Abstract
The aim of this study was to analyze the safety and efficacy of direct-acting antivirals (DAAs) in young children with hepatitis C in a real-life setting. All participants who had received DAAs since August 2019 were included. Young (preschool) children (aged 3–6 years) were compared with those in an older age group (7–17 years). Liver disease progression was determined on the basis of transient elastography evaluation. Fixed doses of DAAs (sofosbuvir/ledipasvir, sofosbuvir/velpatasvir, and glecaprevir/pibrentasvir) were used, adjusted to the patients’ age and weight. The primary endpoints included evaluation of treatment efficacy (defined as a sustained virologic response (SVR12), with undetectable HCV RNA at 12 weeks posttreatment) and adverse event (AE) analysis. A total of 165 patients, including 35 (21%) 3–6-year-olds, were eligible for the study. Children in this group presented with elevated aminotransferase levels more often, but their median liver stiffness value was lower than that of the older age group (3.7 kPa vs. 4.6 kPa, p < 0.0001). Compared with 10/130 (8%) of the older children, no young children had significant fibrosis. In each age group, one child was lost to follow-up after the end of treatment, but all the remaining participants achieved SVR12. Thus, the intention-to-treat SVR12 was 97% in young children and 99% in the older group. There was no difference in the incidence of AEs between the groups (46% and 45%). Younger children reported headaches less often than older participants did (0 vs. 11%, p = 0.04).
Conclusion: This study confirms both the efficacy and tolerability of DAAs for the treatment of hepatitis C in children aged 3–6 years in a clinical setting. Qualification for treatment in this age group should be made individually on the basis of the child’s ability and willingness to swallow medications.
|
What is known: •Young children are underrepresented in studies documenting treatment with direct-acting antivirals (DAAs), which leads to significant gaps in our knowledge concerning the effects of novel antiviral treatment options on HCV treatment in this age group. What is new: • This is one of the few studies that analyzed the safety and efficacy of DAAs in young children with hepatitis C in a real-life setting, which confirmed both the efficacy and tolerability of DAAs for the treatment of hepatitis C in children aged 3–6 years. • We present a new algorithm for therapeutic management in young children with hepatitis C based on individual qualification for treatment according to the child’s ability and willingness to swallow medications. |
Keywords: Children, Direct-acting antivirals, Hepatitis C, Sustained virologic response
Introduction
Modeling estimations indicate that the global hepatitis C virus (HCV) prevalence among children was 0.13% (3,258,000) in 2018, with a lower prevalence among the youngest children (0.06% among 0–2-year-olds and 0.08% among 3–6-year-olds) than among older children (0.12% among 7–11-year-olds) and adolescents (0.21% among 12–18-year-olds) [1]. Chronic hepatitis C (CHC) is considered a slow-progressing disease in children, with an up to 40% chance for spontaneous HCV clearance [2]. However, more recent studies have documented the possibility of significant fibrosis and cirrhosis development during childhood [3, 4]. The implementation of novel interferon-free antiviral therapies based on direct-acting antivirals (DAAs) a decade ago for adult patients revolutionized the treatment of CHC and provided hope for access to effective therapy for children [2, 5]. Approval for the use of DAA in pediatric patients was delayed, but since 2017, subsequent DAA combinations have also been registered for use in children. The first approved DAA regimen for pediatric patients in the USA and Europe was sofosbuvir/ledipasvir (SOF/LDV), followed by the registration of other DAAs (glecaprevir/pibrentasvir, GLE/PIB; sofosbuvir plus ribavirin, SOF + RBV; sofosbuvir/velpatasvir, SOF/VEL; sofosbuvir/velpatasvir/voxilaprevir, SOF/VEL/VOX; and elbasvir/grazoprevir, EBR/GZR) in subsequent years [2, 6–14]. At present, DAA combinations are recommended for the treatment of CHC in children as young as 3 years of age, irrespective of the HCV genotype or the extent of disease progression [15]. However, according to the World Health Organization guidelines updated in 2022, there is only a conditional recommendation to treat HCV in younger children (3–5 years), as very little evidence exists [16]. A recent systematic review with a meta-analysis performed by Indolfi et al. revealed that almost 2500 pediatric patients have received DAA treatment thus far [17]. Over three-quarters of them (1882) were adolescents (12–18 years), whereas only 166 (6.7%) were young children (3–5 years) [17]. These findings indicate that preschool children are underrepresented in studies documenting DAA treatment, which may be due to the low prevalence of CHC in this group, as well as delayed approval of DAAs for children younger than 6 years of age, which occurred in 2019 for SOF/LDV and in 2021 for SOF/VEL and GLE/PIB [7, 10, 13]. This leads to significant gaps in our knowledge concerning the effects of novel antiviral treatment options on HCV treatment in the youngest children. Thus, the aim of this study was to analyze the safety and efficacy of DAAs, as well as disease progression, in young children with hepatitis C in a real-life setting.
Methods
Study design
Our tertiary health care department has conducted therapeutic programs for pediatric patients with CHC using DAAs since August 2019. As of June 2025, 172 patients aged 3 to < 18 years were eligible for treatment with SOF/LDV (40 patients), SOF/VEL (50 patients), or GLE/PIB (82 patients). Prior to April 1, 2025, children in Poland had not been included in the national program for HCV treatment with DAAs. We launched two projects for pediatric patients with CHC, with similar management protocols: the POLAC project and the PANDAA-PED Study. In brief, the POLAC Project (“treatment of Polish children and adolescents with chronic hepatitis C using direct-acting antivirals”) is a real-life therapeutic program launched in August 2019, which is led courtesy of the donation of DAAs (SOF/LDV, GLE/PIB) by pharmaceutical companies [18, 19]. The PANDAA-PED study (“treatment of chronic hepatitis C in children aged 6–18 years using the pangenotypic direct-acting antiviral sofosbuvir/velpatasvir”) was a noncommercial, nonrandomized, open-label study funded by the Medical Research Agency, Warsaw, Poland (grant number 2019/ABM/01/00014). In this project, 50 patients aged 6–18 years were successfully treated for HCV infection between January 2022 and October 2022 via a 12-week course of SOF/VEL fixed-dose adjusted to the body weight [20].
In the current study, we included all participants who completed DAA treatment (irrespective of the regimen) and who had undergone their visit scheduled at 12 weeks posttreatment to evaluate the SVR12 until July 2025.
Participants
Children qualified for treatment on the basis of confirmed CHC (positive HCV PCR RNA testing for at least 6 months), irrespective of the HCV genotype (for pangenotypic regimens), or infected with HCV genotypes 1 or 4 in case of SOF/LDV therapy. Until April 2021, only children aged at least 12 years were eligible, according to the DAA approvals, and subsequently, younger participants, aged at least 3 years, were included. For the purpose of this study, the participants were divided into two age groups: preschool children (3–6 years old) were compared with older children (7–17 years old).
Treatment
In all cases, fixed doses of SOF/LDV and SOF/VEL were used, adjusted to the patients’ age and weight according to the current guidelines of the European Society of Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) [15, 21]. The treatment duration was 8 weeks for the GLE/PIB regimen and 12 weeks for the SOF/LDV and SOF/VEL regimens. In the case of SOF/LDV therapy, the duration of treatment was extended to 24 weeks, and children infected with HCV genotype 1 and presenting with cirrhosis were treated [21]. Before starting treatment, potential drug interactions between DAAs and comedications were excluded via HEP Drug Interactions Checker, University of Liverpool (https://www.hep-druginteractions.org). Patients were screened at baseline and then monitored every 4 weeks during therapy, at the end of treatment (EOT), and at 12 weeks posttreatment to assess SVR12. The primary endpoint included an evaluation of DAA efficacy (defined as SVR12, with undetectable HCV RNA at 12 weeks posttreatment). Nonresponse was defined as persistent HCV during and after treatment, and relapse was defined as the reappearance of HCV RNA after its previous clearance during or after therapy. Nucleic acid testing (HCV RNA) was performed using quantitative real-time polymerase chain reaction (RT‒PCR, Test Cobas 5800 HCV, Roche Diagnostics, Rotkreuz, Switzerland; with a measurement linearity range of 15–1.0 × 108 IU/ml). Biochemical serum testing was performed via commercially available laboratory kits. For both alanine and aspartate aminotransferase (ALT and AST) levels, the upper limits of normal were set at 40 IU/mL. Transient elastography was performed in all participants at baseline via a FibroScan device (Echosens, Paris, France) to assess liver fibrosis and steatosis. All adverse events (AEs) that occurred during treatment were recorded at every patient’s visit and analyzed. Patients’ parents/guardians were also asked to report every observed AE by phone call immediately after its occurrence.
Statistical analysis
Data are presented as numbers (percentages of total) or medians (interquartile ranges, IQRs), as appropriate. Categorical variables were compared via the chi-square test, and continuous data were compared via the Mann‒Whitney test. Statistical analyses were performed via MedCalc Statistical Software version 23.2.8 (MedCalc, Ostend, Belgium). A two-sided p value of < 0.05 was considered significant. p values of ≥ 0.05 were described as nonsignificant (NS).
Results
Study group
One hundred sixty-five children were eligible for the study, including 35 (21%) young children (3–6 years old) and 130 (79%) participants aged 7–17 years. Among the young group, 6 (17%) were 3 years old, 8 (23%) were 5 years old, and 21 (60%) were 6 years old. In both groups, most patients were infected vertically with genotype 1 HCV. The most frequently used DAA regimen was GLE/PIB (75/165, 45% of children), followed by SOF/VEL (50/165, 30%) and SOF/LDV (40/165, 25%). The majority of young children (80%) were treated with GLE/PIB (Fig. 1). The baseline characteristics of the participants in the study group according to age are presented in Table 1.
Fig. 1.
Flowchart of patient selection in the study. (*) Granulate formula; (**) tablets. DAAs, direct-acting antivirals; EOT, end of treatment; GLE/PIB, glecaprevir/pibrentasvir; LTFU, lost to follow-up; SOF/LDV, sofosbuvir/ledipasvir; SOF/VEL, sofosbuvir/velpatasvir; SVR12, sustained virologic response at 12 weeks posttreatment
Table 1.
Baseline characteristics of the study group according to the age of the participants
| Characteristics | All participants (n = 165) |
Young children (n = 35) |
Older children (n = 130) |
p |
|---|---|---|---|---|
| Sex (male/female) | 85 (53%)/80 (47%) | 15 (43%)/20 (57%) | 70 (54%)/60 (46%) | NS |
| Age (years) | 11 (7–13) | 6 (5–6) | 12 (10–14) | < 0.0001 |
| HCV genotype | ||||
| 1 | 121 (73%) | 25 (71%) | 96 (74%) | NS |
| 3 | 26 (16%)* | 7 (20%) | 19 (15%)* | NS |
| 4 | 18 (11%) | 3 (9%) | 15 (11%) | NS |
| Mother-to-child HCV transmission (vertical infection) | 148 (90%) | 34 (97%) | 114 (88%) | NS |
| Treatment experienced** | 20 (12%) | 0 | 20 (15%) | 0.01 |
| BMI (kg/m2) | 17.9 (15.5–21.0) | 14.8 (14.3–15.5) | 18.8 (16.8–22.1) | < 0.0001 |
| BMI z score | 0.23 (− 0.5–0.97) | − 0.35 (− 0.7–0.18) | 0.41 (− 0.38–1.19) | 0.0006 |
| ALT (IU/mL) | ||||
| Median | 45 (33–66) | 52 (42–63) | 43 (32–69) | NS |
| Number of children with ALT > 40 IU/mL | 97 (59%) | 27 (77%) | 70 (54%) | 0.01 |
| AST (IU/mL) | ||||
| Median | 47 (36–61) | 59 (48–73) | 45 (33–55) | < 0.0001 |
| Number of children with AST > 40 IU/mL | 106 (64%) | 32 (91%) | 74 (57%) | 0.0002 |
| Total bilirubin (µmol/L) | 9.6 (7.1–12.9) | 7.5 (6.1–9.2) | 10.2 (7.7–13.4) | 0.0007 |
| HCV viral load (log10 IU/mL) | 5.8 (5.3–6.3) | 6.1 (5.4–6.5) | 5.8 (5.3–6.3) | NS |
| Positive HBsAg or anti-HBc total | 2 (1%) | 0 | 2 (2%) | NS |
| Positive anti-HIV | 2 (1%) | 0 | 2 (2%) | NS |
| LSM (kPa) | 4.4 (3.7–5.4) | 3.7 (3.2–4.4) | 4.6 (3.9–5.6) | < 0.0001 |
| Liver fibrosis (METAVIR scale) | ||||
| F0/F1 | 35 (100%) | 120 (92%) | NS | |
| F ≥ 2 | 0 | 10 (8%) | NS | |
| CAP (dB/m) | 188 (160–211) | 165 (131–198) | 192 (168–213) | 0.001 |
| Number of patients with CAP > 238 dB/m | 6 (4%) | 0 | 6 (5%) | NS |
Data are presented as numbers (%) or medians (IQRs), as appropriate
NS nonsignificant (p ≥ 0.05), ALT alanine aminotransferase, AST aspartate aminotransferase, BMI body mass index, CAP controlled attenuation parameter, LSM liver stiffness measurement
*Including one patient infected with mixed genotypes 3 and 4 HCV
**Previous ineffective treatment with pegylated interferon plus ribavirin
Liver disease progression
Biochemical evaluation revealed that young children presented with elevated levels of both ALT and AST more frequently than older participants: 77% vs. 54% for ALT (p = 0.01) and 91% vs. 57% for AST (p = 0.0002) (Table 1). In contrast, the median total bilirubin concentration was lower in the younger group (7.5 µmol/L vs. 10.2 µmol/L, p = 0.0007). The median logarithmic values of the HCV viral loads did not differ significantly between the two age groups (Table 1). Considering the results of the elastography evaluation, we found lower median values of liver stiffness measurement (LSM) in young children than in older participants (3.7 kPa vs. 4.6 kPa, p < 0.0001). In addition, no child aged 3 to 6 years presented with significant fibrosis (F ≥ 2 on the METAVIR scale) compared with 10/130 (8%) patients in the older group (p = NS). Moreover, median controlled attenuation parameter (CAP) values, which indicate liver steatosis if > 238 dB/m, were lower in the younger group (165 dB/m vs. 192 dB/m, p = 0.001). No child in the younger group presented with steatosis on the basis of the CAP parameter, whereas 6 (5%) of the older patients presented with steatosis.
Treatment efficacy
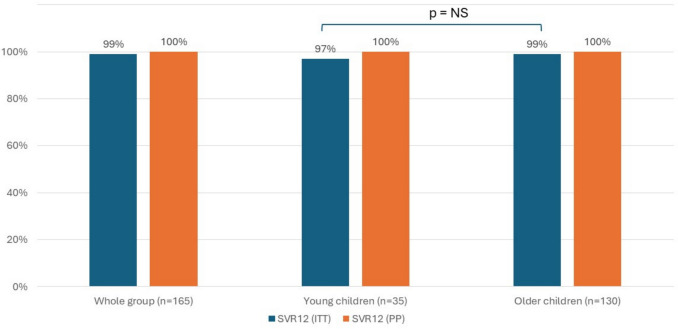
In each age group, one child was lost to follow-up after the end of treatment (one 5-year-old boy in the younger group and one 17-year-old adolescent in the older group), but all the remaining participants achieved SVR12. Thus, the intention-to-treat SVR12 was 97% in young children and 99% in the older group (p = NS). The per-protocol efficacy of treatment was 100% in both groups (Fig. 2). After 4 weeks of treatment, the HCV viral load was still detectable in 4 (11%) patients in the younger group and 15 (12%) in the older group (p = NS). At EOT, all the participants presented with undetectable HCV RNA (except for 6 older patients for whom data were unavailable due to the COVID-19 pandemic). No cases of relapse or nonresponse occurred.
Fig. 2.
Proportion of patients who achieved a sustained virologic response at 12 weeks posttreatment (SVR 12) according to age group. ITT, intention-to-treat; NS, nonsignificant; PP, per protocol; SVR12, sustained virologic response at 12 weeks posttreatment
Treatment safety
There was no difference in the incidence of AEs related to treatment between the two groups (46% and 45%, p = NS) (Table 2). Compared with older participants, younger children reported headache less often (0 vs. 11%, p = 0.04), but the differences in the prevalence of all other AEs among the age groups were insignificant (Table 2). All reported AEs were mild to moderate. No case of treatment discontinuation occurred. No child reported problems with swallowing medications.
Table 2.
Prevalence of adverse events (AEs) related to the use of direct-acting antivirals in the study group according to the participants’ age
| Symptom | All participants (n = 165) |
Young children (n = 35) |
Older children (n = 130) |
p |
|---|---|---|---|---|
| Number of children with any AE* | 74 (45%) | 16 (46%) | 58 (45%) | NS |
| Abdominal pain | 17 (10%) | 6 (17%) | 9 (7%) | NS |
| Fatigue/asthenia | 16 (10%) | 1 (3%) | 15 (12%) | NS |
| Headache | 14 (8%) | 0 | 14 (11%) | 0.04 |
| Diarrhea | 10 (6%) | 3 (9%) | 7 (5%) | NS |
| Somnolence | 9 (5%) | 0 | 9 (7%) | NS |
| Pruritus | 7 (4%) | 2 (6%) | 5 (4%) | NS |
| Vomiting | 7 (4%) | 3 (9%) | 4 (3%) | NS |
| Rash | 7 (4%) | 3 (9%) | 4 (3%) | NS |
| Nausea | 3 (2%) | 0 | 3 (2%) | NS |
| Dizziness | 3 (2%) | 0 | 3 (2%) | NS |
| Epistaxis | 3 (2%) | 2 (6%) | 1 (1%) | NS |
| Other** | 7 (4%) | 0 | 7 (5%) | NS |
*There were children who reported more than one AE
NS nonsignificant (p ≥ 0.05)
**Include the following AEs that occurred in no more than two children: syncope, hair loss, calf cramps, abnormal taste, and chest pain
Discussion
The main goal of antiviral therapy in children with CHC is to cure the infection and, as a consequence, prevent the progression of HCV-related liver disease and its complications [15]. Young children, who usually present with no liver fibrosis or CHC complications and rarely suffer from comorbidities requiring additional treatment, which may cause drug interactions with DAAs, seem ideal candidates for anti-HCV therapy. However, this age group constitutes a minority of participants in clinical trials regarding DAA use in children [17]. A recent meta-analysis performed by Indolfi et al. revealed that the overall treatment efficacy of different DAAs, defined as SVR12, was 100% (95% confidence interval (CI) 96–100%); however, it was significantly lower in the youngest children (96%, 95% CI 90–99%) than in 99% (97–100%) of older children aged 6–11 years and 100% (99–100%) of adolescents aged 12–18 years [17]. In particular, the lowest SVR12 rates were observed in patients treated with SOF/VEL. This reflects the results of a trial by Jonas et al. involving 216 children treated with SOF/VEL, where the SVR12 rates were 95% (97/102) for participants aged 12–17 years, 93% (68/73) for children aged 6–11 years, and only 83% (34/41) for 3–5-year-olds [7]. In this study, two of the nonresponders experienced virologic failure, 7 were lost to follow-up, and another 8 discontinued treatment for various reasons [7]. Interestingly, no cases of virologic failure were reported in the youngest group, but 7/41 (17%) of the children aged 3–5 years discontinued treatment or were lost to follow-up [7]. In a second part of the DORA study investigating the efficacy of GLE/PIB in children aged 3–12 years, the SVR12 in 3–5-year-olds was 23/14 (96%), and the efficacy was similar to that in other age groups [13]. In this study, one 3-year-old participant refused to swallow the granule formulation and prematurely discontinued the study after day 1 [13]. These observations indicate that DAA efficacy is lower in the youngest age group than in the older children and adolescents, which seems to be a concern for whether patients in this group should receive anti-HCV treatment or whether it should be postponed. In most cases, the lack of SVR12 achievement in this age group was not related to virological failure but was a result of treatment discontinuation or participants being lost to follow-up [17]. Discontinuation of treatment is mainly because medicines containing DAAs have a bitter taste and should not be chewed or split, which is often difficult for young children. In addition, no syrup formulations are available. These observations contrast, to some extent, with our clinical experience. In our study, 97% of the participants aged 3 to 6 years achieved SVR12, and no problems with their compliance were reported. Only one 5-year-old boy was lost to follow-up after EOT, when his HCV RNA was undetectable. We recommended that our patients take DAA oral pellets with food, e.g., yogurt, nut cream, or soft cheese, and not chew the medicine to avoid a bitter taste, but instead swallow the whole pellet.
In our opinion, based on our experience, the decision to implement DAA treatment in young children should be made individually, according to the clinical evaluation of liver disease and, in particular, the child’s ability and willingness to swallow medications, which is concordant with the ESPGHAN guidelines that recommend swallowing assessment before starting treatment [15]. Our proposed algorithm for therapeutic management in young children with hepatitis C is presented in Fig. 3. If DAAs are locally available in formulations dedicated to young children and if the child is able to swallow them, treatment should be implemented. In children who are unable to swallow medications, careful evaluation of liver disease should be performed. In the case of normal liver function and, in particular, no signs of liver fibrosis on elastography, antiviral treatment may be postponed. In our experience, we had two children (a 3-year-old girl and a 6-year-old boy) unable to swallow medications, without significant liver abnormalities, in which DAA inclusion was postponed until the child was ready to take medications. Notably, CHC progression is usually slow, and the risk of developing significant fibrosis during the first years of life is relatively low. In our cohort, no child in the younger group (and even those up to 10 years of age) presented with significant fibrosis, which supports this watchful waiting strategy. In addition, in children up to 7 years of age, there is still a possibility of spontaneous clearance of HCV without antiviral treatment implementation. We observed this phenomenon in two patients (aged 6 and 10 years) who had been diagnosed with CHC and had undetectable HCV RNA at the qualification for treatment. On the other hand, in children unable to swallow medications, who have developed signs of liver disease, particularly fibrosis, active support in training the child to swallow medicines (training with little candies or confectionary sprinkles that are similar to DAA granulate formulas) should be advised to hasten treatment implementation (Fig. 3). We should always consider advantages of DAA treatment in young children that include elimination of the possibility of stigma prior to school entrance, decrease in transmission risk, and the cost effectiveness of treating the young both by eliminating the risk of chronic liver disease and even in the need for use of smaller amounts of drug.
Fig. 3.
Proposed algorithm for therapeutic management in young children with hepatitis C. ALT, alanine aminotransferase; DAA, direct-acting antiviral; PI, prothrombin index
Another issue that should be considered before starting DAA treatment in young patients is the potential risk of AEs. According to the meta-analysis by Indolfi et al., the summarized frequency of AEs decreases with increasing age from 72% in 3–5-year-olds to 53% in 6–11-year-olds and 50% in children aged 12–18 years [17]. Severe AEs were more common in the youngest children (3%) than in those aged < 1% [17]. This is in contrast to our observations, which revealed a similar frequency of AEs in both age groups and only one difference in the occurrence of particular AEs (e.g., headache, which was less often observed in young children). In addition, we did not observe any AEs leading to treatment discontinuation. This is also in contrast to other papers, e.g., in the study by Jonas et al., in which treatment discontinuation occurred in 6/41 (15%) 3–5-year-olds and 2/73 (3%) 6–11-year-olds treated with SOF/VEL [7].
There is also limited experience with DAA treatment in children younger than 3 years of age. Li et al. reported two cases in which 2-year-old children were infected with genotype 1b HCV [22]. Both children were underweight, had elevated ALT levels, and suffered from diarrhea, which are considered symptoms of CHC. Thus, they both received a 12-week course of SOF/LDV (granulate formulation), which is officially not recommended and off-label in children aged below 3 years. Patients achieved SVR12 without obvious treatment-related adverse effects (AEs), and both gained weight [22]. These cases may indicate the safety and efficacy of DAAs in even children younger than 3 years of age; however, the demand for such early treatment does not seem to be very frequent.
There is still a global problem of a lack of population screening for HCV, or even for high-risk children. There are no such systematic screening programs in Poland, and the decision on testing for HCV infection is made individually based on the clinical or epidemiological issues. In the case of our patients, who are mainly infected via their mothers, screening practices for pregnant women are of particular importance. In Poland, testing for HCV infection is routinely recommended until the 10 weeks of pregnancy (in all women) and between the 33 and 37 weeks (only in risk groups). Children of the HCV-infected mothers should be tested for HCV infection using the PCR method for HCV RNA detection during the first year of life or by anti-HCV assessment over 18 months of life. However, as DAA treatment is not indicated during pregnancy, a significant number of HCV-infected mothers and their children seem to be lost to follow-up after birth.
Our study was performed on a significant number of patients and is one of the few studies that analyzed the effects of DAA treatment in the youngest age groups. However, some limitations should be noted. First, there was a different distribution of DAA regimens across the two age groups, which might have influenced the results obtained between the groups. Second, there were no 4-year-olds in our study group, although they qualified for treatment. In addition, we included 6-year-old children in the younger age group, as they are also considered preschool-aged in Poland, and in our opinion, their compliance is usually similar to that of younger children.
In conclusion, our study confirms both the efficacy and tolerability of DAA treatment for CHC in children aged 3–6 years in a real-life setting, which is comparable to the treatment results in older age groups. Qualification for the treatment of young children should be made individually on the basis of the child’s ability and willingness to swallow medications.
Abbreviations
- DAAs
Direct-acting antivirals
- SVR
Sustained virologic response
- AEs
Adverse events
- HCV
Hepatitis C virus
- CHC
Chronic hepatitis C
- SOF/LDV
Sofosbuvir/ledipasvir
- GLE/PIB
Glecaprevir/pibrentasvir
- RBV
Ribavirin
- SOF/VEL
Sofosbuvir/velpatasvir
- VOX
Voxilaprevir
- EBR/GZR
Elbasvir/grazoprevir
- ESPGHAN
European Society of Pediatric Gastroenterology, Hepatology, and Nutrition
- EOT
End of treatment
- CI
Confidence interval
- IQR
Interquartile range
- NS
Nonsignificant
- LSM
Liver stiffness measurement
- CAP
Controlled attenuation parameter
Authors’ contributions
MPŚ contributed to the study conception and design. Material preparation and data collection were performed by MPŚ, AD, ET and MA. Data analysis were performed by MPŚ and AD. The first draft of the manuscript was written by MPŚ and all authors commented on previous versions of the manuscript. MPŚ prepared the revision of the manuscript. MM supervised the work. All authors read and approved the final manuscript.
Funding
The PANDAA-PED research project was funded by the Medical Research Agency, Warsaw, Poland (project number 2019/ABM/01/00014); the POLAC project is a real-life, noncommercial study without any external funding.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval
The investigation was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments. Written informed consent was obtained from all the patients and/or their parents/guardians before their inclusion in the study. The local ethics committee of the Medical University of Warsaw approved both the PANDAA-PED Study and the POLAC Project (approval numbers KB/87/2019, 13 May 2019; KB/136/2020, September 14, 2020; No KB/91/A2020, 14 October 2020; and KB/30/A2021, April 19, 2021; No KB/73/A2022, November 7, 2022).
Consent to participate
Informed consent was obtained from all participants involved in the study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schmelzer J, Dugan E, Blach S, Coleman S, Cai Z, DePaola M et al (2020) Global prevalence of hepatitis C virus in children in 2018: a modelling study. Lancet Gastroenterol Hepatol 5(4):374–392 [DOI] [PubMed] [Google Scholar]
- 2.Indolfi G, Easterbrook P, Dusheiko G, El-Sayed MH, Jonas MM, Thorne C et al (2019) Hepatitis c virus infection in children and adolescents. Lancet Gastroenterol Hepatol 4(6):477–487 [DOI] [PubMed] [Google Scholar]
- 3.Modin L, Arshad A, Wilkes B, Benselin J, Lloyd C, Irving WL et al (2019) Epidemiology and natural history of hepatitis C virus infection among children and young people. J Hepatol 70(3):371–378 [DOI] [PubMed] [Google Scholar]
- 4.Pokorska-Śpiewak M, Dobrzeniecka A, Lipińska M, Tomasik A, Aniszewska M, Marczyńska M (2021) Liver fibrosis evaluated with transient elastography in 35 children with chronic hepatitis C virus infection. Pediatr Infect Dis J 40(2):103–108 [DOI] [PubMed] [Google Scholar]
- 5.Malik F, Bailey H, Chan P, Collins IJ, Mozalevskis A, Thorne C et al (2021) Where are the children in national hepatitis C policies? A global review of national strategic plans and guidelines. JHEP Rep 3(2):100227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balistreri WF, Murray KF, Rosenthal P, Bansal S, Lin CH, Kersey K et al (2017) The safety and effectiveness of ledipasvir-sofosbuvir in adolescents 12–17 years old with hepatitis C virus genotype 1 infection. Hepatology 66(2):371–378 [DOI] [PubMed] [Google Scholar]
- 7.Jonas MM, Romero R, Rosenthal P, Lin CH, Verucchi G, Wen J et al (2024) Sofosbuvir-velpatasvir in children 3–17 years old with hepatitis C virus infection. J Pediatr Gastroenterol Nutr 78(6):1342–1354 [DOI] [PubMed] [Google Scholar]
- 8.Murray KF, Balistreri WF, Bansal S, Whitworth S, Evans HM, Gonzalez-Peralta RP et al (2018) Safety and efficacy of ledipasvir-sofosbuvir with or without ribavirin for chronic hepatitis C in children ages 6–11. Hepatology 68(6):2158–2166 [DOI] [PubMed] [Google Scholar]
- 9.Rosenthal P, Schwarz KB, Gonzalez-Peralta RP, Lin CH, Kelly DA, Nightingale S et al (2020) Sofosbuvir and ribavirin therapy for children aged 3 to <12 years with hepatitis C virus genotype 2 or 3 infection. Hepatology 71(1):31–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarz KB, Rosenthal P, Murray KF, Honegger JR, Hardikar W, Hague R et al (2020) Ledipasvir-sofosbuvir for 12 weeks in children 3 to <6 years old with chronic hepatitis c. Hepatology 71(2):422–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jonas MM, Squires RH, Rhee SM, Lin CW, Bessho K, Feiterna-Sperling C et al (2020) Pharmacokinetics, safety, and efficacy of glecaprevir/pibrentasvir in adolescents with chronic hepatitis C virus: part 1 of the DORA study. Hepatology 71(2):456–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez-Peralta RP, Wirth S, Squires RH, Mutschler F, Lang T, Pawlowska M et al (2023) Elbasvir/grazoprevir in children aged 3–18 years with chronic HCV genotype 1 or 4 infection: a pharmacokinetic modeling study. Hepatol Commun 7(3):e0031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jonas MM, Rhee S, Kelly DA, Del Valle-Segarra A, Feiterna-Sperling C, Gilmour S et al (2021) Pharmacokinetics, safety, and efficacy of glecaprevir/pibrentasvir in children with chronic HCV: part 2 of the DORA study. Hepatology 74(1):19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Indolfi G, Kelly D, Nebbia G, Iorio R, Mania A, Giacomet V et al (2022) Sofosbuvir-velpatasvir-voxilaprevir in adolescents 12 to 17 years old with HCV infection. Hepatology 76(2):445–455 [DOI] [PubMed] [Google Scholar]
- 15.Indolfi G, Gonzalez-Peralta RP, Jonas MM, Sayed MH, Fischler B, Sokal E (2024) ESPGHAN recommendations on treatment of chronic hepatitis C virus infection in adolescents and children including those living in resource-limited settings. J Pediatr Gastroenterol Nutr. 10.1002/jpn3.12160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Updated recommendations on treatment of adolescents and children with chronic HCV infection: policy brief. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO.
- 17.Indolfi G, Easterbrook P, Giometto S, Malik F, Chou R, Lucenteforte E (2024) Efficacy and safety of DAA in children and adolescents with chronic HCV infection: a systematic review and meta-analysis. Liver Int. 10.1111/liv.15827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pokorska-Śpiewak M, Dobrzeniecka A, Aniszewska M, Marczyńska M (2021) Real-life experience with ledipasvir/sofosbuvir for the treatment of chronic hepatitis C virus infection with genotypes 1 and 4 in children aged 12 to 17 years-results of the POLAC project. J Clin Med. 10.3390/jcm10184176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pokorska-Śpiewak M, Dobrzeniecka A, Ogrodnik A (2022) Efficacy and safety of the treatment of chronic hepatitis C with sofosbuvir/ledipasvir in children aged 5 to 10 years with comorbidities-a brief report. Infect Dis Rep 14(4):574–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pokorska-Śpiewak M, Talarek E, Aniszewska M, Pluta M, Dobrzeniecka A, Marczyńska M et al (2023) Efficacy and safety of treatment with sofosbuvir/velpatasvir in patients aged 6–18 years with chronic hepatitis C-results of the PANDAA-PED study. Liver Int. 10.1111/liv.15637 [DOI] [PubMed] [Google Scholar]
- 21.Indolfi G, Hierro L, Dezsofi A, Jahnel J, Debray D, Hadzic N et al (2018) Treatment of chronic hepatitis C virus infection in children: a position paper by the Hepatology Committee of European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 66(3):505–515 [DOI] [PubMed] [Google Scholar]
- 22.Li M, Wulayin K, Lin S, Wu C, Chen L (2023) Should they wait? Two children under 3 years old infected by HCV 1b successfully treated by ledipasvir/sofosbuvir: a report of two cases. Liver Res 7(4):361–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.