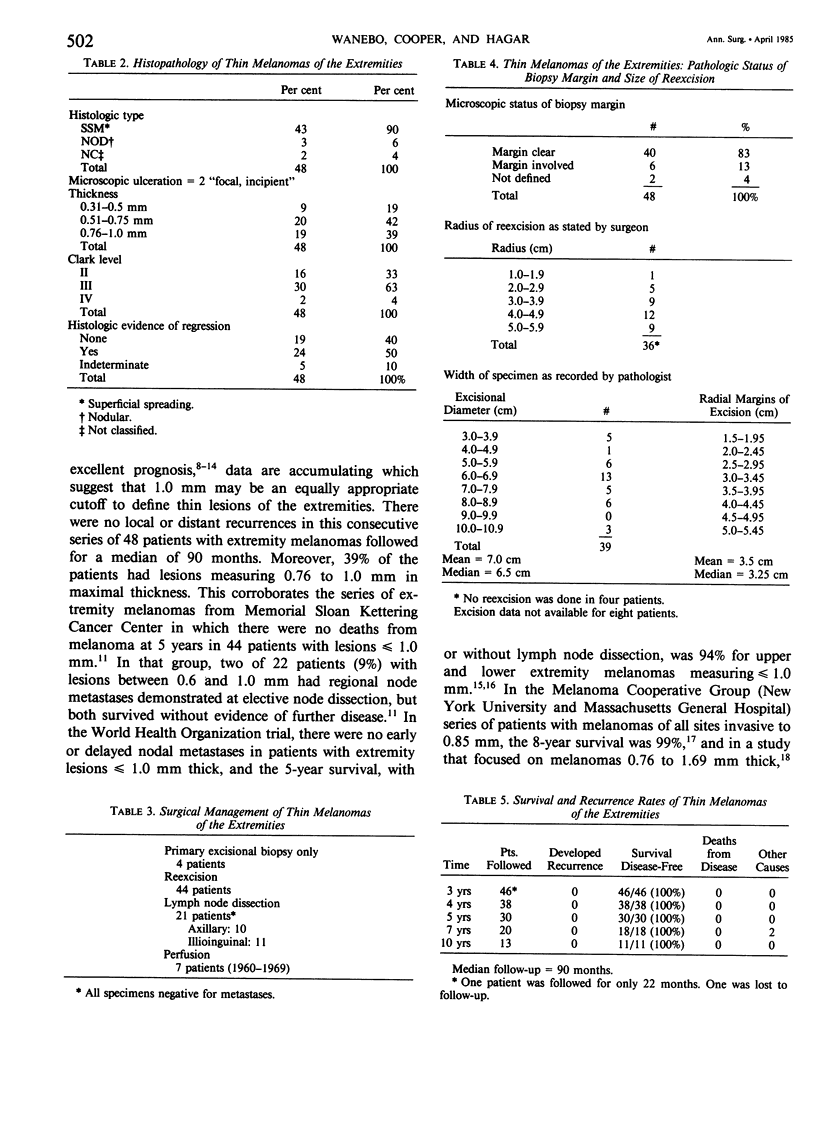
Although a thickness of less than or equal to 0.76 mm has been used to define biologically favorable (thin) melanoma, there is evidence that 1 mm may be a reasonable cutoff to categorize favorable extremity melanomas. This is tempered, however, by the claim that histologic regression in thin melanomas is associated with an increased metastatic rate. We have therefore addressed the following questions: Is 1 mm an appropriate cutoff point to define thin melanoma on the extremities? Does regression in a thin lesion truly signify a poor prognosis? Is the width of excision (narrow vs. wide) related to recurrence rates in these lesions? To address these issues we reviewed 48 patients with extremity melanomas, less than or equal to 1 mm in maximum thickness, treated at this institution during a 20-year period. Pathologic features included histologic type: superficial spreading (90%), nodular (6%), and not classified (4%); thickness: less than 0.76 mm (61%) and 0.76 to 1 mm (39%); and Clark's level: II (33%), III (63%), and IV (4%). A moderate or marked lymphoid infiltrate occurred in 75%, and histologic tumor regression was found in 50%. The median margin of excision, as stated by the surgeon, was 4 cm. The median margin measured by the pathologist in unfixed specimens was 3.5 cm. Although 13% had atypical melanocytic hyperplasia in the initial excisional biopsy margin, all reexcisions were clear. Of 21 patients having node dissections, none had nodal metastases. There were no recurrences or deaths due to melanoma (median follow-up: 90 months). We conclude that melanomas less than or equal to 1 mm in thickness on the extremities can be defined as biologically highly favorable, "thin" lesions. Foci of regression do not alter their behavior. Their favorable prognosis justifies conservative excision in most cases.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagley F. H., Cady B., Lee A., Legg M. A. Changes in clinical presentation and management of malignant melanoma. Cancer. 1981 May 1;47(9):2126–2134. doi: 10.1002/1097-0142(19810501)47:9<2126::aid-cncr2820470904>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Balch C. M., Murad T. M., Soong S. J., Ingalls A. L., Richards P. C., Maddox W. A. Tumor thickness as a guide to surgical management of clinical stage I melanoma patients. Cancer. 1979 Mar;43(3):883–888. doi: 10.1002/1097-0142(197903)43:3<883::aid-cncr2820430316>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Balch C. M., Soong S. J., Murad T. M., Ingalls A. L., Maddox W. A. A multifactorial analysis of melanoma. II. Prognostic factors in patients with stage I (localized) melanoma. Surgery. 1979 Aug;86(2):343–351. [PubMed] [Google Scholar]
- Breslow A. Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg. 1970 Nov;172(5):902–908. doi: 10.1097/00000658-197011000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow A. Tumor thickness, level of invasion and node dissection in stage I cutaneous melanoma. Ann Surg. 1975 Nov;182(5):572–575. doi: 10.1097/00000658-197511000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosimi A. B., Sober A. J., Mihm M. C., Fitzpatrick T. B. Conservative surgical management of superficially invasive cutaneous melanoma. Cancer. 1984 Mar 15;53(6):1256–1259. doi: 10.1002/1097-0142(19840315)53:6<1256::aid-cncr2820530607>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Day C. L., Jr, Lew R. A., Mihm M. C., Jr, Harris M. N., Kopf A. W., Sober A. J., Fitzpatrick T. B. The natural break points for primary-tumor thickness in clinical Stage I melanoma. N Engl J Med. 1981 Nov 5;305(19):1155–1155. doi: 10.1056/NEJM198111053051916. [DOI] [PubMed] [Google Scholar]
- Day C. L., Jr, Mihm M. C., Jr, Sober A. J., Fitzpatrick T. B., Malt R. A. Narrower margins for clinical stage I malignant melanoma. N Engl J Med. 1982 Feb 25;306(8):479–482. doi: 10.1056/NEJM198202253060810. [DOI] [PubMed] [Google Scholar]
- Day C. L., Jr, Mihm M. C., Jr, Sober A. J., Harris M. N., Kopf A. W., Fitzpatrick T. B., Lew R. A., Harrist T. J., Golomb F. M., Postel A. Prognostic factors for melanoma patients with lesions 0.76 - 1.69 mm in thickness. An appraisal of "thin" level IV lesions. Ann Surg. 1982 Jan;195(1):30–34. doi: 10.1097/00000658-198201001-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day C. L., Jr, Sober A. J., Kopf A. W., Lew R. A., Mihm M. C., Jr, Golomb F. M., Hennessey P., Harris M. N., Gumport S. L., Raker J. W. A prognostic model for clinical stage I melanoma of the lower extremity. Location on foot as independent risk factor for recurrent disease. Surgery. 1981 May;89(5):599–603. [PubMed] [Google Scholar]
- Day C. L., Jr, Sober A. J., Kopf A. W., Lew R. A., Mihm M. C., Jr, Hennessey P., Golomb F. M., Harris M. N., Gumport S. L., Raker J. W. A prognostic model for clinical stage I melanoma of the upper extremity. The importance of anatomic subsites in predicting recurrent disease. Ann Surg. 1981 Apr;193(4):436–440. doi: 10.1097/00000658-198104000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromet M. A., Epstein W. L., Blois M. S. The regressing thin malignant melanoma: a distinctive lesion with metastatic potential. Cancer. 1978 Nov;42(5):2282–2292. doi: 10.1002/1097-0142(197811)42:5<2282::aid-cncr2820420528>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Hansen M. G., McCarten A. B. Tumor thickness and lymphocytic infiltration in malignant melanoma of the head and neck. Am J Surg. 1974 Oct;128(4):557–561. doi: 10.1016/0002-9610(74)90275-x. [DOI] [PubMed] [Google Scholar]
- McGovern V. J., Shaw H. M., Milton G. W., Farago G. A. Prognostic significance of the histological features of malignant melanoma. Histopathology. 1979 Sep;3(5):385–393. doi: 10.1111/j.1365-2559.1979.tb03020.x. [DOI] [PubMed] [Google Scholar]
- McLean D. I., Lew R. A., Sober A. J., Mihm M. C., Jr, Fitzpatrick T. B. On the prognostic importance of white depressed areas in the primary lesion of superficial spreading melanoma. Cancer. 1979 Jan;43(1):157–161. doi: 10.1002/1097-0142(197901)43:1<157::aid-cncr2820430123>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Milton G. W., Shaw H. M., Farago G. A., McCarthy W. H. Tumour thickness and the site and time of first recurrence in cutaneous malignant melanoma (stage I). Br J Surg. 1980 Aug;67(8):543–546. doi: 10.1002/bjs.1800670804. [DOI] [PubMed] [Google Scholar]
- Paladugu R. R., Yonemoto R. H. Biologic behavior of thin malignant melanomas with regressive changes. Arch Surg. 1983 Jan;118(1):41–44. doi: 10.1001/archsurg.1983.01390010031008. [DOI] [PubMed] [Google Scholar]
- Shafir R., Hiss J., Tsur H., Bubis J. J. The thin malignant melanoma: changing patterns of epidemiology and treatment. Cancer. 1982 Aug 15;50(4):817–819. doi: 10.1002/1097-0142(19820815)50:4<817::aid-cncr2820500434>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Smith J. L., Jr, Stehlin J. S., Jr Spontaneous regression of primary malignant melanomas with regional metastases. Cancer. 1965 Nov;18(11):1399–1415. doi: 10.1002/1097-0142(196511)18:11<1399::aid-cncr2820181104>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Stage I melanoma of the skin: the problem of resection margins. W.H.O. Collaborating Centres for Evaluation of Methods of Diagnosis and Treatment of Melanoma. Eur J Cancer. 1980 Aug;16(8):1079–1085. [PubMed] [Google Scholar]
- Trau H., Kopf A. W., Rigel D. S., Levine J., Rogers G., Levenstein M., Bart R. S., Mintzis M. M., Friedman R. J. Regression in malignant melanoma. J Am Acad Dermatol. 1983 Mar;8(3):363–368. doi: 10.1016/s0190-9622(83)70040-x. [DOI] [PubMed] [Google Scholar]
- Van Der Esch E. P., Cascinelli N., Preda F., Morabito A., Bufalino R. Stage I melanoma of the skin: evaluation of prognosis according to histologic characteristics. Cancer. 1981 Oct 1;48(7):1668–1673. doi: 10.1002/1097-0142(19811001)48:7<1668::aid-cncr2820480732>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Veronesi U., Adamus J., Bandiera D. C., Brennhovd O., Caceres E., Cascinelli N., Claudio F., Ikonopisov R. L., Javorski V. V., Kirov S. Delayed regional lymph node dissection in stage I melanoma of the skin of the lower extremities. Cancer. 1982 Jun 1;49(11):2420–2430. doi: 10.1002/1097-0142(19820601)49:11<2420::aid-cncr2820491133>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Wanebo H. J., Woodruff J., Fortner J. G. Malignant melanoma of the extremities: a clinicopathologic study using levels of invasion (microstage). Cancer. 1975 Mar;35(3):666–676. doi: 10.1002/1097-0142(197503)35:3<666::aid-cncr2820350320>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]




