Abstract
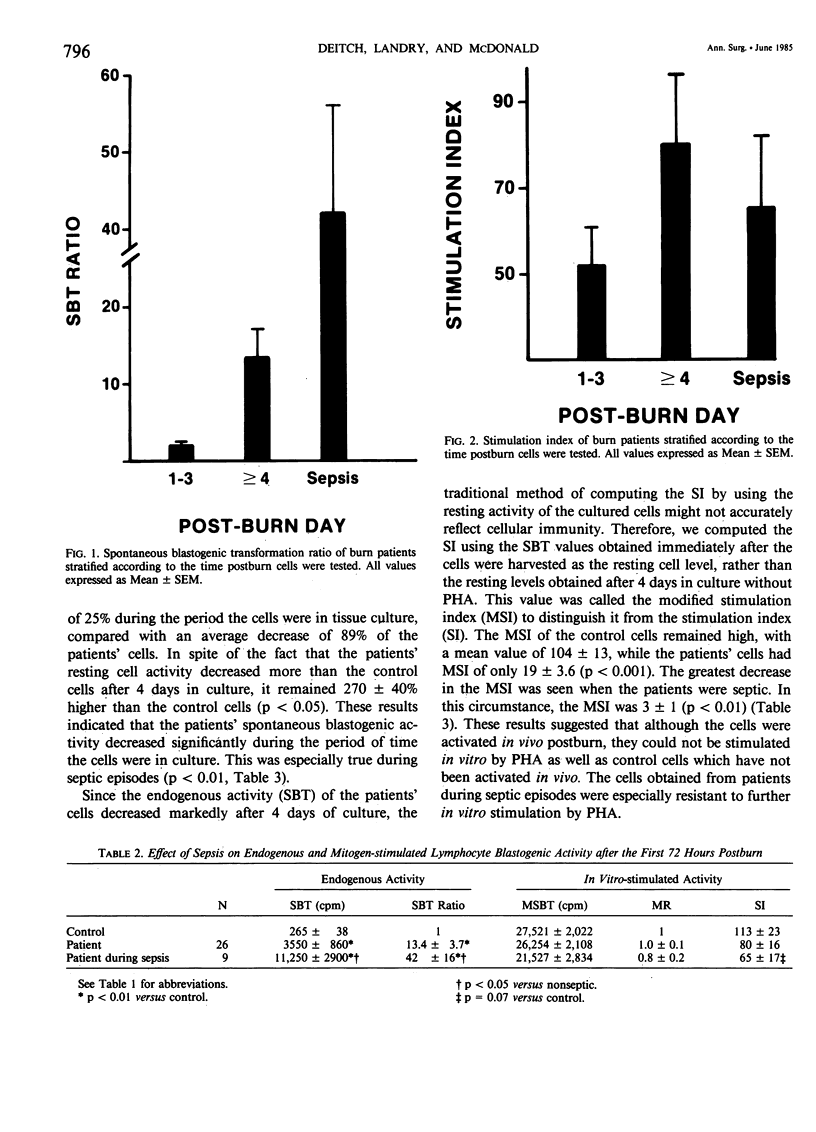
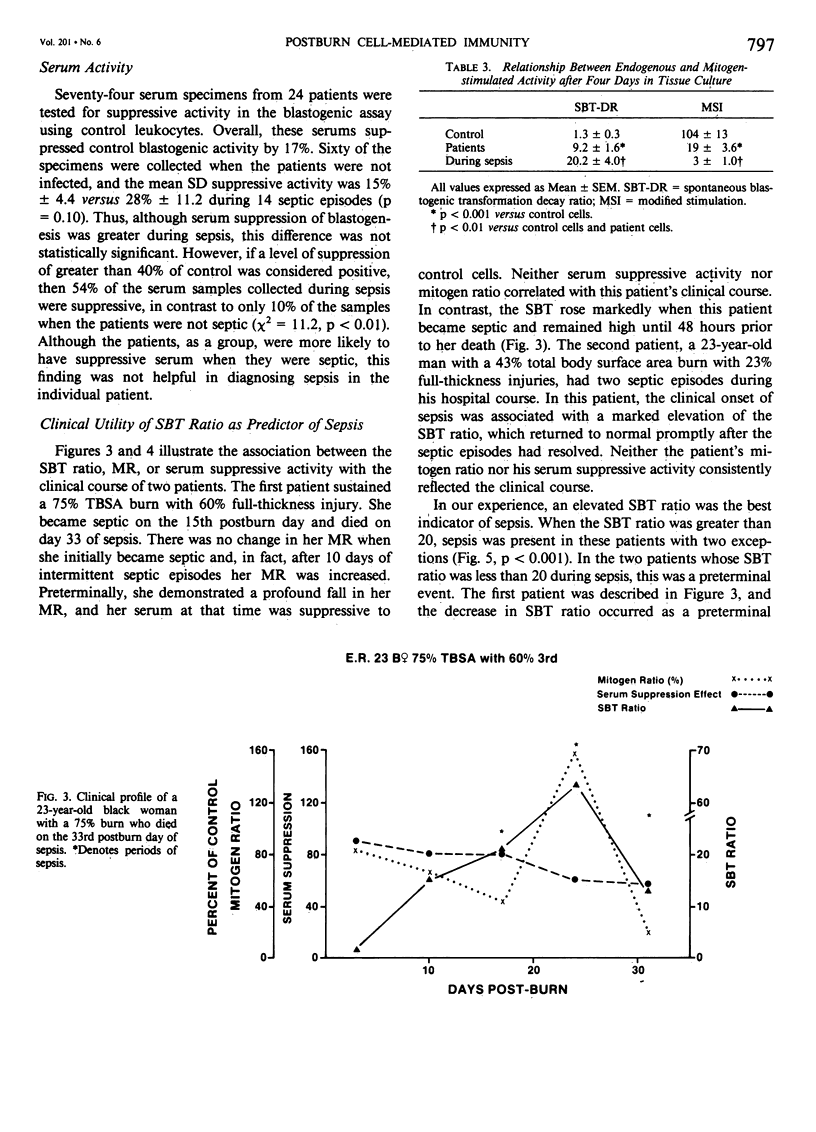
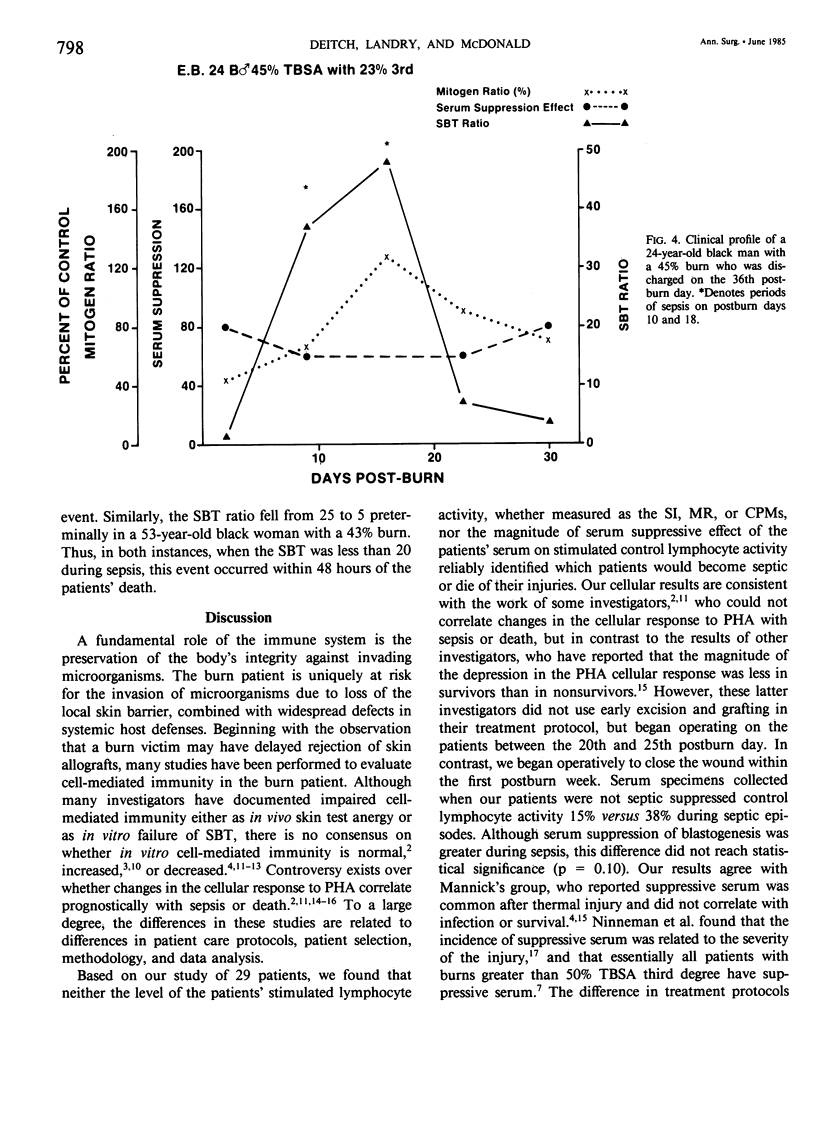
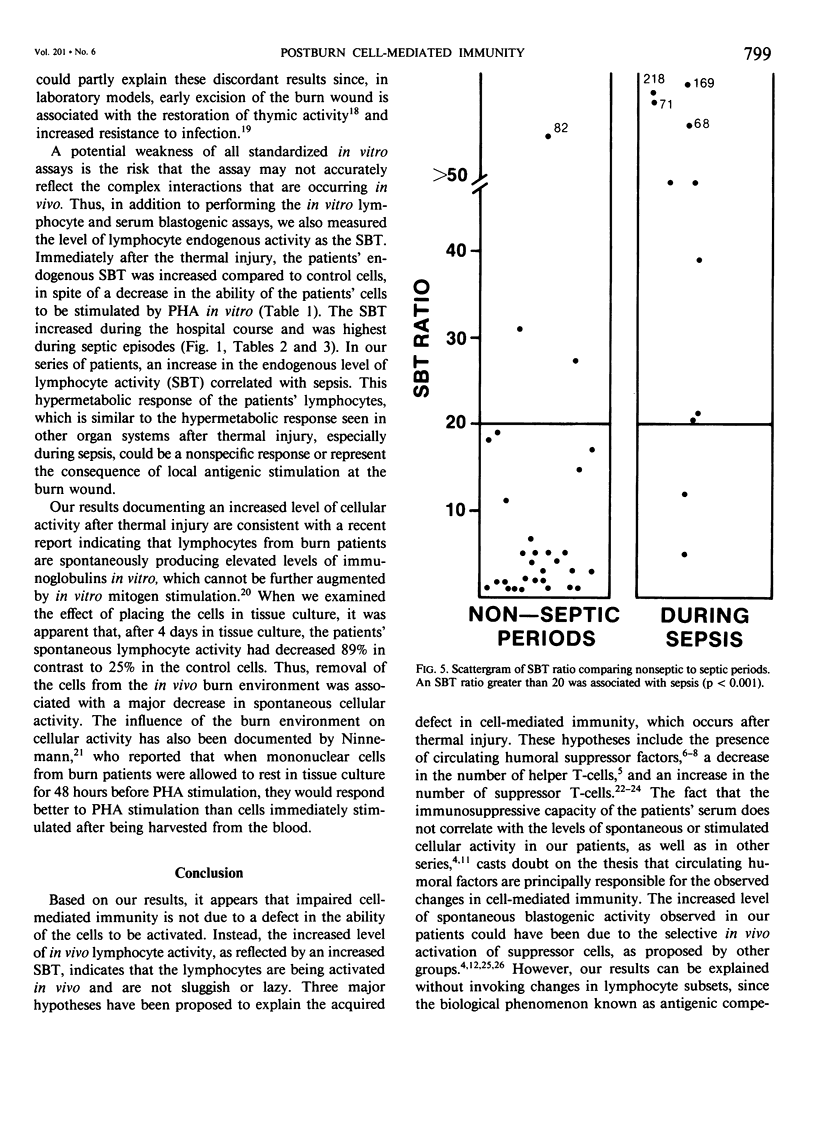
After major trauma, including burns, patients develop a multitude of immunologic alterations, including impaired cellular immunity (CMI). Because of conflicting reports on the relationship of in vitro lymphocyte activity to the clinical course of burn patients, we studied CMI in 29 patients with a mean burn size of 41% and a mean age of 32 years. The patients' cellular response to the mitogen phytohemagglutinin and the ability of the patients' serum to suppress a normal lymphocyte mitogenic response were measured. The endogenous level of lymphocyte activity spontaneous blastogenic transformation (SBT) was measured immediately after the cells were harvested from the blood. During the first 72 hours postburn, the ability of the patients' cells to respond to mitogens in vitro decreased, while the endogenous activity (SBT) increased. Subsequent changes in the SBT, but not the mitogen-stimulated response, predicted sepsis. Although the patients' serum was mildly suppressive, these changes were not of statistical or clinical significance. It is postulated that the in vivo and in vitro CMI defects are not primarily due to a defect in the ability of the cell to be activated, but instead are due to exhaustion, desensitization, or down-regulation of these in vivo-activated cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonacci A. C., Reaves L. E., Calvano S. E., Amand R., De Riesthal H. F., Shires G. T. Flow cytometric analysis of lymphocyte subpopulations after thermal injury in human beings. Surg Gynecol Obstet. 1984 Jul;159(1):1–8. [PubMed] [Google Scholar]
- Baker C. C., Miller C. L., Trunkey D. D., Lim R. C., Jr Identity of mononuclear cells which compromise the resistance of trauma patients. J Surg Res. 1979 May;26(5):478–487. doi: 10.1016/0022-4804(79)90037-4. [DOI] [PubMed] [Google Scholar]
- Baker C. C., Miller C. L., Trunkey D. D. Predicting fatal sepsis in burn patients. J Trauma. 1979 Sep;19(9):641–648. doi: 10.1097/00005373-197909000-00001. [DOI] [PubMed] [Google Scholar]
- Constantian M. B. Association of sepsis with an immunosuppressive polypeptide in the serum of burn patients. Ann Surg. 1978 Aug;188(2):209–215. doi: 10.1097/00000658-197808000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantian M. B. Impaired reactivity of burn patient lymphocytes to phytohemagglutinin in autologous serum: failure to improve responsiveness by washing in vitro. J Surg Res. 1979 Aug;27(2):84–92. doi: 10.1016/0022-4804(79)90114-8. [DOI] [PubMed] [Google Scholar]
- Deitch E. A., Gelder F., McDonald J. C. Sequential prospective analysis of the nonspecific host defense system after thermal injury. Arch Surg. 1984 Jan;119(1):83–89. doi: 10.1001/archsurg.1984.01390130065012. [DOI] [PubMed] [Google Scholar]
- Echinard C. E., Sajdel-Sulkowska E., Burke P. A., Burke J. F. The beneficial effect of early excision on clinical response and thymic activity after burn injury. J Trauma. 1982 Jul;22(7):560–565. doi: 10.1097/00005373-198207000-00006. [DOI] [PubMed] [Google Scholar]
- Keane R. M., Munster A. M., Birmingham W., Shatney C., Winchurch R. A. Suppressor cell activity after major injury: indirect and direct functional assays. J Trauma. 1982 Sep;22(9):770–773. doi: 10.1097/00005373-198209000-00009. [DOI] [PubMed] [Google Scholar]
- Levine N. S., Salisbury R. E., Mason A. D., Jr The effect of early surgical excision and homografting on survival of burned rats and of intraperitoneally-infected burned rats. Plast Reconstr Surg. 1975 Oct;56(4):423–429. doi: 10.1097/00006534-197510000-00009. [DOI] [PubMed] [Google Scholar]
- Mahler D., Batchelor J. R. Phytohaemagglutinin transformation of lymphocytes in burned patients. Transplantation. 1971 Nov;12(5):409–411. doi: 10.1097/00007890-197111000-00015. [DOI] [PubMed] [Google Scholar]
- McDonald J. C., Lee J. H. "Spontaneous" blastogenic transformation. A monitor of immunosuppression. Arch Surg. 1974 Aug;109(2):320–325. doi: 10.1001/archsurg.1974.01360020180035. [DOI] [PubMed] [Google Scholar]
- McIrvine A. J., O'Mahony J. B., Saporoschetz I., Mannick J. A. Depressed immune response in burn patients: use of monoclonal antibodies and functional assays to define the role of suppressor cells. Ann Surg. 1982 Sep;196(3):297–304. doi: 10.1097/00000658-198209000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. L., Baker C. C. Changes in lymphocyte activity after thermal injury. The role of suppressor cells. J Clin Invest. 1979 Feb;63(2):202–210. doi: 10.1172/JCI109290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster A. M. Post-traumatic immunosuppression is due to activation of suppressor T cells. Lancet. 1976 Jun 19;1(7973):1329–1330. doi: 10.1016/s0140-6736(76)92658-1. [DOI] [PubMed] [Google Scholar]
- Munster A. M., Winchurch R. A., Birmingham W. J., Keeling P. Longitudinal assay of lymphocyte responsiveness in patients with major burns. Ann Surg. 1980 Dec;192(6):772–775. doi: 10.1097/00000658-198012000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninnemann J. L., Condie J. T., Davis S. E., Crockett R. A. Isolation immunosuppressive serum components following thermal injury. J Trauma. 1982 Oct;22(10):837–844. doi: 10.1097/00005373-198210000-00006. [DOI] [PubMed] [Google Scholar]
- Ninnemann J. L., Fisher J. C., Wachtel T. L. Thermal injury-associated immunosuppression: occurrence and in vitro blocking effect of post recovery serum. J Immunol. 1979 May;122(5):1736–1741. [PubMed] [Google Scholar]
- Ninnemann J. L. Immunosuppression following thermal injury through B cell activation of suppressor T cells. J Trauma. 1980 Mar;20(3):206–213. doi: 10.1097/00005373-198003000-00003. [DOI] [PubMed] [Google Scholar]
- Ninnemann J. L., Stockland A. E. Participation of prostaglandin E in immunosuppression following thermal injury. J Trauma. 1984 Mar;24(3):201–207. doi: 10.1097/00005373-198403000-00003. [DOI] [PubMed] [Google Scholar]
- Shorr R. M., Ershler W. B., Gamelli R. L. Immunoglobulin production in burned patients. J Trauma. 1984 Apr;24(4):319–322. doi: 10.1097/00005373-198404000-00006. [DOI] [PubMed] [Google Scholar]
- Sukhtankar A. Y., Sengupta S. R. Cellular immunity in burns. Burns Incl Therm Inj. 1982 Jan;8(3):168–171. doi: 10.1016/0305-4179(82)90082-1. [DOI] [PubMed] [Google Scholar]
- Wolfe J. H., Saporoschetz I., Young A. E., O'Connor N. E., Mannick J. A. Suppressive serum, suppressor lymphocytes, and death from burns. Ann Surg. 1981 Apr;193(4):513–520. [PMC free article] [PubMed] [Google Scholar]
- Wood J. J., Rodrick M. L., O'Mahony J. B., Palder S. B., Saporoschetz I., D'Eon P., Mannick J. A. Inadequate interleukin 2 production. A fundamental immunological deficiency in patients with major burns. Ann Surg. 1984 Sep;200(3):311–320. doi: 10.1097/00000658-198409000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]


