Abstract
Biochemical analysis of amylose-extender (ae) mutant of rice (Oryza sativa) revealed that the mutation in the gene for starch-branching enzyme IIb (BEIIb) specifically altered the structure of amylopectin in the endosperm by reducing short chains with degree of polymerization of 17 or less, with the greatest decrease in chains with degree of polymerization of 8 to 12. The extent of such change was correlated with the gelatinization properties of the starch granules, as determined in terms of solubility in urea solution. The ae mutation caused a dramatic reduction in the activity of BEIIb. The activity of soluble starch synthase I (SSI) in the ae mutant was significantly lower than in the wild type, suggesting that the mutation had a pleiotropic effect on the SSI activity. In contrast, the activities of BEI, BEIIa, ADP-Glc pyrophosphorylase, isoamylase, isoamylase, pullulanase, and Suc synthase were not affected by the mutation. Therefore, it is stressed that the function of BEIIb cannot be complemented by BEIIa and BEI. These results strongly suggest that BEIIb plays a specific role in the transfer of short chains, which might then be extended by SS to form the A and B1 chains of amylopectin cluster in rice endosperm.
Starch is composed of two types of molecule, namely amylose and amylopectin. Amylose is an essentially linear molecule composed of α(1→4)-linked glucosidic chains, whereas amylopectin is a highly branched glucan with α(1→6) glucosidic bonds that connect linear chains. The α-1,4 chains of amylopectin consist of A chains that carry no additional chains, B chains that carry A chains or other B chains, and a C chain that includes the reducing terminus (Peat et al., 1952). Hizukuri (1986) proposed a cluster model for amylopectin. In this model, A and B1 chains form a single cluster, whereas B2 and B3 chains extend to two and three clusters, respectively. Hanashiro et al. (1996) proposed that, in amylopectin, chains of degree of polymerization (DP) ≦12, 13 ≦ DP ≦ 24, 25 ≦ DP ≦ 36, and DP ≧ 37 correspond to A chains, B1 chains, B2 chains, and B3 and longer chains, respectively.
Amylose is synthesized by ADP-Glc pyrophosphorylase (AGPase) and granule-bound starch synthase I (GBSSI), which is encoded by the Waxy gene. Amylopectin is synthesized by concerted reactions catalyzed by AGPase, soluble starch synthase (SS), starch-branching enzyme (BE), and starch-debranching enzyme. BE is the only enzyme that can introduce α-1,6-glucosidic linkages into α-polyglucans and, therefore, it plays an essential role in the biosynthesis of amylopectin. The BEs from various higher plants appear to be composed of two types, namely BEI and BEII from maize (Zea mays; Boyer and Preiss, 1978a; Fisher and Boyer, 1983; Guan and Preiss, 1993; Guan et al., 1997), wheat (Triticum aestivum; Morell et al., 1997), and barley (Hordeum vulgare; Sun et al., 1997), and the A-type and B-type from pea (Pisum sativum; Burton et al., 1995; Martin and Smith, 1995) and potato (Solanum tuberosum; Larsson et al., 1996, 1998). At least three isoforms of BE have been identified in rice (Oryza sativa) endosperm (Mizuno et al., 1992; Nakamura et al., 1992). Yamanouchi and Nakamura (1992) resolved the BE of the developing endosperm, leaf blade, leaf sheath, culm, and root of rice into two types, BEI and BEII. The BEI type consists of only a single isoform, whereas the BEII type consists of multiple isoforms. The endosperm contains the two isoforms BEIIa (QEIIb) and BEIIb (QEIIa). BEIIb has been detected only in the endosperm, whereas BEIIa has been found in all organs.
Biochemical analysis of purified isoforms of BEI and BEII from maize endosperm indicated that BEI preferentially branches amylose-type, fewly branched polyglucans, as compared with BEII, whereas BEII has a higher capacity than BEI for branching amylopectin-type, highly branched glucans (Guan and Preiss, 1993; Takeda et al., 1993; Guan et al., 1997). These observations strongly suggest that BEI and BEII might play distinct roles in the construction of amylopectin molecules. However, the significance of the multiple isoforms of BEII have not yet been clarified.
BEIIb-deficient mutants have been isolated from maize and pea. In maize, they have been designated as amylose-extender (ae) mutants and have detected in the endosperm (Boyer and Preiss, 1978b; Stinard et al., 1993). The corresponding rugosus (r) mutants in pea have been detected in the embryos (Bhattacharyya et al., 1990). Starches from both ae and r mutants are characterized by a high amylose content (Hilbert and MacMasters, 1946; Banks et al., 1974). The average chain length of amylopectin in ae maize endosperm is significantly longer than that of normal amylopectin (Baba and Arai, 1984). In addition, the temperatures for the initiation of gelatinization of starch granules from ae maize and r pea are higher than those of the normal starches (Yuan et al., 1993; Bogracheva et al., 1995). The starch granules from amylomaize are also very resistant to disintegration in a concentrated solution of urea (Baba et al., 1983). These observations indicate that mutation of genes for BE leads to alterations in the structure of amylopectin and to rheological changes in starch. It has been reported that gelatinization properties are affected by the fine structure of amylopectin (Sanders et al., 1990; Tester and Morrison, 1990; Yuan et al., 1993; Jane et al., 1999), but the relationships between starch-synthesizing enzymes, the fine structure of amylopectin, and gelatinization properties have not been fully clarified. Studies of BE-deficient mutations provide clues to the roles of BE in the synthesis of amylopectin.
The ae starch in rice endosperm has a higher gelatinization temperature and the amylopectin has longer α-1,4-glucan chains than those of the wild-type starch and amylopectin (Yano et al., 1985). We do not understand, however, the way in which biochemical changes affect the structure of starch granules and the functional properties of starch. Mizuno et al. (1993) showed that BEIIb is deficient in ae rice mutants, whereas the level of the BEI transcript is apparently unaffected by the mutation. These observations suggest that the ae mutation might be useful in attempts to clarify not only the role of BEIIb in the biosynthesis of amylopectin in rice but also the effects of the fine structure of amylopectin on the physicochemical properties of starch.
In the present investigations, to eliminate the effects of amylose on the physicochemical properties of starch granules in ae mutant, we produced amylose-free ae mutant lines by crossing ae and waxy (wx) mutants. In addition, to examine the effect of the level of BEIIb on the synthesis of amylopectin, we performed reciprocal crosses to generate F1 seeds with different doses of the Ae allele.
In the present study, we measured gelatinization properties of endosperm starches from ae and amylose-free ae mutant lines using various concentrations of urea in solution. We examined the relationship between the gelatinization properties of starch granules and the structure of amylopectin. To assess the metabolic role of BEIIb, we analyzed the effects of ae mutation on activities of other isoforms of BE, namely BEI and BEIIa, and of starch- and Suc-metabolizing enzymes, such as SS, AGPase, isoamylase, pullulanase, and Suc synthase.
In the present paper, the Ae protein of rice is referred to as starch-branching enzyme IIb (BEIIb) as is that of maize (Preiss and Levi, 1980; Stinard et al., 1993). This enzyme in rice has also been referred to as QEIIa (Yamanouchi and Nakamura, 1992) or RBE3 (Mizuno et al., 1993). The other type of BEII, which is present in all rice tissues, including the developing endosperm and green leaves (Yamanouchi and Nakamura, 1992), is referred to as BEIIa. This enzyme has been called QEIIb (Yamanouchi and Nakamura, 1992) or RBE4 (Mizuno et al., 1992).
RESULTS
Effects of the Concentration of Urea on Gelatinization of Samples of Endosperm Starch from ae Mutant and the Parental Rice Cultivar
Endosperm starch from the amylose-extender (ae) mutant (aeae/WxWx) of rice, EM10, had a higher affinity for iodine, as measured by blue value at 680 nm than that from the wild-type (AeAe/WxWx) cv Kinmaze (0.59 versus 0.42; Table I). The apparent amylose content of the ae mutant, calculated on the basis of the blue value (Juliano, 1971) was estimated to be 26.5%, which was higher than that (15.7%) of the wild type (Table I). Takeda et al. (1987) suggested that the rice amylopectin having long chains showed high affinity for iodine. The blue value at 680 nm showed the affinity of starch, which included both amylose and amylopectin, for iodine. Thus, the amylose content calculated from the blue value at 680 nm merely shows the apparent amylose content. The endosperm starch from the waxy (wx) mutant (AeAe/wxwx) EM21, which contained only amylopectin, had lower affinity for iodine because it lacked GBSSI. The blue value of starch for the double-recessive mutant (aeae/wxwx), AMF44, derived from a cross between the ae mutant EM10 and the wx mutant EM21 was 0.28, which was obviously higher than that of the wx mutant, 0.16. The apparent amylose content of endosperm starch from the double mutant was estimated to be 7.5% despite the absence of amylose. Although the endosperm starch from the wild type was stained dark blue with I2/KI solution, that from the japonica wx mutant was stained lightly reddish brown. The endosperm starch from the double-recessive mutant was stained reddish purple despite the absence of amylose. The maximum wavelength of absorbance of the starch-iodine complex (λmax) of starch from the double mutant was 553 nm, which was 32 nm higher than that from the wx mutant. This result suggests that the increased apparent amylose content of endosperm starch in the ae mutant of rice was caused predominantly by the abnormal structure of amylopectin.
Table I.
Iodine-staining properties of endosperm starch from ae and amylose-free ae mutant lines
| Line | Genotype | BEIIb | GBSSI | λmax | B.V.(680)a | Apparent Amylose Contentb |
|---|---|---|---|---|---|---|
| nm | % | |||||
| Kinmaze | AeAe/WxWx | + | + | 561 | 0.42 | 15.7 |
| EM10 | aeae/WxWx | – | + | 574 | 0.59 | 26.5 |
| EM21 | AeAe/wxwx | + | – | 521 | 0.16 | 0.3 |
| AMF44c | aeae/wxwx | – | – | 553 | 0.28 | 7.5 |
The B.V.(680) showed the absorbance at 680 nm of the starch-iodine complex.
The apparent amylose content was determined by the iodine method (Juliano, 1971), on the base of calibration line, which was obtained from the blue values by changing the ratio of maize (Zea mays) amylose and rice amylopectin in the iodine solution.
AMF44 was a double-recessive mutant line, with the genotype aeae/wxwx, which was derived from a cross between the ae mutant EM10 and the wx mutant EM21. See text for details.
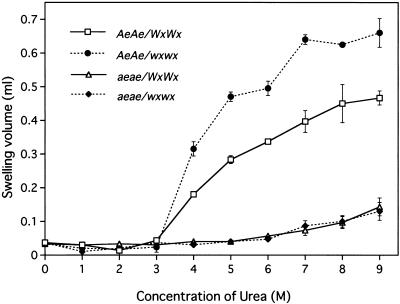
In maize, the amylomaize starch granules differ from wild-type granules in terms of the solubility in reagents such as dimethyl sulfoxide and urea (Adkins et al., 1970; Baba et al., 1983). To examine the effects of the concentration of urea on the gelatinization of starch granules of rice, we prepared endosperm starch from the wild type, the ae mutant, the wx mutant, and the double mutant and mixed them with solutions of urea at concentrations from 0 to 9 m (Fig. 1). The starch from the mature endosperm of the wild type and the wx mutant started to gelatinize in urea solution between 3 and 4 m. The swelling of both preparations of starch exhibited a biphasic response with increases in the concentration of urea. However, the extent of swelling at a given concentration of urea was more significant in the case of the glutinous wx starch than in that of the non-glutinous starch. In contrast, the granules of endosperm starch from the ae mutant hardly gelatinized in urea up to 6 m but they began to gelatinize at 7 m. The starch granules from the double mutant, which consisted exclusively of ae-type amylopectin, also gelatinized in 7 m urea. These results indicate that the starting concentration of urea for gelatinization of endosperm starch granules depends on the structure of the amylopectin rather than on the level or structure of amylose and, moreover, that the amylopectin in the ae endosperm is markedly more resistant to gelatinization in urea than that in the wild-type endosperm.
Figure 1.
Effects of the concentration of urea on gelatinization of starch granules from the ae mutant EM10 and the wild-type cv Kinmaze and from lines with the ae and Ae genes on the waxy background. After incubation of starch granules in solutions of urea for 24 h, swelling was examined by measuring the volume of the swollen starch sediment. □, Kinmaze (wild type, AeAe/WxWx); ●, EM21 (wx mutant, AeAe/wxwx); ▵, EM10 (ae mutant, aeae/WxWx); ♦, AMF44 (double-recessive mutant, aeae/wxwx). Results are means ± sd of results from three replicate experiments.
Effect of the ae Mutation on the Fine Structure of Amylopectin in the Mature Endosperm
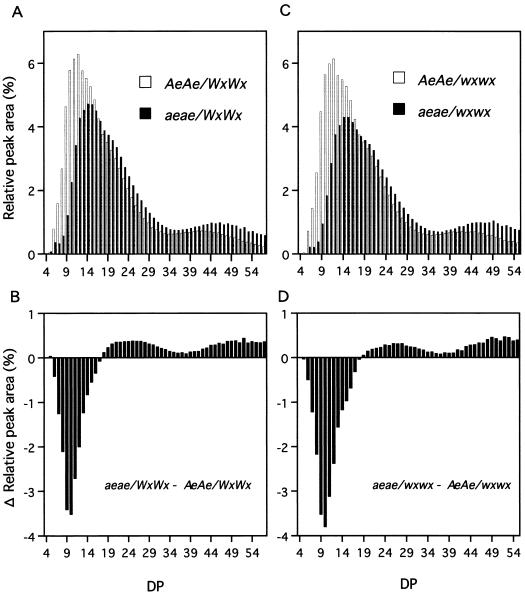
To examine the structural changes of ae amylopectin, we treated starch granules from mature seeds of the wild type, the ae mutant, the wx mutant, and the double mutant with the debranching by isoamylase from Pseudomonas amyloderamosa and then examined the distribution of chain lengths by high-performance anion-exchange chromatography (HPAEC)-pulsed amperometric detector (PAD; Fig. 2). In the ae mutant, the proportion of short chains was dramatically lower than in the wild type (Fig. 2A). In contrast, the proportion of longer chains in the ae mutant was higher than in the wild type. In particular, the proportion of short chains with DP ≦ 17 was markedly depressed in the endosperm starch of the ae mutant, whereas proportions of long chains with DP ≧ 38 were greatly elevated (Fig. 2B). The proportion of intermediate-length chains (18 ≦ DP ≦ 36) was also significantly higher in the ae mutant than in the wild type. The same trend was obtained from the amylose-free wx mutant and the amylose-free aeae/wxwx mutant (Fig. 2, C and D). These results suggest a reduced rate of synthesis of short chains in the amylopectin in the ae mutant EM10, and they are consistent with the hypothesis that the high affinity for iodine of the starch from the ae endosperm is caused by an abnormal structure of amylopectin, which is enriched with long chains and depleted of short ones.
Figure 2.
Distribution of chain length of total α-polysaccharides from wild-type, ae, wx, and aeae/wxwx rice endosperm as determined by HPAEC-PAD. Amylopectin was debranched with isoamylase from P. amyloderamosa, reduced with sodium borohydride, and then fractionated on a Carbopac PA1 column. The α-1,4-glucan chains were eluted with a gradient of sodium hydroxide and sodium acetate and monitored with a PAD. A, The distribution of α-1,4-glucan chains in amylopectin from the wild type (AeAe/WxWx) and the ae mutant (aeae/WxWx). B, The difference in amylopectin chain lengths between the ae mutant and the wild type. The columns show the area of each peak for the ae mutant minus the area of the corresponding peak with the same DP for the wild type. C, The distribution of α-1,4-glucan chains in amylopectin from the wx mutant (AeAe/wxwx) and the double-recessive mutant (aeae/wxwx). D, The difference in amylopectin chain lengths between the double mutant and the wx mutant. The results show representatives from three experiments that gave similar results.
Dosage Effects of the Ae Locus
The ae mutation of rice is a mutation of a structural gene for the endosperm-specific branching enzyme BEIIb (RBE3; Mizuno et al., 1993). Because the endosperm is the triploid tissue, we can generate F1 seeds with different doses of the Ae allele by making reciprocal crosses between the Kinmaze and EM10 lines. It seems likely that the gene dosage effect might be useful to understand the effects of the level of BEIIb protein on the proportion of short chains in amylopectin, on the gelatinization properties of starch granules in urea, and on the starch content.
Levels of the BEIIb
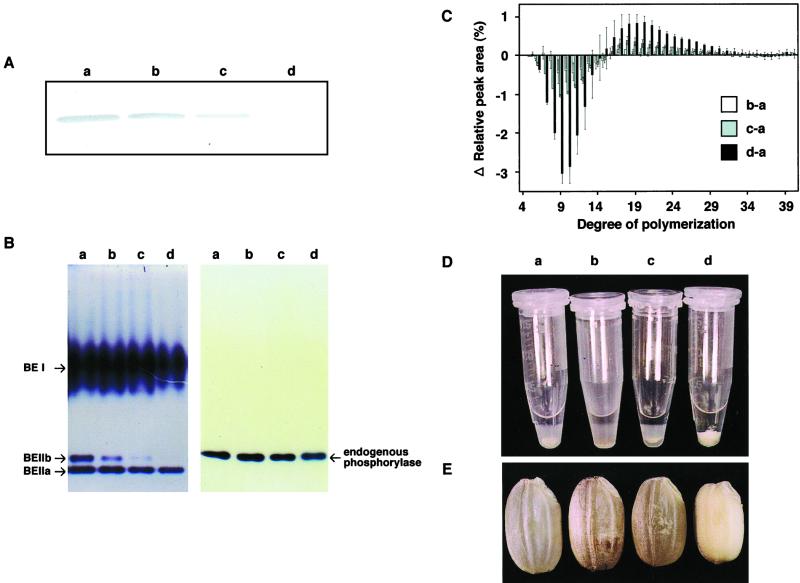
The endosperm of the ae mutant EM10 having the nulliplex genotype contained no BEIIb protein or only negligible amounts (Fig. 3A). There was a significant difference in the amount of BEIIb protein, as measured by western-blotting analysis, among the triplex (AeAeAe: wild type), duplex (AeAeae), simplex (Aeaeae), and nulliplex (aeaeae) genotypes (Fig. 3A). Densitometeric measurement indicated that the amount of BEIIb protein relative to the wild type (AeAeAe) was 74% in the duplex (AeAeae), 26% in the simplex (Aeaeae), and 0% in nulliplex (aeaeae) genotype (Table II). Thus, the level of BEIIb protein appeared to increase almost linearly with increases in the number of dominant Ae alleles. The same trend in the gene dosage effect on the BEIIb activity was found (Fig. 3B). However, it is noted that the activities of BEIIa and BEI were at the similar levels in any genotypes (Fig. 3B), indicating that the ae mutation specifically affects the BEIIb activity, but not the BEIIa and BEI activities.
Figure 3.
Gene dosage effects of the Ae allele on the level and activity of BEIIb, chain length distribution, gelatinization properties, and grain morphology. A, Western-blotting analysis of BEIIb in mature rice kernels. Protein was extracted from 20 mg of rice powder. The immunoblot was developed with antiserum raised against BEIIb from rice endosperm (Nakamura et al., 1992) at a dilution of 1:500. B, Native PAGE/activity stainings of BEs (left) and endogenous phosphorylase (right) in the endosperm of four genotypes. The migration and identification of each band corresponding to three BE isoforms (BEI, BEIIa, and BEIIb) and phosphorylase were according to our previous report (Yamanouchi and Nakamura, 1992). The volumes of crude enzyme extract applied were 0.67 and 6.7 μL for BE and phosphorylase, respectively. Note that the phosphorylase band was not detected under the lower protein concentration (0.67 μL of crude extract). C, Differences in the distribution of α-1,4-glucan chains among the four genotypes. The columns show the peak areas for each glucan chain from each genotype minus that from the wild-type cv Kinmaze. The sd was given from three separate experiments. D, Effects of 4 m urea on the swelling of starch granules from the endosperm of four genotypes. Ten milligrams of rice powder in an Eppendorf tube was mixed with 0.5 mL of 4 m urea, and shaken for 24 h at 25°C. After centrifugation, samples were allowed to stand for 1 h. E, Kernels from the four genotypes. a through d, Results for the four genotypes, AeAeAe, AeAeae, Aeaeae, and aeaeae, respectively.
Table II.
Gene dosage effects of the Ae allele on the various parameters of rice endosperm
| Genotype | Combination | Relative BEIIb Contenta | 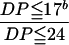 |
Concentration of Ureac | Grain Size
|
|||
|---|---|---|---|---|---|---|---|---|
| Wtd | Length | Width | Thickness | |||||
| % | m | mg | mm | |||||
| AeAeAe | Kinmaze | 100 | 75.4 ± 0.8 (10) | 4 | 23.7 ± 1.6 (100) | 5.3 ± 0.2 | 3.0 ± 0.2 | 2.1 ± 0.2 |
| AeAeae | Kinmaze × EM10 | 74 | 74.1 ± 1.4 (8) | 4 | 23.4 ± 1.4 (99) | 5.2 ± 0.2 | 3.0 ± 0.2 | 2.1 ± 0.1 |
| Aeaeae | EM10 × Kinmaze | 26 | 71.7 ± 0.3 (6) | 4 | 23.0 ± 1.1 (97) | 5.1 ± 0.1 | 2.8 ± 0.1 | 2.0 ± 0.2 |
| aeaeae | EM10 | 0 | 65.8 ± 1.7 | 8 | 15.5 ± 1.7 (65) | 4.8 ± 0.3 | 2.6 ± 0.2 | 1.7 ± 0.1 |
The BEIIb content was determined from the intensity of the immunoreactive band (Fig. 3A) with a densitometer.
The percentage of short chains with DP ≦ 17 within a cluster (DP ≦ 17/DP ≦ 24; Hanashiro et al., 1996) is given as the mean ± sd of results of three separate experiments. The difference from the aeaeae genotype (EM10) is indicated in parentheses.
The lowest concentration of urea for gelatinization of starch granules.
Details of grain size are given as means ± sd of results of more than seven separate sets of measurements of mature seeds. The kernel wt is given as a percentage of the wild-type wt in parentheses.
Distribution of Chain Length in Amylopectin
We observed a marked difference among the gene dosage groups in terms of the profiles of chain lengths in the isoamylolysates of amylopectin from rice endosperm (Fig. 3C). Proportions of short chains, in particular those with DP ≦ 17, were remarkably depressed with decreases in the number of Ae alleles. All changes in the proportion of short chains of amylopectin were reproducible and reliable although the change was most marked in the nulliplex condition. Although a gene dosage effect of the Ae allele was apparent in the four genotypes, the extent of the gene dosage effect on the chain length distribution was not necessarily related to the effect on the level of BEIIb itself (Fig. 3 and Table II). Hizukuri (1986) proposed that A and B1 chains form a single cluster, and Hanashiro et al. (1996) classified A chains as those with DP ≦ 12 and B1 chains as those with 12 ≦ DP ≦ 24. Based on this classification, we can define a single cluster as one that consists of chains with DP ≦ 24. The proportion of short chains with DP ≦ 17 within a single cluster was 75% in the wild type (AeAeAe), 74% in the duplex (AeAeae), 72% in the simplex (Aeaeae), and 66% in the nulliplex (aeaeae) genotype (Table II). Thus, the proportion of short chains with DP ≦ 17 increased with increasing number of the Ae allele but the relation was not linear.
Gelatinization Properties
The starch granules from the wild type were gelatinized in 4 m urea, whereas those of EM10 were scarcely gelatinized at all in 4 m urea, as shown in Figure 1. Although the granules of endosperm starch from the duplex (AeAeae) genotype were gelatinized in 4 m urea, the solubility, as determined by the volume of the sediment, was slightly lower than that of the wild type, corresponding to about 78% of the wild-type volume (Fig. 3D). Starch granules from the simplex (Aeaeae) genotype were less gelatinized than those of the wild type, and the solubility was only 57% of the wild-type value (Fig. 3D). These results are consistent with the gene dosage effects of the Ae allele on the chain length distribution of amylopectin.
Morphological Phenotype
Figure 3E shows the dosage effects of the Ae allele on the phenotype of the entire mature kernel. The kernels of the ae mutant EM10 were markedly smaller than the wild type, and the dry weight was 65% of the wild-type dry weight (Table II). The weights of the duplex (AeAeae) and simplex (Aeaeae) kernels were 99% and 97% of the wild-type weight, respectively. These results are also consistent with the gene dosage effects of the Ae allele on chain length distribution and gelatinization properties (Table II).
In the F2 population, the deficiency of BEIIb was always associated with the high resistance to gelatinization in urea and smaller kernels, and no segregants were found among them (data not shown). These results indicate that these characteristics, observed in the ae mutant line EM10, are caused by the ae gene. Thus, we can conclude that BEIIb, encoded by the Ae gene, plays a major role in the formation of short chains with DP ≦ 17 that are included in a single cluster of amylopectin, and, furthermore, that the reduction in proportion of short chains with DP ≦ 17 in the ae mutants is responsible for the insolubility of ae starch granules in a solution of urea.
Although the level of BEIIb increased approximately linearly with increasing numbers of Ae alleles, the proportion of short chains with DP ≦ 17, the gelatinization properties, and the starch content of kernels did not increase in parallel with the increase in number of the Ae allele. This observation suggests that BEIIb activity might not be the rate-limiting factor and that BEIIb might be present in excess relative to the rate of starch synthesis in the wild-type endosperm.
Effects of ae Mutation on Levels of Enzymes for the Metabolism of Starch and Suc
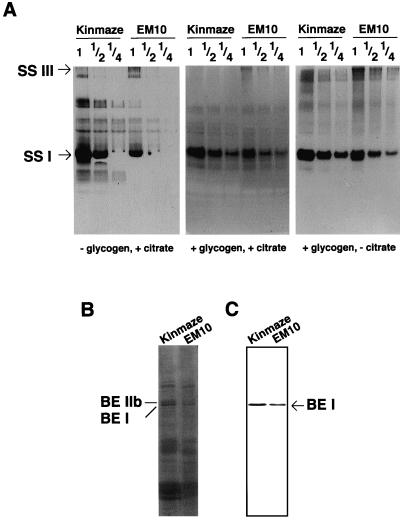
We examined the effects of ae mutation on the activities of other enzymes that are involved in the metabolism of starch and Suc (Table III). Total activities of AGPase, pullulanase, and Suc synthase from the developing endosperm of three ae mutant lines were, respectively, almost the same as those from the wild type, suggesting that ae mutations have no or little effect on the activities of these enzymes. Figure 3B showed that the level of phosphorylase activity was also unaffected by the ae mutation. In contrast, the total SS activity in EM10 endosperm was markedly decreased as compared with that in the wild-type endosperm in any assay conditions (Table III and Fig. 4A). These results suggest the possibility that the ae mutation causes pleiotropic effects on SS(s). The amount of soluble protein on the mutant grain was 91% of that in the wild-type grain. The SDS-PAGE analysis of the total soluble protein in the immature grain showed that the BEIIb protein was specifically absent and BEI protein was present in EM10 endosperm (Fig. 4B). Immunoblotting analysis indicates that the amount of BEI was at the same level in EM10 as in Kinmaze (Fig. 4C). Native PAGE/activity staining of isoamylase showed the activity of isoamylase in EM10 endosperm was also at the same level in Kinmaze (data not shown).
Table III.
Effects of the ae mutation on the activities of enzymes involved in the metabolism of starch and Suc in developing rice endosperm
| Enzyme | Enzymatic Activity (nmol min−1 per endosperm)
|
|
|---|---|---|
| Kinmaze | EM10 | |
| % | ||
| Starch synthase | ||
| +Citrate, +glycogen | 4.78 ± 0.2 (100) | 2.71 ± 0.1 (57) |
| +Citrate, −glycogen | 3.78 ± 0.3 (100) | 1.30 ± 0.1 (34) |
| −Citrate, +glycogen | 0.70 ± 0.1 (100) | 0.21 ± 0.1 (30) |
| AGPase | 44.8 ± 2.3 (100) | 56.7 ± 1.6 (127) |
| Pullulanase | 14.6 ± 1.1 (100) | 14.5 ± 0.2 (99) |
| Suc synthase | 28.5 ± 1.6 (100) | 35.7 ± 1.0 (125) |
The values represent means ± sd of results of three separate experiments. The enzymatic activity obtained in each experiment was the mean of the results of three replicate incubations. The value as a percentage of the wild-type Kinmaze value is shown in parentheses.
Figure 4.
Effects of the ae mutation on other starch-synthesizing enzymes. A, Native PAGE/activity staining of SS in the endosperm of ae mutant EM10 and the wild-type cv Kinmaze. Each lane contained 7.5 μL multiplied by the number given above the lane of the crude enzyme extract. The migration of each band corresponding to two SS isoforms (SSI and SSIII) was according to Abel et al. (1996). B, SDS-PAGE of total protein in rice mature kernel. C, Western-blotting analysis of BEI in mature rice kernels. The immunoblot was developed with antiserum raised against BEI from rice endosperm (Nakamura et al., 1992) at a dilution of 1:1,000.
Effect of ae Mutation on SSs
In an attempt to determine the isoform of SS that is affected by the ae mutation, the activity of each SS isoform was measured after the separation by native PAGE. Figure 4A shows that the SSI isoform accounted for the bulk of the total SS activity in rice endosperm, and that the activity of SSI was distinctly lower (about 50%) in the mutant than that in the wild type. Figure 4A shows that even in the absence of glycogen as glucan primer, 0.5 m citrate supported the SSI activity as in the case of SSI from maize endosperm (Boyer and Preiss, 1981). This primer-independent SSI activity was markedly lower in EM10 than in Kinmaze (Table III and Fig. 4A, left). The reduction of SSI activity was also observed in the presence of glycogen (Table III and Fig. 4A, middle and right). Because BEIIa and BEIIb bands as well as the BEI band were separated from the SSI band, the reduction of SSI activity was not caused by the omission of BE isoforms (data not shown). The deduced SSIII isoform was also present in the rice endosperm, but its activity was to a markedly lesser extent than that of SSI. The SSIII activity in the mutant was at the same or slightly higher level as compared with that in the wild type. Therefore, the decrease in the total activity of SS caused by ae mutation was at least mainly due to decrease in the SSI activity.
Effects of ae Mutation on Levels of Transcripts for Starch-Synthesizing Enzymes
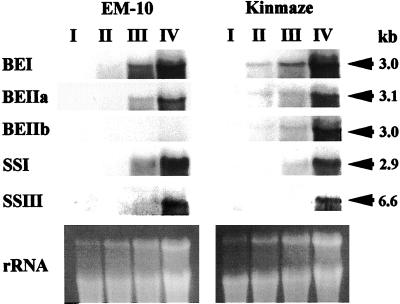
Figure 5 shows transcript levels for genes encoding starch-synthesizing enzymes. The ae mutant EM10 showed a nearly complete suppression of BEIIb expression. In contrast, no major differences in the expression of BEI, BEIIa, SSI, and SSIII could be observed in endosperm between EM10 and its parent cv Kinmaze. The results suggest that the SSI activity was inhibited at the posttranscriptional level by the ae mutation.
Figure 5.
Northern-blot analyses of BEI, BEIIa, BEIIb, SSI, and SSIII transcripts in ae mutant EM10 and the wild-type cv Kinmaze. Total RNA from developing grains, stages I, II, III, and IV, were blotted and probed with specific RNA probes. The lower portion of the figure shows the ethidium bromide-stained RNA gel.
DISCUSSION
There have been a number of studies with BE-deficient mutants from various plant species. It is known that the ae mutant in maize (Stinard et al., 1993) and the r mutant in pea (Bhattacharyya et al., 1990) are caused by lesions in the gene coding for the BEII-type or BE-A-type enzyme. Mizuno et al. (1993) also showed that BEIIb is deficient in ae rice mutants. Our present investigations strongly suggest that the abnormalities of the endosperm starch of ae rice, such as the high affinity for iodine and the apparently high amylose content, are caused by an altered amylopectin structure that is due to mutation in a gene for BEIIb (Table I, Figs. 1–3). Rice ae mutants have been classified in the same type of BE-deficient mutants from other plant species; for example, ae in maize and r in pea, which are characterized by an apparent increase in the amount of amylose in the storage starch (Banks et al., 1974; Jane et al., 1999). However, the blue value and λ-max after I2/KI staining of endosperm starch of the ae mutant with an amylose free waxy background were significantly higher than those of the wild type (Table I), indicating that the apparently elevated amylose content of ae mutants of rice was not caused by an increase in the net amylose content but by the proposed abnormal structure of the amylopectin.
The structure of amylopectin from BE-deficient mutants is clearly distinct from the wild-type structure in various plants. Earlier analysis of chain length distribution showed that the proportion of short chains is markedly lower, whereas that of the longest chains is elevated in the amylopectins from maize ae endosperm (Kasemsuwan et al., 1995) and pea r embryos (Tomlinson et al., 1997). The present study specified the effect of ae mutation on the fine structure of amylopectin by showing that the proportion of short chains with DP ≦ 17 and, in particular, of chains with DP 8 to 12 was remarkably reduced in the amylopectin from the endosperm of rice ae mutant (Fig. 2 and Table II). The specific role of BEIIb is also supported by the good correlations among the gene dosage effects of the Ae gene, the level of BEIIb itself, and the proportion of short chains with DP ≦ 17 (Fig. 3, A–C). These results indicate that the proportion of outer chains, that is to say, mainly the A chains of a cluster, is specifically reduced in ae mutants of rice. The evidence is consistent with the reports that, in maize endosperm, BEI predominantly produces longer chains (DP ≧ 10), whereas BEII preferentially transfers short chains (3 ≦ DP ≦ 9; Guan and Preiss, 1993; Takeda et al., 1993; Guan et al., 1997).
Several investigations have reported that the side chains (A and B1 chains) of a unit cluster of amylopectin form double helices although the minimum chain length for such a double helix might be approximately 10 Glc units, and the length of each double helix correlated with the gelatinization temperature (Gidley and Bulpin, 1987; Cooke and Gidley, 1992; Gidley et al., 1995; Moates et al., 1997; Safford et al., 1998). The gelatinization properties in the gene dosage group were correlated with the extent of the decrease in the short chains with DP ≦ 17 (Fig. 3, C and D). Thus, we conclude that the gelatinization behavior of starch granules in rice is predominantly, if not exclusively, determined by the proportion of short chains to long chains within a single cluster of amylopectin.
In rice endosperm, the activity of BEI is markedly higher than that of the total BEII (BEIIa and BEIIb) when measured by the phosphorylase a stimulation assay (Yamanouchi and Nakamura, 1992). The present study showed that the activity of BEI in rice endosperm was unaffected by the ae mutation (Fig. 3B), suggesting that the metabolic role of BEIIb cannot be compensated by the function of BEI. In cereal endosperm, there are two distinct forms of BEII, namely BEIIa and BEIIb (Boyer and Preiss, 1978a; Yamanouchi and Nakamura, 1992; Sun et al., 1998). BEIIb is specifically or predominantly found in the endosperm, whereas BEIIa is present in almost all tissues of maize (Fisher et al., 1996; Gao et al., 1997), barley (Sun et al., 1998), and rice (Yamanouchi and Nakamura, 1992; Mizuno et al., 1993). Although there are some differences in terms of amino acid sequence between BEIIa and BEIIb, several investigators (Guan and Preiss, 1993; Takeda et al., 1993; Mizuno et al., 2001) reported that the substrate specificities of the two enzymes are indistinguishable. The critical decreases in the proportion of short chains with DP ≦ 17 (Figs. 2 and 3C), despite the maintenance of normal BEIIa activity (Fig. 3B), indicate that the function of BEIIb cannot be compensated by BEIIa during the biosynthesis of amylopectin in rice endosperm.
In the ae mutant, both grain weight and the proportion of short chains with DP ≦ 17 were markedly lower than in the wild type (Table II). Isoforms of BE in rice belong to the family of amylolytic enzymes (Mizuno et al., 1992), cleaving α-1,4-glucosidic linkages and transferring the newly formed nonreducing ends to other α-1,4-linked chains (Borovsky et al., 1976). Thus, the actions of BE result in an increase in the number of nonreducing ends to which Glc moieties from ADP-Glc are bound in the reaction catalyzed by the SS. Therefore, we speculate that the reduction in the activity of BEIIb in the ae mutants caused decreases in the number of reducing ends that, in turn, resulted in a decrease in the synthesis of starch.
The significant proportions of short chains in the amylopectin of wild-type japonica rice cv Kinmaze suggest that the activity of BEIIb for synthesizing short chains of the new amylopectin cluster is in excess of the amylopectin biosynthesis, and that the capacity of some form(s) of SS for elongation of short chains within the amylopectin cluster might be a rate-limiting factor in the endosperm. On the other hand, the activity of BEIIb is greatly depressed in ae mutants and the number of short chains transferred within the cluster is considered to be markedly reduced. These short chains are then fully elongated by SS(s). The result alters the pattern of side chains from the normal pattern to the ae-type pattern with a considerable decrease in the proportion of short chains (DP ≦ 17).
The activity of SS in the ae mutant EM10 was markedly decreased as compared with that of the wild type, whereas the activities of the other starch-synthesizing enzymes, such as AGPase, pullulanase, and Suc synthase, were unaffected (Table III). The decrease in the total activity of SS induced by ae mutation was due to the decrease in SSI by approximately 50%, the major SS isoform in rice endosperm (Fig. 4A). The result seems to be consistent with the previous report by Boyer and Preiss (1978b) that the ae mutation of maize causes a reduction in the activity of SSI, as well as in the activity of BEIIb, whereas the activity of SSII is unaffected in the mutant endosperm. These results might suggest that BEIIb is associated with SSI in maize and rice endosperm.
The gelatinization property is one of the most important rheological indicators of the cooking quality and processing characteristics of rice starch. Numerous investigations have shown that the rheological properties of starch, such as gelatinization, retrogradation, and pasting properties are affected by amylopectin structure (Kalichevsky et al., 1990; Sanders et al., 1990; Tester and Morrison, 1990; Shi and Seib, 1992; Yuan et al., 1993; Lu et al., 1997; Safford et al., 1998; Jane et al., 1999). It has been noted that there is a correlation between the crystalline structure of starch and its rheological properties and that A chains play an important role of the formation of the crystalline structure. Jane et al. (1999) reported that the chain length and distribution of amylopectin branches determine the gelatinization temperature of starch, enthalpy changes, and pasting properties, and that the gelatinization temperature of starch increases with increasing the chain length. The results and the present studies support the view that alterations in amylopectin structure, in particular in short chains within clusters, might play a critical role in the rheological properties of starch. It is, therefore, likely that genetic modification of the gene for BEIIb will lead to the synthesis of novel types of starch as new materials for the food and starch industries.
MATERIALS AND METHODS
Plant Materials
In this study, we used an amylose-extender (ae) mutant line EM10 that was generated by treatment of fertilized egg cells of japonica rice (Oryza sativa) cv Kinmaze (Satoh and Omura, 1979) with N-methyl-N-nitrosourea. We also used an amylose-free ae mutant line, AMF44, in which the endosperm starch consists of only ae mutant-type amylopectin. AMF44 is a double-recessive mutant for ae and waxy (wx), with the aeae/wxwx genotype. It was derived from a cross between the ae mutant line EM10 and the wx mutant line EM21. The endosperm starch of EM21 contains no amylose and consists solely of amylopectin. The original parental cv Kinmaze and the wx mutant line EM21 were used as controls. For analysis of gene dosage effects, F1 seeds with two and one copies of the Ae allele were generated by reciprocal crosses between Kinmaze and EM10.
Determination of Apparent Amylose Content
Twenty milligrams of starch was gelatinized by treatment with 2 mL of 1 n NaOH and stood for 24 h at room temperature. After addition of 4 mL of 1 n CH3COOH and 4 mL of distilled water, the solution was homogenized. An aliquot (0.8 mL) of the solution was taken and added by 0.2 mL of 0.2% (w/v) I2, 2% (w/v) KI, and 4 mL of distilled water. An A680 (the blue value) and λ-max were measured. The apparent amylose content was determined according to the method of Juliano (1971), on the base of calibration line, which was obtained from the blue value at 680 nm by changing the ratio of maize (Zea mays) amylose and rice amylopectin in the iodine solution.
Measurement of Gelatinization Properties
Five kernels of mature seeds of average size, which had been removed from embryos with forceps, were ground with a mortar and pestle, and 20 mg of the powder was mixed with 1 mL of a solution of 0 to 9 m urea, the pH of which had been adjusted to 6.0 with acetic acid, in an Eppendorf tube. The mixture was incubated at 25°C for 24 h. The suspension was centrifuged for 20 min at 8,000g at room temperature and then allowed to stand for 1 h. The solubility of the granules in the urea solution was expressed in terms of the volume of the swollen sediment, which was calculated by subtracting the volume of supernatant from the urea solution (1 mL).
Determination of the Distribution of Lengths of α-1,4-Glucan Chains in α-Polysaccharides by HPAEC-PAD
The embryo and pericarp were removed from three dehulled grains of average size. The endosperms were ground with a mortar and pestle and 5 mg of the resulting powder were suspended in 5 mL of methanol and boiled for 10 min. The homogenate was centrifuged at 2,500g for 5 min. The pelleted polyglucan was washed twice with 5 mL of 90% (v/v) methanol, suspended in 5 mL of distilled water, and then boiled for 60 min. An aliquot (1.0 mL) of the sample of gelatinized polyglucan was added to 50 μL of 600 mm sodium-acetate buffer (pH 4.4) and 10 μL of 2% (w/v) NaN3 and then hydrolysis was achieved by addition of 10 μL of isoamylase from Pseudomonas amyloderamosa (1,400 units; Seikagaku Corporation, Tokyo) and incubation at 37°C for 24 h. The hydroxyl groups of the debranched glucans were reduced by treatment with 0.5% (w/v) of sodium borohydride under alkaline conditions for 20 h by the method of Nagamine and Komae (1996).
The preparation was dried in vacuo at room temperature and allowed to dissolve in 20 μL of 1 m NaOH for 60 min. Then, the solution was diluted with 180 μL of distilled water. An aliquot (25 μL) of the preparation was injected into a BioLC (System model DX-500, Dionex, Sunnyvale, CA) equipped with a PAD and a Carbopac PA-1 column (4-mm i.d.× 25 cm). Size fractionation of α-1,4-glucans was performed with a linear gradient of sodium acetate (50–500 mm) in 0.1 m NaOH at a flow rate of 1 mL min−1.
Extraction of Proteins from Mature Seeds
The protein from mature seeds was analyzed for the gene dosage effect of the Ae allele on the level of BEIIb. Twenty milligrams of mature seed were powdered with a pair of pliers and added to 700 μL of sample buffer, which contained 4% (w/v) SDS, 4 m urea, 5% (v/v) 2-mercaptoethanol, and 0.125 m Tris-HCl (pH 6.8). After shaking at room temperature for 12 h, the sample was centrifuged at 10,000g for 20 min at 4°C. An aliquot of the supernatant (10 μL) was taken and subjected to SDS-PAGE.
Western Blotting
SDS-PAGE was performed on a resolving gel (6 × 9 × 0.1 cm3) prepared with 10% (w/v) acrylamide and 0.035% (w/v) bisacrylamide as described by Laemmli (1970). The bands of proteins were blotted onto a polyvinylidenedifluoride membrane (Millipore, Bedford, MA) with a transblotter (Nihon Eido Co., Tokyo) and the membrane was incubated with rabbit antiserum that contained polyclonal antibodies raised against purified BEIIb and BEI from developing rice endosperm (Nakamura et al., 1992). Immunoreactive proteins were detected by incubation with horseradish peroxidase-conjugated antibodies against rabbit IgG (Bio-Rad, Hercules, CA) and finally with 4-chloro-1-naphthol, as described by Towbin et al. (1979).
Preparation of Enzymes Involved in the Metabolism of Starch and Suc
All the following procedures were carried out at 0°C to 4°C, unless otherwise noted.
For analysis of native-PAGE/activity stainings of BE and phosphorylase, an immature rice grain at the late-milking stage that was removed by the hull, pericarp, and embryo was homogenized with a mortar and pestle in 10-fold volume of a solution, which consisted of 50 mm HEPES (N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid)-NaOH (pH 7.4), 2 mm MgCl2, 50 mm 2-mercaptoethanol, and 12.5% (v/v) glycerol. The homogenate was centrifuged twice at 15,000g for 15 min. The supernatant was used as the crude enzyme extract.
For analysis of native PAGE/activity staining of SS, an immature rice grain at the late-milking stage removed from the hull, pericarp, and embryo was homogenized with a mortar and pestle in the same volume of solution, which consisted of 50 mm Tris-HCl (pH 7.5), 2 mm EDTA-2Na, 5 mm dithiothreitol (DTT), 0.4 mm PMSF, and 10% (v/v) glycerol. The homogenate was centrifuged at 15,000g for 15 min. The supernatant was used as the crude enzyme extract. The protein content was measured by the method of Bradford (1976).
For enzyme assay of SS, five immature rice grains at the late-milking stage removed from the hull, pericarp, and embryo were homogenized with a mortar and pestle in 1 mL of the same buffer as described above, and the supernatant was used as the crude enzyme extract.
For assays of the other enzymes, five immature rice grains at the late-milking stage that were removed by the hull, pericarp, and embryo were homogenized with a mortar and pestle in 1 mL of solution, which consisted of 50 mm HEPES-NaOH (pH 7.4), 2 mm MgCl2, 50 mm 2-mercaptoethanol, and 12.5% (v/v) glycerol. The homogenate was centrifuged twice at 15,000g for 15 min. The supernatant was used as the crude enzyme extract.
Native PAGE/Activity Stainings of BE and Phosphorylase
Native PAGE was performed on a slab gel prepared with 5% (w/v; resolving gel) and 3.3% (w/v; stacking gel) acrylamide by a modified version of the method described by Davis (1964). Electrophoresis was carried out at 4°C at a constant current of 20 mA. After electrophoresis, the gel was rinsed with 20 mL of a solution of 50 mm HEPES-NaOH buffer (pH 7.4) and 20% (v/v) glycerol for 5 min on ice. The gel for detection of BE was incubated for 5 to 6 h at 30°C in 20 mL of the reaction mixture, which consisted of 50 mm HEPES-NaOH buffer (pH 7.4), 50 mm Glc-1-P, 2.5 mm AMP, 10% (v/v) glycerol, and rabbit muscle phosphorylase a (about 50 units; Sigma, St.Louis), whereas the gel for detection of phosphorylase was incubated for 5 to 6 h at 30°C in 20 mL of the same reaction mixture except that rabbit muscle phosphorylase a was omitted. The gels were placed in an iodine solution that consisted of 0.1% (w/v) I2 and 1% (w/v) KI.
Native PAGE/Activity Staining of SS
Native PAGE was performed on a slab gel prepared with 7.5% (w/v; resolving gel) containing 0% or 0.8% (w/v) oyster glycogen (Type II; Sigma) and 3.3% (w/v; stacking gel) acrylamide by a modified version of the method described by Davis (1964). Electrophoresis was carried out at 4°C at a constant current of 15 mA. After electrophoresis, the gel was rinsed twice with 35 mL of a solution of 100 mm Bicine-NaOH buffer (pH 7.5), 0.5 mm EDTA, 2 mm DTT, 10% (v/v) glycerol, and either in the presence or absence of 0.5 m citrate-Na buffer (pH 7.5) for 15 min on ice and then it was incubated for 12 h at 30°C in 35 mL of the reaction mixture, which consisted of 100 mm Bicine-NaOH buffer (pH 7.5), 0.5 mm EDTA, 2 mm DTT, 10% (v/v) glycerol, 1 mm ADP-Glc, and either in the presence or absence of 0.5 m citrate-Na buffer (pH 7.5). The gel was placed in an iodine solution that consisted of 0.1% (w/v) I2 and 1% (w/v) KI.
Assays of Enzymatic Activities
Soluble SS
The assay was conducted at 30°C under three different conditions in: (a) 0.5 m citrate-Na (pH 7.5), 50 mm Bicine-NaOH (pH 7.5), 1.7 mm ADP-Glc, 0.7 mg oyster glycogen (Type II, Sigma), 16.7 mm DTT, and the crude enzyme extract in a reaction volume of 300 μL; (b) 0.5 m citrate-Na (pH7.5), 50 mm Bicine-NaOH (pH 7.5), 1.7 mm ADP-Glc, 16.7 mm DTT, and the crude enzyme extract in a reaction volume of 300 μL; or (c) 50 mm Bicine-NaOH (pH 7.5), 1.7 mm ADP-Glc, 0.7 mg oyster glycogen, 16.7 mm DTT, and the crude enzyme extract in a reaction volume of 300 μL. Twenty minutes after the start of the reaction, enzymes were inactivated by placing the mixture in a boiling water bath for 2 min. Then, the mixture was supplemented with 100 μL of a solution of 50 mm HEPES-NaOH (pH 7.4), 10 mm phosphocreatine, 200 mm KCl, 10 mm MgCl2, and 10 μL of 5 mg mL−1 creatine phosphokinase (5 units; Type I, Sigma), and incubated for 30 min at 30°C to convert ADP to ATP. The resulting solution was heated in a boiling water bath for 2 min and then subjected to centrifugation at 15,000g at 4°C for 10 min. The supernatant (300 μL) was mixed with a solution of 125 mm HEPES-NaOH (pH 7.4), 10 mm Glc, 20 mm MgCl2, and 150 μg NADP+. The enzymatic activity was recorded as the increase in A340 after the addition of 1μL each of hexokinase (1.5 units; Roche Diagnostics, Tokyo) and G6P dehydrogenase (0.5 units; Type XV, Sigma).
AGPase
The assay was conducted at 30°C in 100 mm HEPES-NaOH (pH 7.4), 3 mm 3-phosphoglycerate, 1.2 mm ADP-Glc, 3 mm sodium pyrophosphate, 5 mm MgCl2, 4 mm DTT, and the preparation of enzymes in a reaction mixture of 250 μL. Twenty minutes after the start of the reaction, enzymes were inactivated by placing the mixture in a boiling water bath for 2 min. After addition of 350 μL of distilled water, the mixture was subjected to centrifugation at 15,000g at 2°C for 10 min. The supernatant (500 μL) was mixed with 0.15 mg NADP+. The enzymatic activity was recorded as the increase in A340 after the addition of 1 μL each of phosphoglucomutase (0.4 units; Roche Diagnostics) and G6P dehydrogenase (0.5 units; Type XV, Sigma).
Pullulanase
The assay was conducted at 40°C in 50 mm MES (2-N-morpholinoethanesulfonic acid)-NaOH (pH 6.2), 2 mg pullulan, 20 mm CaCl2, and the preparation of enzyme in a reaction mixture of 200 μL. Twenty minutes after the start of the reaction, enzymes were inactivated by placing the mixture in a boiling-water bath for 2 min. Activity was determined as the increase in level of reducing sugars by measuring A520. The method was based on those described by Nelson (1944) and Somogyi (1952).
Suc Synthase
The assay was conducted at 25°C in 50 mm HEPES-NaOH (pH 7.4), 7.5 mm UDP-Glc, 7.5 mm Fru, 15 mm MgCl2, and the preparation of enzyme in a reaction mixture of 140 μL. After 10 min, the reaction was terminated by placing the mixture in a boiling water bath for 2 min and then it was centrifuged at 13,000g at 2°C for 5 min. The supernatant (100 μL) was mixed with a solution of 15 mm HEPES-NaOH (pH 8.0), 0.4 mm phosphoenolpyruvate, 5 mm KCl, 1 mm MgCl2, and 0.1 mm NADH. The enzymatic activity was determined as the increase in A340 after the addition of 5 μL pyruvate kinase (2 units; Roche Diagnostics) and 1 μL lactate dehydrogenase (5.5 units; Roche Diagnostics).
Preparation of Total RNA from Rice Seeds and Northern-Blot Analysis
The following stages in grain development were used in northern-blot analysis: I, maximum grain length (fresh weight of grain: Kinmaze, 4.3 mg; EM10, 5.2 mg); II, about two-thirds grain width (Kinmaze, 9.6 mg; EM10, 9.5 mg); III, maximum grain width (Kinmaze, 14.5 mg; EM10, 14.0 mg); IV, and maximum grain thickness (Kinmaze, 20.2 mg; EM10, 17.5 mg).
Total RNA from rice developing seeds was prepared as follows (Chang et al., 1993). Rice seeds were ground with a mortar and pestle in liquid nitrogen, and incubated at 65°C for 10 min in 7 mL of 2× cetyltrimethylammonium bromide (2% [w/v] cetyltrimethylammonium bromide, 0.1 m Tris [pH 9.5], 20 mm EDTA, 1.4 m NaCl, and 1% [w/v] β-mercaptoethanol) with vigorous shaking. The mixture was extracted two times with 7 mL of chloroform. Adding 0.25 volumes of 10 m LiCl to the aqueous phase and storing on ice for 3 h, the total RNA was selectively precipitated. After centrifugation, the total RNA pellet was resuspended in 500 μL Tris-EDTA buffer (pH 8.0), and extracted with an equal volume of chloroform. The total RNA was precipitated by adding 0.1 volumes of 3 m Na-acetate (pH 5.2) and 2 volumes of ethanol to the aqueous phase, then chilled at −70°C for 30 min, and collected by centrifugation and dissolved in water.
Twenty micrograms of total RNA was electrophoresed on a denaturing 1.2% (w/v) agarose gel containing 0.66 m formaldehyde and then transferred to a NYTRAN nylon membrane (Schleicher and Schuell, Keene, NH). The membrane was hybridized with a digoxygenin-labeled RNA probe and washed as recommended by the manufacturer (Roche Diagnostics).
RNA Probe
A digoxygenin-labeled RNA probe was prepared according to the manufacturer (Roche Diagnostics). DNA fragment for the preparation of BEI, BEIIa, BEIIb, SSI, and SSIIIa gene-specific probes were amplified by PCR. The DNA fragments were amplified from a cDNA (expressed sequence tags) of rice cv Nipponbare using pairs of one forward primer (f), including EcoRI site at the 5′ end, and one reverse primer (r), including KpnI site at the 5′ end. Genes, primers used, and amplified DNA fragment size were in the following: BEI gene, primer sbe1-f 5′-TACGAATTCCCCGAGGGAATGCCAGGAGTA-3′ and primer sbe1-r 5′-TACGGTACCAACTATACATAAAGTTCATAT-3′(441 bp); BEIIa gene, sbe2a-f 5′-TACGAATTCACTTAC-AGAGGACTAATGATC-3′ and sbe2a-r 5′-TACGGTACC-CTGTCGAACAGATTATTCATA-3′ (359 bp); BEIIb gene, sbe2b-f 5′-TACGAATTCTCCAGCGGAATGAGAACA-CCA-3′and sbe2b-r 5′-TACGGTACCCAAGATGTACAGA-AGTGCAGA-3′ (331 bp); SSI gene, ss1-f 5′-TACGAATT-CTTCATGGATCAACCATATGTC-3′ and ss1-r 5′-TACG-GTACCGTCTTCACCTTAAGACTCAAC-3′ (457 bp); and SSIII gene, ss3-f 5′-TACGAATTCAAGACCGGGCTCG-AGTTCTAG-3′ and ss3-r 5′-TACGGTACCACCTT-CATT-TTACTTGCAATT-3′(529 bp). After digesting with EcoRI and KpnI, the fragments were cloned into pBluescript II SK+. The resulting plasmid was used for the in vitro production of digoxygenin-labeled specific RNA probe with T7 RNA polymerase after linearization with EcoRI.
ACKNOWLEDGMENTS
The authors thank Dr. Akiko Kubo and Ms. Kazuko Kimura (National Institute of Agrobiological Resources) for growing the plant materials, and also thank Dr. Naoko Fujita (Akita Prefectural University) for technical advice for measurement of SS activity.
Footnotes
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010127.
LITERATURE CITED
- Abel GJW, Springer F, Willmitzer L, Kossmann J. Cloning and functional analysis of a cDNA encoding a novel 139 kDa starch synthase from potato (Solanum tuberosum L.) Plant J. 1996;10:981–991. doi: 10.1046/j.1365-313x.1996.10060981.x. [DOI] [PubMed] [Google Scholar]
- Adkins GK, Greenwood CT, Hourston DJ. Studies on starches of high amylose content: XI. Some physicochemical properties of dispersions of amylomaize starch, and observations on the nature of high-amylose starches. Cereal Chem. 1970;47:13–18. [Google Scholar]
- Baba T, Arai Y. Structural characterization of amylopectin and intermediate material in amylomaize starch granules. Agric Biol Chem. 1984;48:1763–1775. [Google Scholar]
- Baba T, Kim S-J, Arai Y. Treatment of amylomaize starch granules with urea: comparison with normal maize starch. Carbohydr Res. 1983;124:344–348. [Google Scholar]
- Banks W, Greenwood CT, Muir DD. Studies on starches of high amylose content: part 17. A review of current concepts. Starch. 1974;26:289–300. [Google Scholar]
- Bhattacharyya MK, Smith AM, Ellis THN, Hedley C, Martin C. The wrinkled-seed character of pea described by Mendel is caused by a transposon-like insertion in a gene encoding starch-branching enzyme. Cell. 1990;60:115–122. doi: 10.1016/0092-8674(90)90721-p. [DOI] [PubMed] [Google Scholar]
- Bogracheva TY, Davydova NI, Genin YV, Hedley CL. Mutant genes at the r and rb loci affect the structure and physico-chemical properties of pea seed starches. J Exp Bot. 1995;46:1905–1913. [Google Scholar]
- Borovsky D, Smith EE, Whelan WJ. On the mechanism of amylose branching by potato Q-enzyme. Eur J Biochem. 1976;62:307–312. doi: 10.1111/j.1432-1033.1976.tb10162.x. [DOI] [PubMed] [Google Scholar]
- Boyer CD, Preiss J. Multiple forms of (1→4)-α-D-glucan, (1→4)-α-D-glucan-6-glycosyl transferase from developing Zea mays L. kernels. Carbohydr Res. 1978a;61:321–334. [Google Scholar]
- Boyer CD, Preiss J. Multiple forms of starch branching enzyme of maize: evidence for independent genetic control. Biochem Biophys Res Commun. 1978b;80:169–175. doi: 10.1016/0006-291x(78)91119-1. [DOI] [PubMed] [Google Scholar]
- Boyer CD, Preiss J. Evidence for independent genetic control of the multiple forms of maize endosperm branching enzymes and starch synthases. Plant Physiol. 1981;67:1141–1145. doi: 10.1104/pp.67.6.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burton RA, Bewley JD, Smith AM, Bhattacharyya MK, Tatge H, Ring S, Bull V, Hamilton WDO, Martin C. Starch branching enzymes belonging to distinct enzyme families are differentially expressed during pea embryo development. Plant J. 1995;7:3–15. doi: 10.1046/j.1365-313x.1995.07010003.x. [DOI] [PubMed] [Google Scholar]
- Chang S, Puryear J, Cairney J. A simple and efficient method for isolation RNA from pine trees. Plant Mol Biol Rep. 1993;11:113–116. [Google Scholar]
- Cooke D, Gidley MJ. Loss of crystalline and molecular order during starch gelatinisation: origin of the enthalpic transition. Carbohydr Res. 1992;227:103–112. [Google Scholar]
- Davis BJ. Disc electrophoresis II: method and application of human serum proteins. Ann NY Acad Sci. 1964;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Fisher MB, Boyer CD. Immunological characterization of maize starch branching enzymes. Plant Physiol. 1983;72:813–816. doi: 10.1104/pp.72.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DK, Gao M, Kim K-N, Boyer CD, Guiltinan MJ. Allelic analysis of the maize amylose-extender locus suggests that independent genes encode starch-branching enzymes IIa and IIb. Plant Physiol. 1996;110:611–619. doi: 10.1104/pp.110.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Fisher DK, Kim K-N, Shannon JC, Guiltinan MJ. Independent genetic control of maize starch-branching enzymes IIa and IIb: isolation and characterization of Sbe2a cDNA. Plant Physiol. 1997;114:69–78. doi: 10.1104/pp.114.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidley MJ, Bulpin PV. Crystallisation of malto-oligosaccharides as models of the crystalline forms of starch: minimum chain-length requirement for the formation of double helices. Carbohydr Res. 1987;161:291–300. [Google Scholar]
- Gidley MJ, Cooke D, Darke AH, Hoffmann RA, Russell AL, Greenwell P. Molecular order and structure in enzyme-resistant retrograded starch. Carbohydr Polym. 1995;28:23–31. [Google Scholar]
- Guan H, Li P, Imparl-Radosevich J, Preiss J, Keeling P. Comparing the properties of Escherichia coli branching enzyme and maize branching enzyme. Arch Biochem Biophys. 1997;342:92–98. doi: 10.1006/abbi.1997.0115. [DOI] [PubMed] [Google Scholar]
- Guan HP, Preiss J. Differentiation of the properties of the branching isozymes from maize (Zea mays) Plant Physiol. 1993;102:1269–1273. doi: 10.1104/pp.102.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanashiro I, Abe J, Hizukuri S. A periodic distribution of the chain length of amylopectin as revealed by high-performance anion-exchange chromatography. Carbohydr Res. 1996;283:151–159. [Google Scholar]
- Hilbert GE, MacMasters MM. Pea starch, a starch of high amylose content. J Biol Chem. 1946;162:229–238. [PubMed] [Google Scholar]
- Hizukuri S. Polymodal distribution of the chain lengths of amylopectins, and its significance. Carbohydr Res. 1986;147:342–347. [Google Scholar]
- Jane J, Chen YY, Lee LF, McPherson AE, Wong KS, Radosavljevic M, Kasemsuwan T. Effects of amylopectin branch chain length and amylose content on the gelatinization and pasting properties of starch. Cereal Chem. 1999;76:629–637. [Google Scholar]
- Juliano BO. A simplified assay for milled-rice amylose. Cereal Sci Today. 1971;16:334–340. [Google Scholar]
- Kalichevsky MT, Orford PD, Ring SG. The retrogradation and gelation of amylopectins from various botanical sources. Carbohydr Res. 1990;198:49–55. [Google Scholar]
- Kasemsuwan T, Jane J, Schnable P, Stinard P, Robertson D. Characterization of the dominant mutant Amylose-extender (Ae1–5180) maize starch. Cereal Chem. 1995;72:457–464. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larsson C-T, Hofvander P, Khoshnoodi J, Ek B, Rask L, Larsson H. Three isoforms of starch synthase and two isoforms of branching enzyme are present in potato tuber starch. Plant Sci. 1996;117:9–16. [Google Scholar]
- Larsson C-T, Khoshnoodi J, Ek B, Rask L, Larsson H. Molecular cloning and characterization of starch-branching enzyme II from potato. Plant Mol Biol. 1998;37:505–511. doi: 10.1023/a:1005908305456. [DOI] [PubMed] [Google Scholar]
- Lu S, Chen L-N, Lii C-Y. Correlations between the fine structure, physicochemical properties, and retrogradation of amylopectins from Taiwan rice varieties. Cereal Chem. 1997;74:34–39. [Google Scholar]
- Martin C, Smith AM. Starch biosynthesis. Plant Cell. 1995;7:971–985. doi: 10.1105/tpc.7.7.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K, Kawasaki T, Shimada H, Satoh H, Kobayashi E, Okumura S, Arai Y, Baba T. Alteration of the structural properties of starch components by the lack of an isoform of starch branching enzyme in rice seeds. J Biol Chem. 1993;268:19084–19091. [PubMed] [Google Scholar]
- Mizuno K, Kimura K, Arai Y, Kawasaki T, Shimada H, Baba T. Starch branching enzymes from immature rice seeds. J Biochem. 1992;112:643–651. doi: 10.1093/oxfordjournals.jbchem.a123953. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Kobayashi E, Tachibana M, Kawasaki T, Fujimura T, Funae K, Kobayashi M, Baba T. Characterization of an isoform of rice starch branching enzyme, RBE4, in developing seeds. Plant Cell Physiol. 2001;42:349–357. doi: 10.1093/pcp/pce042. [DOI] [PubMed] [Google Scholar]
- Moates GK, Noel TR, Parker R, Ring SG. The effect of chain length and solvent interactions on the dissolution of the B-type crystalline polymorph of amylose in water. Carbohydr Res. 1997;298:327–333. [Google Scholar]
- Morell MK, Blennow A, Kosar-Hashemi B, Samuel MS. Differential expression and properties of starch branching enzyme isoforms in developing wheat endosperm. Plant Physiol. 1997;113:201–208. doi: 10.1104/pp.113.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamine T, Komae K. Improvement of a method for chain-length distribution analysis of wheat amylopectin. J Chromatogr. 1996;732:255–259. [Google Scholar]
- Nakamura Y, Takeichi T, Kawaguchi K, Yamanouchi H. Purification of two forms of starch branching enzyme (Q-enzyme) from developing rice endosperm. Physiol Plant. 1992;84:329–335. [Google Scholar]
- Nelson N. A photometric adaptation of Somogyi method for the determination of glucose. J Biol Chem. 1944;153:375–380. [Google Scholar]
- Peat S, Whelan WJ, Thomas GJ (1952) Evidence of multiple branching in waxy maize starch. J Chem Soc 4546–4548
- Preiss J, Levi C. Starch biosynthesis and degradation. In: Preiss J, editor. Carbohydrates: Structure and Function, The Biochemistry of Plants. Vol. 3. New York: Academic Press; 1980. pp. 371–423. [Google Scholar]
- Safford R, Jobling SA, Sidebottom CM, Westcott RJ, Cooke D, Tober KJ, Strongitharm BH, Russell AL, Gidley MJ. Consequences of antisense RNA inhibition of starch branching enzyme activity on properties of potato starch. Carbohydr Polym. 1998;35:155–168. [Google Scholar]
- Sanders EB, Thompson DB, Boyer CD. Thermal behavior during gelatinization and amylopectin fine structure for selected maize genotypes as expressed in four inbred lines. Cereal Chem. 1990;67:594–602. [Google Scholar]
- Satoh H, Omura T. Induction of mutation by the treatment of fertilized egg cell with N-methyl-N-nitrosourea in rice. J Fac Agr Kyushu Univ. 1979;24:165–174. [Google Scholar]
- Shi Y-C, Seib PA. The structure of four waxy starches related to gelatinization and retrogradation. Carbohydr Res. 1992;227:131–145. [Google Scholar]
- Somogyi M. Notes on sugar determination. J Biol Chem. 1952;195:19–23. [PubMed] [Google Scholar]
- Stinard PS, Robertson DS, Schnable PS. Genetic isolation, cloning, and analysis of a Mutator-induced, dominant antimorph of the maize amylose extender1 locus. Plant Cell. 1993;5:1555–1566. doi: 10.1105/tpc.5.11.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Sathish P, Ahlandsberg S, Deiber A, Jansson C. Identification of four starch-branching enzymes in barley endosperm: partial purification of forms I, IIa, and IIb. New Phytol. 1997;137:215–222. doi: 10.1046/j.1469-8137.1997.00796.x. [DOI] [PubMed] [Google Scholar]
- Sun C, Sathish P, Ahlandsberg S, Jansson C. The two genes encoding starch-branching enzymes IIa and IIb are differentially expressed in barley. Plant Physiol. 1998;118:37–49. doi: 10.1104/pp.118.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y, Guan H-P, Preiss J. Branching of amylose by the branching isoenzymes of maize endosperm. Carbohydr Res. 1993;240:253–263. [Google Scholar]
- Takeda Y, Hizukuri S, Juliano BO. Structure of rice amylopectins with low and high affinity for iodine. Carbohydr Res. 1987;168:79–88. [Google Scholar]
- Tester RF, Morrison WR. Swelling and gelatinization of cereal starches: II. Waxy rice starches. Cereal Chem. 1990;67:558–563. [Google Scholar]
- Tomlinson KL, Lloyd JR, Smith AM. Importance of isoforms of starch-branching enzyme in determining the structure of starch in pea leaves. Plant J. 1997;11:31–43. [Google Scholar]
- Towbin H, Staehelin T, Gorden J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanouchi H, Nakamura Y. Organ specificity of isoforms of starch branching enzyme (Q-enzyme) in rice. Plant Cell Physiol. 1992;33:985–991. [Google Scholar]
- Yano M, Okuno K, Kawakami J, Satoh H, Omura T. High amylose mutants of rice, Oryza sativa L. Theor Appl Genet. 1985;69:253–257. doi: 10.1007/BF00662436. [DOI] [PubMed] [Google Scholar]
- Yuan RC, Thompson DB, Boyer CD. Fine structure of amylopectin in relation to gelatinization and retrogradation behavior of maize starches from three wx-containing genotypes in two inbred lines. Cereal Chem. 1993;70:81–89. [Google Scholar]