Abstract
Ascorbic acid has numerous and diverse roles in plant metabolism. We have used the vtc-1 mutant of Arabidopsis, which is deficient in ascorbate biosynthesis, to investigate the role of ascorbate concentration in growth, regulation of photosynthesis, and control of the partitioning of antioxidative enyzmes. The mutant possessed 70% less ascorbate in the leaves compared with the wild type. This lesion was associated with a slight increase in total glutathione but no change in the redox state of either ascorbate or glutathione. In vtc-1, total ascorbate in the apoplast was decreased to 23% of the wild-type value. The mutant displayed much slower shoot growth than the wild type when grown in air or at high CO2 (3 mL L−1), where oxidative stress is diminished. Leaves were smaller, and shoot fresh weight and dry weight were lower in the mutant. No significant differences in the light saturation curves for CO2 assimilation were found in air or at high CO2, suggesting that the effect on growth was not due to decreased photosynthetic capacity in the mutant. Analysis of chlorophyll a fluorescence quenching revealed only a slight effect on non-photochemical energy dissipation. Hydrogen peroxide contents were similar in the leaves of the vtc-1 mutant and the wild type. Total leaf peroxidase activity was increased in the mutant and compartment-specific differences in ascorbate peroxidase (APX) activity were observed. In agreement with the measurements of enzyme activity, the expression of cytosolic APX was increased, whereas that for chloroplast APX isoforms was either unchanged or slightly decreased. These data implicate ascorbate concentration in the regulation of the compartmentalization of the antioxidant system in Arabidopsis.
Ascorbate fulfills many key functions in plant biology. As well as being the most abundant low-Mr antioxidant in the plant cell, it participates in the regulation of mitosis and cell expansion (Noctor and Foyer, 1998a; Smirnoff and Wheeler, 2000). Ascorbate is also a substrate for key enzymatic reactions, for example in the production of ethylene (McGarvey and Christoffersen, 1992). It is a cofactor for violaxanthin de-epoxidase in the xanthophyll cycle, a process that is involved in the protective dissipation of absorbed light as heat and that can be measured as a non-photochemical quenching of chlorophyll a fluorescence (NPQ).
Leaves often have as much ascorbate as chlorophyll but the extent of ascorbate accumulation depends on developmental and on environmental cues that may act via the rate of production of active oxygen species (AOS; Conklin et al., 1996; Grace and Logan, 1996; Gillham and Dodge, 1987). AOS are produced in aerobic organisms during the course of metabolism and are processed by the antioxidant system, which comprises enzymes and compounds of low Mr (Noctor and Foyer 1998a). Although numerous processes in leaves are capable of producing AOS such as superoxide and hydrogen peroxide (H2O2), those with the highest capacity under most conditions are the Mehler reaction and photorespiration (Foyer and Noctor, 2000). The flux through both of these pathways varies but is generally favored by high light and low CO2 availability.
H2O2 is eliminated from plant cells by the action of catalases and peroxidases. Catalase is largely restricted to the peroxisomes and the ascorbate peroxidase (APX) family of isoenzymes is crucial in maintaining H2O2 contents at nontoxic concentrations in many of the compartments of the cell. APX exists in the chloroplast as thylakoid-bound and stroma-soluble forms (Groden and Beck, 1979; Kelly and Latzko, 1979), and also as a specific cytosolic isoform (Chen and Asada, 1989; Mittler and Zilinskas, 1991). Other forms are associated with the membranes of glyoxysomes and leaf peroxisomes (Yamaguchi et al., 1995; Zhang et al., 1997). The isoforms are encoded by distinct genes and differ in size, specificity for electron donor, and sensitivity to inactivation (Chen and Asada, 1989; Yoshimura et al., 1998). Both chloroplast isoforms are rapidly inactivated (half-time = about 10–20 s) at ascorbate concentrations below 20 μm, particularly in the presence of H2O2 (Hossain and Asada, 1984). In contrast, the cytosolic isoform is more stable (Chen and Asada, 1989; Yoshimura et al., 1998). The differential sensitivity to inactivation of the chloroplastic and cytosolic isoforms can be exploited in whole leaf extracts as an investigative tool to determine the relative extractable activities attributable to these compartments (Amako et al., 1994).
Recent studies have led to a revision of the notion that enhanced AOS production has only negative effects on metabolism and growth. It is now becoming clear that oxidants are important in signal transduction processes (e.g. Levine et al., 1994). Compounds such as H2O2 may function directly as signals of cellular redox balance, or they could act via knock-on changes in antioxidant pools. In the latter case, interplay between the antioxidant system and processes generating AOS would have widespread repercussions for gene expression and the integration of cellular physiology (Levine et al., 1994; Foyer et al., 1997; Noctor et al., 2000). In view of the multiple roles of ascorbate, changes in its concentration may have important consequences for cellular and metabolic regulation. For example, it has been shown recently that antisense suppression of chloroplastic 2-cys peroxiredoxin modifies both ascorbate redox state and the abundance of transcripts encoding several chloroplast-specific antioxidative enzymes (Baier et al., 2000). However, an evaluation of the influence of ascorbate concentration in development and in response to the environment must overcome the difficulty of separating ascorbate-mediated effects from those due to concomitant changes in other factors.
In the present study, we have exploited the potential of the ascorbate-deficient Arabidopsis mutant, vtc-1 (Conklin et al., 1996) to evaluate the effects of leaf ascorbate content on overall physiology and the antioxidant system. This mutant, isolated via its sensitivity to ozone exposure, enabled the elucidation of the pathway of ascorbate biosynthesis in plants (Wheeler et al., 1998; Conklin et al., 1999). It possesses low activity of GDP-Man pyrophosphorylase and accumulates about 30% of the ascorbate in the leaves compared with the wild type (Conklin et al., 1996, 1999). The present study demonstrates that low ascorbate contents in this mutant do not significantly perturb either the photosynthetic function of leaves or lead to detectable oxidative stress under optimal conditions. Despite this, the mutant suffers a marked decrease in biomass production and shows a redistribution of the intracellular antioxidative system in favor of the cytosol. These results present evidence that changes in leaf ascorbate content are involved in the intracellular distribution of antioxidative enzymes.
RESULTS
Biomass and Morphology
When grown in air, the ascorbate-deficient vtc-1 mutant showed a modified shoot morphology (Fig. 1A) and markedly decreased shoot biomass (Table I). Five weeks after sowing, the vtc-1 plants had a smaller rosette, which weighed only approximately 50% of the wild-type rosette fresh weight (Table I). The effect was not ameliorated by growth with CO2 enrichment (Fig. 1 B). In both air and at elevated CO2, the leaves of the vtc-1 mutant were smaller and more elongated than those of the wild type (Fig. 1). The rosettes of mutant leaves entered senescence earlier than those of the wild type, often losing chlorophyll before the flowering stage, which also started a week later than in the wild type (Fig. 1 C). In agreement with the observations of Conklin et al. (1996), the fresh weight to dry weight ratio was lower in the mutant (Table I). Therefore, the reduction in biomass reflects partly a change in dry matter, partly a change in water content.
Figure 1.
Effect of the vtc-1 mutation on phenotype in air and at high CO2. A, Plants grown in air for 5 weeks. B, Plants germinated and grown for 3 weeks in air, followed by 2 weeks at elevated CO2 (3,000 μL L−1). C, Plants at 10 weeks after germination: The mutant displays retarded flowering and the leaves senesce earlier.
Table I.
Biomass is modified in the ascorbate-deficient Arabidopsis mutant vtc-1
| Plant Type | Rosette Biomass Fresh Wt | Dry Wt | Diameter of Rosette | Leaf Biomass Fresh Wt | Intercellular Washing Fluid (WF) |
|---|---|---|---|---|---|
| g | cm | mg | μL g−1 fresh wt | ||
| Col-0 | 2.14 ± 0.24 | 0.146 ± 0.032 | 11.3 ± 0.7 | 91.6 ± 10.9 | 222 ± 13 |
| vtc-1 | 1.04 ± 0.18 | 0.095 ± 0.019 | 8.1 ± 0.5 | 43.7 ± 6.6 | 160 ± 15 |
All analyses were performed 5 weeks after sowing. Growth conditions as described in “Materials and Methods.” Values are means ± sd of three to nine data sets. Individual leaves represent mature leaves taken from the middle of the rosette.
Chlorophyll and Protein
The IWF values obtained after vacuum infiltration of the leaves suggested that the airspace in the leaves was decreased by about 30% in the mutant compared with the wild type (Table I). The vtc-1 mutant had higher amounts of total leaf soluble protein than the wild type (Table II). This difference was accentuated in the mutant apoplast, from which about three times more protein was recovered than from the apoplast of the wild type (Table II). In both plant types, negligible contamination by cytoplasmic proteins was observed, as assessed by Glc-6-phosphate dehydrogenase activity (data not shown). No significant differences were observed in the leaf chlorophyll contents but the leaf protein to chlorophyll ratio was slightly increased in the mutant relative to the wild type. The profile of soluble proteins was modified in the mutant compared with the wild type, some bands being absent from the mutant compared with the wild type and vice versa (data not shown).
Table II.
Soluble protein and chlorophyll in leaves of vtc-1 and Col-0
| Plant Type | Total Soluble Leaf Protein | Soluble Protein in IWF | Leaf Chlorophyll |
|---|---|---|---|
| mg g−1 fresh wt | μg g−1 fresh wt | mg g−1 fresh wt | |
| Col-0 | 7.26 ± 1.06 | 39.7 ± 6.6 | 1.03 ± 0.10 |
| vtc-1 | 9.24 ± 0.57 | 112.9 ± 9.5 | 1.12 ± 0.08 |
Values are means ± sd of seven independent extractions.
Leaf Photosynthesis
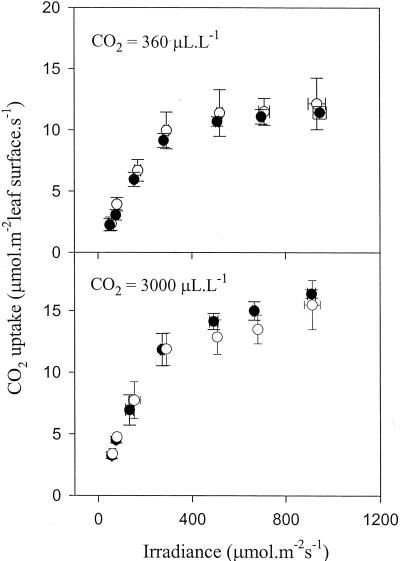
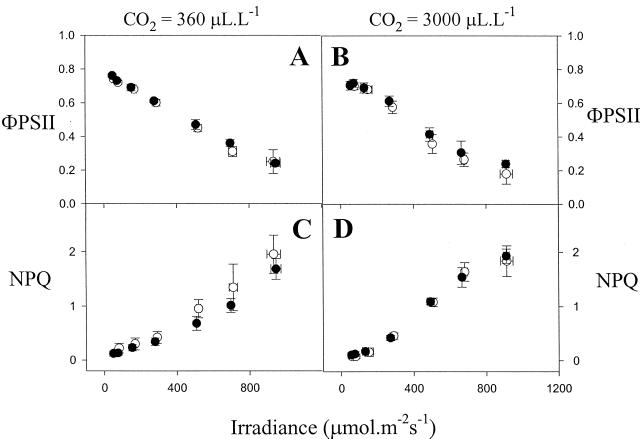
The light saturation curves for CO2 assimilation were comparable in the wild-type and mutant leaves when measured in air or in elevated CO2 (Fig. 2). No differences in the rate of photosynthesis were observed between the lines in air. Although high CO2 stimulated photosynthesis in Arabidopsis by 20% to 30%, there was no significant difference in rate between the lines (Fig. 2). Analysis of chlorophyll fluorescence showed that the photochemical yield of photosystem II was not affected in the mutant (Fig. 3, A and B). A slight decrease in NPQ was observed in the mutant in air, at light intensities above 300 μmol m−2 s−1 (Fig. 3C).
Figure 2.
Light saturation curves for CO2 uptake in the vtc-1 mutant and the wild type. Plants were grown in air. Top, Photosynthesis measured at atmospheric CO2 concentration. Bottom, Photosynthesis measured at elevated CO2. White symbols, Col-0; black symbols, vtc-1. Each value is the mean ± sd of three to eight leaves on different plants.
Figure 3.
Photochemical yield of photosystem II (ΦPSII, top) and non-photochemical quenching of chlorophyll fluorescence (NPQ, bottom) in vtc-1 and Col-0 plants as a function of irradiance. Plants were grown in air. Left, Measurements at atmospheric CO2 concentration. Right, Measurements at elevated CO2. White symbols, Col-0; black symbols, vtc-1. Each value is the mean ± sd of three to eight leaves on different plants.
Leaf Antioxidants and H2O2
In accordance with previously published results (Conklin et al., 1996), the mutant leaves contained 30% of the reduced ascorbate (Asc) of the wild type (Table III). The ascorbate pool was about 95% reduced in both lines. A slight increase in the total amount of glutathione was observed in the mutant compared with the wild type, but no change in the glutathione redox state was observed (Table III). In line with the unchanged redox states of ascorbate and glutathione, we found that leaf H2O2 content was similar in the mutant and the wild type (Table III). Because the vtc-1 mutant was isolated via its sensitivity to ozone, whose first point of contact with the leaf mesophyll cell is the apoplast, we investigated the ascorbate content in this compartment in the mutant and wild type. Only about 4% of the total leaf ascorbate (Asc plus DHA) was found in the IWF from the leaves of the wild type, Columbia 0 (Col-0). Total apoplastic ascorbate was reduced in the mutant to 23% of the wild-type value, i.e. somewhat more than the decrease in total leaf ascorbate. In both lines, a very low proportion of apoplastic ascorbate was found in the reduced form, consistent with measurements in other systems (e.g. Vanacker et al., 1998).
Table III.
Antioxidants and H2O2 in leaves and apoplast of vtc-1 and Col-0
| Plant Type | Ascorbate Whole Leaf Total Content | % Reduced | Apoplast Total Content | % Reduced | Glutathione Whole Leaf Total Glutathione | % Reduced | H2O2 Whole Leaf |
|---|---|---|---|---|---|---|---|
| μmol g−1 fresh wt | nmol g−1 fresh wt | nmol g−1 fresh wt | nmol g−1 fresh wt | ||||
| Col-0 | 2.64 ± 0.16 | 95 ± 11 | 105.6 ± 11.5 | 5.5 ± 2.8 | 203 ± 3 | 99 ± 1 | 63.6 ± 4.1 |
| vtc-1 | 0.79 ± 0.28 | 95 ± 32 | 24.5 ± 0.3 | n.d. | 264 ± 3 | 99 ± 1 | 66.4 ± 7.1 |
Each value is the mean ± sd of three independent extractions. The percentage reduction values were calculated as 100 × ascorbate + dehydroascorbate [DHA]) and 100 × (total glutathione − 2 glutathione disulphide)/total glutathione; n.d., reduced form not detected.
Antioxidative Enzymes
Total extractable activities of glutathione reductase (GR) were not significantly changed in vtc-1 (Table IV; Conklin et al., 1997). Likewise, catalase activity was similar, and DHAR was only slightly decreased in the mutant (Table IV). POX was significantly enhanced in the mutant, when assayed with guaiacol or pyrogallol (Table IV).
Table IV.
Antioxidative enzymes in the leaves vtc-1 and Col-0
| Plant Type | Total APX | Nonspecific Peroxidase Activity
|
Catalase | Dehydroascorbate Reductase (DHAR) | GR | |
|---|---|---|---|---|---|---|
| Guaiacol-peroxidase | Pyrogallol-peroxidase | |||||
| μmol mg−1 protein min−1 | ||||||
| Col-0 | 3.64 ± 0.36 | 0.19 ± 0.04 | 0.45 ± 0.04 | 183 ± 13 | 0.41 ± 0.05 | 0.074 ± 0.010 |
| vtc-1 | 4.34 ± 0.30 | 0.59 ± 0.12 | 1.13 ± 0.24 | 197 ± 9 | 0.31 ± 0.04 | 0.074 ± 0.007 |
Each value is the mean ± sd of three to four independent leaf extractions.
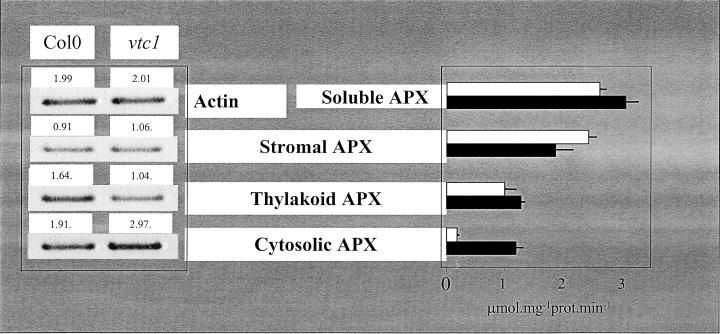
Total APX activity of the leaves was similar in both plant types (Table IV). However, this activity is a composite of thylakoid-bound, stroma-soluble, and cytosol-soluble isoforms. We separated these activities by centrifugation and by specific inhibition of the stromal form according to Amako et al. (1994). The activity of cytosolic APX isoform was greatly increased in the mutant leaves, whereas that of the stromal form was slightly decreased (Fig. 4, right). Thylakoid-bound APX activity was comparable in the mutant and the wild type (Fig. 4).
Figure 4.
Changes in the intracellular distribution of APX in vtc-1. Left, Transcript abundance. Right, Enzyme activities. White columns, Col-0; black columns, vtc-1. For transcript quantitation, reverse transcriptase (RT)-PCR products were followed using a cycle number in the linear range (25 cycles). Sample loading (20–25 μL) was as indicated. Relative band intensities are indicated by the numbers on top of each band. For activities, the soluble forms of APX were distinguished by measuring activities before and after different periods of incubation in the presence of 5 μm H2O2 and 1 μm ascorbate. Activity data are means ± sd of three separate extractions. For both transcripts and activities, similar results were obtained in two other experiments performed on different days.
Quantitative RT-PCR was used to quantify the relative abundance of transcripts encoding the cytosolic, thylakoid and stromal APX isoforms. The abundance of stromal APX transcripts was similar in the mutant and wild type (Fig. 4, left). Transcripts for the thylakoid isoform were decreased in the mutant leaves compared with the wild type, but the amount of cytosolic APX transcripts was higher in the vtc-1 mutant than in the wild type (Fig. 4).
DISCUSSION
The present results demonstrate: (a) that the vtc-1 mutant produces considerably less biomass than the wild type, (b) that the apoplastic ascorbate content is reduced in the mutant even more severely than the overall decrease in leaf content, (c) that nonspecific peroxidase activity is induced in the mutant, and (d) that the balance between chloroplastic and cytosolic APX is shifted in favor of the latter in the mutant, through a differential effect on gene expression.
Decreased Biomass Production: An Effect of Low Ascorbate?
In close agreement with previous observations (Conklin et al., 1996, 1999), the vtc-1 mutant was found to have only 30% of the wild-type foliar ascorbate content. The vtc-1 mutant is deficient in the activity of GDP-Man pyrophosphorylase, an enzyme found in the initial part of the biosynthetic pathway before it becomes committed to ascorbate synthesis (Wheeler et al., 1998). Decreased biomass production might conceivably reflect an effect on the supply of C skeletons for cell wall biosynthesis. Another Arabidopsis mutant, deficient in Fuc, has also been shown to exhibit decreased shoot growth (Reiter et al., 1993), suggesting that part of the effect in vtc-1 could be attributable to decreased C supply to cell wall synthesis.
In view of the many correlations between ascorbate and growth and the putative role of ascorbate in the plant cell cycle, we may conjecture that part of the effect of low ascorbate could also be due to the disruption of control mechanisms involved in cell division and/or elongation (Noctor and Foyer, 1998a). One mechanism may be accelerated cross linking of cell wall components, which limits cell expansion in the mutant. This notion is supported by our observations of a substantial increase in total leaf POX. Analysis of extracellular POX showed that this activity was also higher in the IWF of the mutant than in the wild type (data not shown). In addition to the effect of POX induction, the lower extracellular Asc concentration in the mutant (Table III) will create an environment that markedly favors cross linking. A future approach to distinguish between the mechanisms linking low ascorbate to decreased growth could involve a systematic comparison of the effect of ascorbate and precursors on growth. For example, restoration of growth merely by ascorbate addition would suggest that the effect of the low ascorbate contents in the vtc-1 mutant is not due to inadequate supply of precursors for cell wall biosynthesis. Such experiments are, however, more complicated than they appear because careful analysis reveals that ascorbate is not stable in either nutrient solutions or agar medium.
Decreased growth was not accompanied by changes in leaf H2O2 or antioxidant redox states, suggesting that atmospheric pollution is not a major source of oxidative load on the plants in our growth conditions. Atmospheric conditions at Rothamsted are typical of agricultural conditions in northern Europe and therefore provide an appropriate environment in which to conduct growth experiments. Moreover, the lack of a beneficial effect of high CO2 on growth suggests that the effect is: (a) not linked to oxidative stress, and (b) not due to pollutants, given the effect of high CO2 on stomatal conductance.
Low Ascorbate Does Not Lead to Oxidative Stress
Effects on growth due to diminished leaf ascorbate concentrations conceivably could be due to disruption of cellular homeostasis caused by oxidative stress. Several of our observations argue strongly against exacerbated oxidative stress in the mutant. First, no difference was found in leaf H2O2 contents. Second, the redox states of the major leaf antioxidant pools were unchanged. Third, the effects on growth could not be ameliorated by growth at elevated CO2, where AOS production is slowed. Fourth, no general up-regulation of antioxidative enzymes was observed, in agreement with previous observations (Conklin et al., 1997). Fifth, photosynthetic capacity and photochemical efficiency were unchanged. Sixth, effects on energy dissipation in the thylakoid membrane (NPQ) were trivial. This last observation suggests that ascorbate is not limiting for the de-epoxidation of violaxanthin, that violaxanthin de-epoxidation is not limiting NPQ, or that ascorbate is preferentially accumulated in the chloroplast in the mutant. In any case, the maintenance of a high NPQ capacity allows the mutant to dissipate light energy safely, and thereby avoid the production of harmful species such as singlet oxygen.
Failure to Maintain Sufficient Apoplastic Ascorbate Explains the Enhanced Sensitivity to Ozone
Although leaf ascorbate is decreased to 30% in the mutant, it is not known whether this decrease occurs proportionally in all cellular compartments. The final step of ascorbate synthesis takes place in the inner mitochondrial membrane with ascorbate being released into the inter-membrane space (Bartoli et al., 2000). Ascorbate must be transported to all the other compartments of the cell including the chloroplast and apoplast. Plant cells contain multiple transporters for Asc and DHA (Horemans et al., 1999). Upake of ascorbate across the chloroplast envelope (Foyer and Lelandais, 1996) may maintain the stromal ascorbate pool at wild-type concentrations, leading to a considerable deficit in the cytosol. This would explain the lack of effect of ascorbate depletion on photosynthesis and energy dissipation in the mutant.
Although the chloroplasts in the mutant may be able to maintain ascorbate closer to wild-type concentrations than total leaf contents would suggest, our measurements show that the apoplast is unable to do likewise. The apoplastic ascorbate pool was only 23% of wild-type values, and all of this was in the oxidized form. The absence from plants of mechanisms to recycle ascorbate in the apoplast suggests that the reduced form must be supplied by transport from the cytosol (Horemans et al., 2000). It is clear from our measurements that transport of Asc from the cytosol is not able to maintain apoplastic ascorbate at the expense of the intracellular pool. Because the apoplastic pool of ascorbate is thought to be critical in defense against ozone, the inadequate supply of ascorbate to the apoplast likely accounts for the enhanced ozone sensitivity of the vtc-1 mutant.
Leaf Ascorbate Content and the Intracellular Distribution of the Antioxidant System
Although total leaf activities of antioxidative enzymes were not changed in the mutant, closer inspection revealed significant changes in the intracellular distribution of APX, the first enzyme in the reductive detoxification of H2O2. We have recently discussed the extent of coupling between the ascorbate and glutathione pools, and the way in which independent changes in the redox state or content of these antioxidants could mediate changes in gene expression (Noctor et al., 2000). Preliminary investigations of this mutant suggested changes in the distribution of leaf APX activity (Noctor et al., 2000). Here, these results are elaborated and, more importantly from a signaling perspective, we show differential regulation of APX transcript abundance is linked to changes in Asc concentration independent of redox changes. As a result, the proportion of the enzyme activity in the cytosol was enhanced relative to that in the chloroplast. This suggests that ascorbate deficiency may trigger alarm signals that increase antioxidant activity specifically in the cytosol. The effect is clearly not linked to H2O2 contents, or to the redox state of leaf ascorbate and glutathione, because these parameters were similar in the wild type and mutants.
Transcripts for the inducible cytosolic APX have been shown to be particularly enhanced by high-light treatment in wild-type Arabidopsis (Karpinski et al., 1997), although the signals that relay information from the chloroplast to the nucleus have not been identified. In some species, foliar ascorbate content is also under light control, increasing markedly with growth irradiance (Gillham and Dodge, 1987; Grace and Logan, 1996). Our data suggest that ascorbate concentration plays a key role in maintaining homeostasis in the antioxidative system. In wild-type Arabidopsis, high light is required to overcome the stabilizing effect of ascorbate and to induce significant cytosolic APX expression (Karpinski et al., 1997). In contrast, in the vtc-1 mutant, where there is constitutive ascorbate deficiency, the cytosolic APX appears to be “turned up” even during growth at low light. In species where ascorbate content changes significantly with growth irradiance, ascorbate concentration is likely to play a part in the intracellular coordination of the antioxidative system in response to changing light intensity.
MATERIALS AND METHODS
Plant Growth
Seeds of Arabidopsis Columbia (Col) ecotype, wild type and the mutant vtc-1 (Conklin et al., 1996) were germinated on 1% (w/v) agar containing one-quarter-strength Hoagland's nutrient solution. Seedlings were grown to the age of 10 d (8-h photoperiod, 200 μmol quanta m−2 s−1, 60% [v/v] relative humidity, and day/night temperature of 23°C/18°C) and then transferred to pots containing a mixture of compost:sand (3:1) under the same growth conditions. Fully developed leaves attached to 5- to 6-week-old plants at rosette stage were used for gas exchange and fluorescence measurements. For measurements of proteins, antioxidants, H2O2, and enzymes, leaves from the middle of the rosette were harvested in the growth chamber 4 h into the light period, frozen in liquid nitrogen, and stored at −80°C until analysis.
Gas Exchange and Chlorophyll Fluorescence Measurements
Attached leaves were enclosed in a multicuvette open-circuit infrared gas exchange system that allowed measurement of steady-state photosynthesis in four leaves on different plants during a single analysis. The composition of the gas mixture (0.79 L L−1 N2, 340 μL L−1 [or 3,000 μL L−1] CO2, and 0.21 L L−1 O2) was adjusted by mass flow controllers (Bronkhorst HI-TECH B.V., Ruurlo, The Netherlands). Transpirational water loss and CO2 exchange were recorded on an infrared gas exchange analyzer (model WA-225-MK3, ADC, Hoddesdon, Hertfordshire, UK). For gas exchange and fluorescence measurements, leaves enclosed in the cuvettes were predarkened for at least 30 min. Actinic light was provided by metal halide lamps (FGL Lighting Ltd, Iver Heath, Berkshire, UK), positioned above the leaves. To measure a dependency of CO2 assimilation rate (A) on irradiance, neutral density sheets were progressively withdrawn so that the photon flux density was increased in steps, with 15 to 20 min at each photon flux density. Modulated chlorophyll fluorescence was measured with a pulse chlorophyll fluorometer (Waltz, Effeltrich, Germany). The photochemical yield of photosystem II (ΦPSII) was calculated empirically as the fluorescence parameter (Fm′ − F)/Fm′ (Genty et al., 1989). NPQ was calculated as a Stern-Vollmer-type quenching (Bilger and Björkman, 1990). The minimal “dark” fluorescence level following illumination (Fo′) was measured in the presence of a background far-red light to favor rapid oxidation of intersystem electron carriers.
Antioxidative Enzymes
All enzymes were measured in discs taken from fully developed leaves in the middle of the rosettte. DHAR and GR were measured as by Foyer and Halliwell (1976). Catalase was measured as the absorbance decrease at 240 nm (ε = 0.04 mm−1 cm−1) in 50 mm KH2 PO4 and 20 mm H2O2. POX was measured by monitoring oxidation of either 20 mm pyrogallol (ε430 = 2.47 mm−1 cm−1) or 4 mm guaiacol (ε470 = 22.6 mm−1 cm−1) in 50 mm potassium-phosphate buffer (pH 6.5), following addition of 1 mm H2O2.
For APX activity, leaf material was ground in liquid N2 and homogenized in 50 mm potassium phosphate buffer (pH 7.2), 1 mm Asc, 1 mm EDTA, 0.1% (w/v) phenylmethylsulfonyl fluoride, and 2% (w/v) polyvinyl pyrrolidone. The homogenate was strained through one layer of Miracloth and centrifuged at 15,000g for 15 min. APX activities were measured by following the oxidation of Asc at 290 nm (ε = 2.8 mm−1 cm−1) in the presence of 1 mm Asc. The reaction was initiated by addition of 0.1 mm H2O2. Soluble isoforms of APX were measured in the first supernatant. The pellet was resuspended in 300 μL of extraction medium with 1% (w/v) 3-[(3-Cholamidopropyl) dimethylammonio]-2-hydroxy-1-propanesulfonate and, after centrifugation at 15,000g for 10 min, the second supernatant was used for measuring the thylakoid-bound form of APX. Different enzyme forms of soluble APXs (from the supernatant) were distinguished by their sensitivity to inactivation induced by H2O2 using a modification of the procedure described by Amako et al. (1994). Inactivation of APXs was initiated by addition of 5 μm H2O2 in the absence of Asc. The process was terminated by addition of 1 mm Asc and residual activities of APX were plotted against incubation time. Two phases in the decreasing of APX activity were obtained. The first phase, with a half-time for inactivation of 10 s, represents the stromal isoform, which is rapidly inactivated by sensitivity to H2O2 (Hossain et al., 1984). The activity that could be measured after 90 s of incubation was taken to represent the cytosolic isoform, which is more resistant to inactivation by H2O2 (Chen and Asada, 1989; Yoshimura et al., 1998).
Assay of Ascorbate, Glutathione, and H2O2
Leaf samples were ground in N2 and then in 1.0 n HClO4. Aliquots were withdrawn for chlorophyll assay, and the remaining extract was clarified by centrifugation. An aliquot of the supernatant was neutralized with K2CO3. Insoluble KClO4 was removed by centrifugation and aliquots of the supernatant used for assay of Asc and DHA as in Foyer et al. (1983) or total glutathione using the GR recycling assay as in Noctor and Foyer (1998b). Glutathione disulphide was measured as in Griffith (1980). Chlorophyll was measured in 80% (v/v) acetone at 666 and 655 nm, assuming stoichiometric conversion to pheophytin. H2O2 was measured by a method modified from Okuda et al. (1991).
Extraction of IWF
Soluble apoplastic enzymes and those ionically bound to the cell walls were extracted by vacuum infiltration in 10 mm sodium phosphate (pH 6.0) with 50 mm KCl and subsequent centrifugation of leaves at 2,000g for 10 min at 4°C. For assay of Asc and DHA contents, leaves were vacuum infiltrated with 50 mm KCl, 10 mm sodium phosphate (pH 4.0), and centrifuged in Eppendorf tubes containing 200 μL of 0.1 m HClO4. Asc and DHA were then determined as described above. Cytoplasmic contamination was assessed by Glc-6-phosphate dehydrogenase activity; routine extraction of IWF by the above methods in our laboratory has shown that estimation of contamination by Glc-6-phosphate dehydrogenase and Glc-6-phosphate yields similar results (less than 1% whole leaf activity or content in IWF).
Total RNA Extraction and RT-PCR
Total RNA was extracted using RNAWIZ (Ambion, Inc., Abingdon, Oxfordshire UK) according to the supplier's recommendation. Residual DNA was removed with DNase I, Amp Grade (GIBCO-BRL, Paisley, Strathclyde, UK). DNase was inactivated by addition of EDTA to a final concentration of 2.5 mm and incubation at 65°C for 10 min. The absence of DNA contamination in the samples was confirmed by a PCR of 35 cycles using the primers BAS-O1 and BAS-O4 (Baier and Dietz, 1999), which give a 585-bp product with genomic DNA. One microgram total RNA was reverse transcribed using 0.5 μg Oligo (dT)12–18 (GIBCO-BRL), 0.5 mm deoxy-nucleotide phosphate, 10 mm dithiothreitol, and 200 units Superscript II (GIBCO-BRL) following the supplier's recommendation. cDNA samples were standardized by PCR for actin content using the gene-specific primers Atact-S (5′-GAGAAGATGACTCAGATC-3′) and Atact-A (5′-ATCCT-TCCTGATATCGAC-3′). Primer sequences for stromal APX and thylakoid APX where obtained from Baier et al. (2000). Gene-specific primers were designed for cytosolic APX1 on the basis of the published sequence (Kubo et al., 1992; X59600; 5′-AAGGCTGTTGAGAAGTGC-3′ and 5′-TTAAGCATCA-GCAAACCC-3′). The PCR conditions were optimized empirically by testing various annealing temperatures. The identity of the PCR products was verified by single-strand sequencing (ABI PRISM, 310 Genetic Analyzer, Perkin-Elmer, Warrington, Cheshire, UK). For quantitative RT-PCR, the cycle number was reduced to the linear range (25 cycles). RT-PCR products (20–25 μL) were loaded on 2% (w/v) agarose gel containing 0.5 μg mL−1 ethidium bromide. The bands were detected and the band intensities quantified with the Eagle Eye II, Stratagene.
ACKNOWLEDGMENTS
We thank Professor Robert Last (Cornell University, Ithaca, NY) for the kind gift of vtc-1 seed, Dr. Margarete Baier (University of Bielefeld, Germany) for kind donation of APX cDNAs, and Simon Driscoll (Rothamsted, UK) for technical assistance.
Footnotes
This work was supported by the UK Biotechnology Sciences Research Council and by the Royal Society, London, UK (fellowship to S.D.V.-J.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010141.
LITERATURE CITED
- Amako K, Chen G-X, Asada K. Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol. 1994;35:497–504. [Google Scholar]
- Baier M, Dietz KJ. Protective function of chloroplast 2-cysteine peroxiredoxin in photosynthesis. Evidence from transgenic Arabidopsis. Plant Physiol. 1999;119:1407–1414. doi: 10.1104/pp.119.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier M, Noctor G, Foyer CH, Dietz K-J. Antisense suppression of 2-cystein peroxiredoxin in Arabidopsis specifically enhances the activities and expression of enzymes associated with ascorbate metabolism but not glutathione metabolism. Plant Physiol. 2000;124:823–832. doi: 10.1104/pp.124.2.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli C, Pastori GM, Foyer CH. Ascorbate biosynthesis in mitochondria is linked to the electron transport chain between complexes III and IV. Plant Physiol. 2000;123:335–343. doi: 10.1104/pp.123.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilger W, Björkman O. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth Res. 1990;25:173–185. doi: 10.1007/BF00033159. [DOI] [PubMed] [Google Scholar]
- Chen G, Asada K. Ascorbate peroxidase in tea leaves: occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol. 1989;30:987–998. [Google Scholar]
- Conklin PL, Norris SR, Wheeler GL, Williams EH, Smirnoff N, Last RL. Genetic evidence for the role of GDP-mannose in plant ascorbic acid (vitamin C) biosynthesis. Proc Natl Acad Sci USA. 1999;96:4198–4203. doi: 10.1073/pnas.96.7.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL, Pallanca J, Last RL, Smirnoff N. L-ascorbic acid metabolism in the ascorbate-deficient Arabidopsis mutant vtc1. Plant Physiol. 1997;115:1277–1285. doi: 10.1104/pp.115.3.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL, Williams EH, Last RL. Environmental stress sensitivity of an ascorbic acid-deficient Arabidopsis mutant. Proc Natl Acad Sci USA. 1996;93:9970–9974. doi: 10.1073/pnas.93.18.9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 1976;133:21–25. doi: 10.1007/BF00386001. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Lelandais M. A comparison of the relative rates of transport of ascorbate and glucose across the thylakoid, chloroplast and plasma membranes of pea leaf mesophyll cells. J Plant Physiol. 1996;148:391–398. [Google Scholar]
- Foyer CH, Lopez-Delgado H, Dat JF, Scott IM. Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signaling. Physiol Plant. 1997;100:241–254. [Google Scholar]
- Foyer CH, Noctor G. Oxygen processing in photosynthesis: regulation and signaling. New Phytol. 2000;146:359–388. [Google Scholar]
- Foyer CH, Rowell J, Walker D. Measurements of the ascorbate content of spinach leaf protoplasts and chloroplasts during illumination. Planta. 1983;157:239–244. doi: 10.1007/BF00405188. [DOI] [PubMed] [Google Scholar]
- Genty B, Briantais J-M, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and the quenching of chlorophyll fluorescence. Biochim Biophys Acta. 1989;990:87–92. [Google Scholar]
- Gillham DJ, Dodge AD. Chloroplast superoxide and hydrogen peroxide scavenging systems from pea leaves: seasonal variations. Plant Sci. 1987;50:105–109. [Google Scholar]
- Grace SC, Logan BA. Acclimation of foliar antioxidant systems to growth irradiance in three broad-leaved evergreen species. Plant Physiol. 1996;112:1631–1640. doi: 10.1104/pp.112.4.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Groden D, Beck E. H2O2 destruction by ascorbate-dependent systems from chloroplasts. Biochim Biophys Acta. 1979;546:426–435. doi: 10.1016/0005-2728(79)90078-1. [DOI] [PubMed] [Google Scholar]
- Horemans N, Foyer CH, Asard H. The functions of ascorbate and ascorbate transport systems in plant membranes. In: Denke A, Dornisch K, Fleischmann F, Graβmann J, Heiser I, Hippeli S, Oβwald W, Schempp H, editors. Different Pathways through Life: Biochemical Aspects of Plant Biology and Medicine. Munich: Lincom Europa; 1999. pp. 217–238. [Google Scholar]
- Horemans N, Foyer CH, Asard H. Transport and action of ascorbate at the plant plasma membrane. Trends Plant Sci. 2000;5:263–267. doi: 10.1016/s1360-1385(00)01649-6. [DOI] [PubMed] [Google Scholar]
- Hossain MA, Asada K. Inactivation of ascorbate peroxidase in spinach chloroplasts on dark addition of hydrogen peroxide: its protection by ascorbate. Plant Cell Physiol. 1984;25:1285–1295. [Google Scholar]
- Karpinski S, Escobar C, Karprinska B, Creissen G, Mullineaux PM. Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. Plant Cell. 1997;9:627–640. doi: 10.1105/tpc.9.4.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly GJ, Latzko E. Soluble ascorbate peroxidase. Naturwissenschaften. 1979;66:377–382. doi: 10.1007/BF00405128. [DOI] [PubMed] [Google Scholar]
- Kubo A, Saji H, Tanaka K, Tanaka K, Kondo N. Cloning and sequencing of a cDNA encoding ascorbate peroxidase from Arabidopsis thaliana. Plant Mol Biol. 1992;18:691–701. doi: 10.1007/BF00020011. [DOI] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- McGarvey DJ, Christoffersen RE. Characterization and kinetic parameters of ethylene-forming enzyme from avodaco fruit. J Biol Chem. 1992;267:5964–5967. [PubMed] [Google Scholar]
- Mittler R, Zilinskas BA. Purification and characterization of pea cytosolic ascorbate peroxidase. Plant Physiol. 1991;97:962–968. doi: 10.1104/pp.97.3.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998a;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Noctor G, Foyer CH. Simultaneous measurement of foliar glutathione and amino acids by high-performance liquid chromatography: a comparison with two other methods for assay of glutathione. Anal Biochem. 1998b;264:98–110. doi: 10.1006/abio.1998.2794. [DOI] [PubMed] [Google Scholar]
- Noctor G, Velojovic-Jovanovic S, Foyer CH. Peroxide processing in photosynthesis: antioxidant coupling and redox signaling. Philos Trans R Soc Lond. 2000;355:1465–1475. doi: 10.1098/rstb.2000.0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda T, Matsuda Y, Yamanaka A, Sagisaka S. Abrupt increase in the level of hydrogen peroxide in leaves of winter wheat is caused by cold treatment. Plant Physiol. 1991;97:1265–1267. doi: 10.1104/pp.97.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter W-D, Chapple CCS, Somerville CR. Altered growth and cell walls in a fucose-deficient mutant of Arabidopsis. Science. 1993;261:1032–1035. doi: 10.1126/science.261.5124.1032. [DOI] [PubMed] [Google Scholar]
- Smirnoff N, Wheeler GL. Ascorbic acid in plants: biosynthesis and function. Crit Rev Plant Sci. 2000;19:267–290. doi: 10.1080/10409230008984166. [DOI] [PubMed] [Google Scholar]
- Vanacker H, Carver TLW, Foyer CH. Pathogen-induced changes in the antioxidant status of the apoplast in barley leaves. Plant Physiol. 1998;117:1103–1114. doi: 10.1104/pp.117.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler GL, Jones MA, Smirnoff N. The biosynthetic pathway of vitamin C in higher plants. Nature. 1998;393:365–369. doi: 10.1038/30728. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Mori H, Nishimura M. A novel isozyme of ascorbate peroxidase localized on glyoxysomal and leaf peroxisomal membranes in pumpkin. Plant Cell Physiol. 1995;36:1157–1162. doi: 10.1093/oxfordjournals.pcp.a078862. [DOI] [PubMed] [Google Scholar]
- Yoshimura K, Ishikawa T, Nakamura Y, Tamoi M, Takeda T, Tada T, Nishimura K, Shigeoka S. Comparative study on recombinant chloroplastic and cytosolic ascorbate peroxidase isozymes of spinach. Arch Biochem Biophys. 1998;353:55–63. doi: 10.1006/abbi.1997.0612. [DOI] [PubMed] [Google Scholar]
- Zhang H, Wang J, Nickel U, Allen RD, Goodman HM. Cloning and expression of an Arabidopsis gene encoding a putative peroxisomal ascorbate peroxidase. Plant Mol Biol. 1997;34:967–971. doi: 10.1023/a:1005814109732. [DOI] [PubMed] [Google Scholar]