Abstract
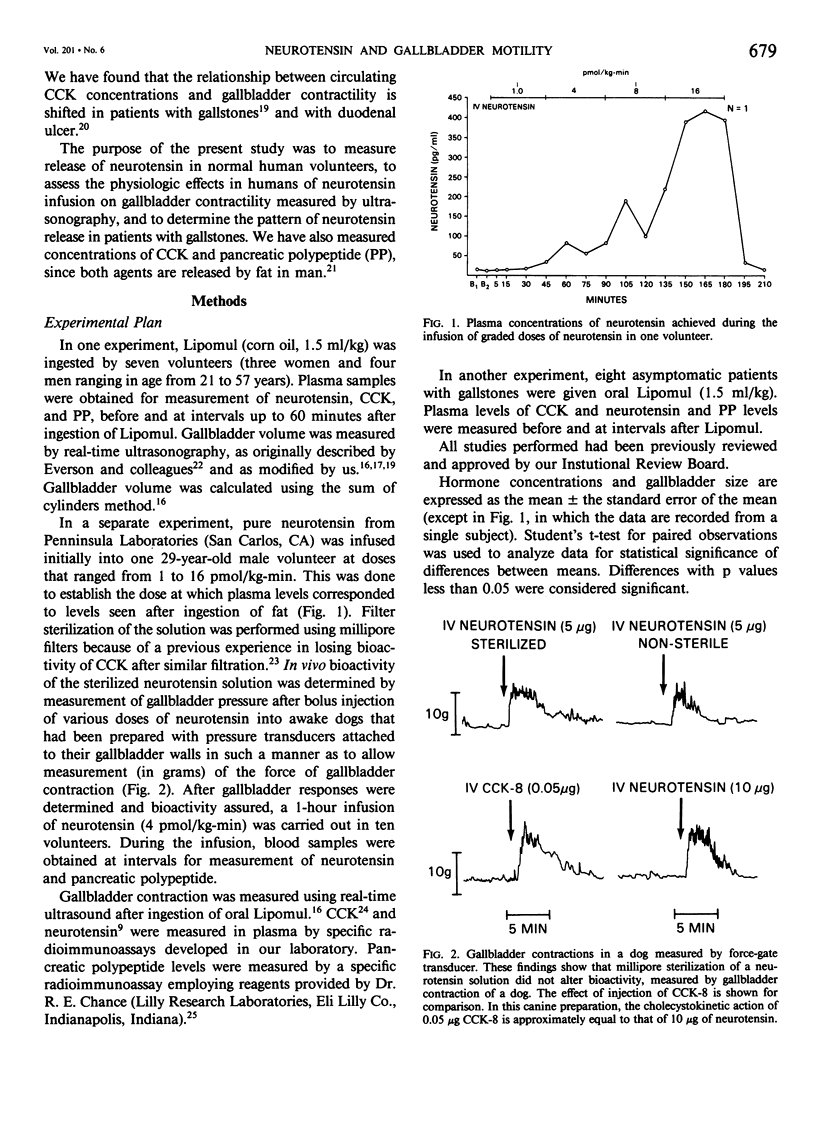
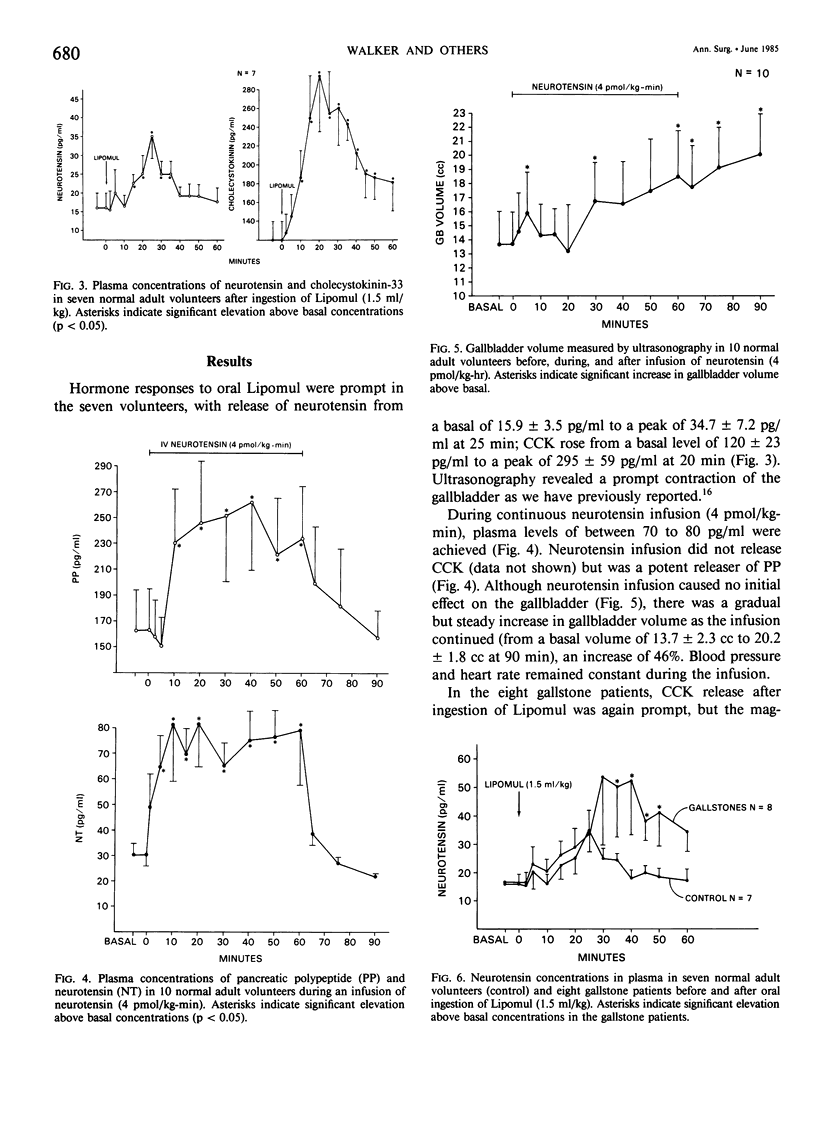
Gallbladder contraction in response to a fatty meal is thought to be caused by release of cholecystokinin (CCK). We have previously demonstrated a close correlation between circulating concentrations of CCK and contraction of the gallbladder in normal humans and in gallstone patients. Recent studies in animals, however, have shown that other potentially cholecystokinetic hormonal agents are released by a fatty meal, which suggests that other hormones may be involved in postprandial gallbladder contraction. Neurotensin, a 13-amino acid peptide, is released by fat; we have shown it to cause gallbladder contraction in dogs. In the present study, we measured release of neurotensin in seven normal adult volunteers. We determined the effects of infused neurotensin (4 pmol/kg-min) on gallbladder contractility, measured by ultrasonography in 10 adult volunteers, and we evaluated release of neurotensin in eight patients with gallstones. After ingestion of fat, we found significant release of neurotensin in normal volunteers from a mean basal concentration of 15.9 +/- 3.5 pg/ml to a maximum of 34.7 +/- 0.2 pg/ml. In the gallstone patients after fat ingestion, neurotensin rose from a basal of 16.8 +/- 3.1 pg/ml to a maximum of 53.4 +/- 28.1 pg/ml, which was a significantly greater release than in controls. Intravenous infusion of neurotensin produced dilatation of the gallbladder (from a mean basal volume of 13.7 +/- 2.3 cc to 20.0 +/- 1.8 cc). Neurotensin causes relaxation of the gallbladder in humans and, by contributing to stasis, may be involved in the formation of gallstones.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baca I., Feurle G. E., Schwab A., Mittmann U., Knauf W., Lehnert T. Effect of neurotensin on exocrine pancreatic secretion in dogs. Digestion. 1982;23(3):174–183. doi: 10.1159/000198725. [DOI] [PubMed] [Google Scholar]
- Blackburn A. M., Fletcher D. R., Bloom S. R., Christofides N. D., Long R. G., Fitzpatrick M. L., Baron J. H. Effect of neurotensin on gastric function in man. Lancet. 1980 May 10;1(8176):987–989. doi: 10.1016/s0140-6736(80)91434-8. [DOI] [PubMed] [Google Scholar]
- Calam J., Unwin R., Peart W. S. Neurotensin stimulates defaecation. Lancet. 1983 Apr 2;1(8327):737–738. doi: 10.1016/s0140-6736(83)92028-7. [DOI] [PubMed] [Google Scholar]
- Carraway R., Leeman S. E. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem. 1973 Oct 10;248(19):6854–6861. [PubMed] [Google Scholar]
- Fried G. M., Ogden W. D., Fagan C. J., Inoue K., Greeley G., Thompson J. C. Plasma concentrations of cholecystokinin in patients with duodenal ulcer disease. Surgery. 1984 Jan;95(1):27–33. [PubMed] [Google Scholar]
- Fried G. M., Ogden W. D., Swierczek J., Greeley G. H., Jr, Rayford P. L., Thompson J. C. Release of cholecystokinin in conscious dogs: correlation with simultaneous measurements of gallbladder pressure and pancreatic protein secretion. Gastroenterology. 1983 Nov;85(5):1113–1119. [PubMed] [Google Scholar]
- Go V. L., Demol P. Role of nutrients in the gastrointestinal release of immunoreactive neurotensin. Peptides. 1981;2 (Suppl 2):267–269. doi: 10.1016/0196-9781(81)90043-7. [DOI] [PubMed] [Google Scholar]
- Greenberg G. R., McCloy R. F., Adrian T. E., Chadwick V. S., Baron J. H., Bloom S. R. Inhibition of pancreas and gallbladder by pancreatic polypeptide. Lancet. 1978 Dec 16;2(8103):1280–1282. doi: 10.1016/s0140-6736(78)92042-1. [DOI] [PubMed] [Google Scholar]
- Hammer R. A., Carraway R. E., Leeman S. E. Elevation of plasma neurotensinlike immunoreactivity after a meal. Characterization of the elevated components. J Clin Invest. 1982 Jul;70(1):74–81. doi: 10.1172/JCI110605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer R. A., Leeman S. E., Carraway R., Williams R. H. Isolation of human intestinal neurotensin. J Biol Chem. 1980 Mar 25;255(6):2476–2480. [PubMed] [Google Scholar]
- Hellström P. M., Rosell S. Effects of neurotensin, substance P and methionine-enkephalin on colonic motility. Acta Physiol Scand. 1981 Oct;113(2):147–154. doi: 10.1111/j.1748-1716.1981.tb06875.x. [DOI] [PubMed] [Google Scholar]
- Helmstaedter V., Feurle G. E., Forssmann W. G. Ultrastructural identification of a new cell type--the N-cell as the source of neurotensin in the gut mucosa. Cell Tissue Res. 1977 Nov 23;184(4):445–452. doi: 10.1007/BF00220968. [DOI] [PubMed] [Google Scholar]
- Konturek S. J., Jaworek J., Cieszkowski M., Pawlik W., Kania J., Bloom S. R. Comparison of effects of neurotensin and fat on pancreatic stimulation in dogs. Am J Physiol. 1983 Jun;244(6):G590–G598. doi: 10.1152/ajpgi.1983.244.6.G590. [DOI] [PubMed] [Google Scholar]
- Lonovics J., Suddith R. L., Guzman S., Rayford P. L., Thompson J. C. Loss of biologic and radioimmunologic activity of cholecystokinin during sterilization by nitrocellulose filtration. N Engl J Med. 1979 Nov 8;301(19):1066–1066. doi: 10.1056/nejm197911083011918. [DOI] [PubMed] [Google Scholar]
- Miller R. J. Neurotensin as a gastrointestinal hormone. Med Biol. 1981 Apr;59(2):65–68. [PubMed] [Google Scholar]
- Pomeranz I. S., Davison J. S., Shaffer E. A. In vitro effects of pancreatic polypeptide and motilin on contractility of human gallbladder. Dig Dis Sci. 1983 Jun;28(6):539–544. doi: 10.1007/BF01308156. [DOI] [PubMed] [Google Scholar]
- Reasbeck P. G., Barbezat G. O., Shulkes A., Leader J. Secretion of neurotensin and its effects on the jejunum in the dog. Gastroenterology. 1984 Jun;86(6):1552–1556. [PubMed] [Google Scholar]
- Rosell S., Thor K., Rökaeus A., Nyquist O., Lewenhaupt A., Kager L., Folkers K. Plasma concentration of neurotensin-like immunoreactivity (NTLI) and lower esophageal sphincter (LES) pressure in man following infusion of (Gln4)-neurotensin. Acta Physiol Scand. 1980 Aug;109(4):369–375. doi: 10.1111/j.1748-1716.1980.tb06609.x. [DOI] [PubMed] [Google Scholar]
- Ryan J., Cohen S. Effect of vasoactive intestinal polypeptide on basal and cholecystokinin-induced gallbladder pressure. Gastroenterology. 1977 Oct;73(4 Pt 1):870–872. [PubMed] [Google Scholar]
- Sakamoto T., Newman J., Fujimura M., Greeley G. H., Jr, Townsend C. M., Jr, Thompson J. C. Role of neurotensin in pancreatic secretion. Surgery. 1984 Aug;96(2):146–153. [PubMed] [Google Scholar]
- Skov Olsen P., Holst Pedersen J., Kirkegaard P., Stadil F., Fahrenkrug J., Christiansen J. Neurotensin inhibits meal-stimulated gastric acid secretion in man. Scand J Gastroenterol. 1983 Nov;18(8):1073–1076. doi: 10.3109/00365528309181843. [DOI] [PubMed] [Google Scholar]
- Thompson J. C., Fried G. M., Ogden W. D., Fagan C. J., Inoue K., Wiener I., Watson L. C. Correlation between release of cholecystokinin and contraction of the gallbladder in patients with gallstones. Ann Surg. 1982 May;195(5):670–676. doi: 10.1097/00000658-198205000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. C., Marx M. Gastrointestinal hormones. Curr Probl Surg. 1984 Jun;21(6):1–80. doi: 10.1016/0011-3840(84)90005-4. [DOI] [PubMed] [Google Scholar]
- Thor K., Rosell S., Rökaeus A., Kager L. (Gln4)-neurotensin changes the motility pattern of the duodenum and proximal jejunum from a fasting-type to a fed-type. Gastroenterology. 1982 Sep;83(3):569–574. [PubMed] [Google Scholar]
- Vagne M., Grossman M. I. Cholecystokinetic potency of gastrointestinal hormones and related peptides. Am J Physiol. 1968 Oct;215(4):881–884. doi: 10.1152/ajplegacy.1968.215.4.881. [DOI] [PubMed] [Google Scholar]
- Vagne M., Troitskaja V. Effect of secretin, glucagon and VIP on gallbladder contraction. Digestion. 1976;14(1):62–67. doi: 10.1159/000197799. [DOI] [PubMed] [Google Scholar]
- Wiener I., Inoue K., Fagan C. J., Lilja P., Watson L. C., Thompson J. C. Release of cholecystokinin in man: correlation of blood levels with gallbladder contraction. Ann Surg. 1981 Sep;194(3):321–327. doi: 10.1097/00000658-198109000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiklund B., Liljeqvist L., Rökaeus A. Neurotensin increases net fluid secretion and transit rate in the small intestine of man. Regul Pept. 1984 Jan;8(1):33–39. doi: 10.1016/0167-0115(84)90026-0. [DOI] [PubMed] [Google Scholar]


