Abstract
Inward-rectifying potassium (K+in) channels in guard cells have been suggested to provide a pathway for K+ uptake into guard cells during stomatal opening. To test the proposed role of guard cell K+in channels in light-induced stomatal opening, transgenic Arabidopsis plants were generated that expressed dominant negative point mutations in the K+in channel subunit KAT1. Patch-clamp analyses with transgenic guard cells from independent lines showed that K+in current magnitudes were reduced by approximately 75% compared with vector-transformed controls at −180 mV, which resulted in reduction in light-induced stomatal opening by 38% to 45% compared with vector-transformed controls. Analyses of intracellular K+ content using both sodium hexanitrocobaltate (III) and elemental x-ray microanalyses showed that light-induced K+ uptake was also significantly reduced in guard cells of K+in channel depressor lines. These findings support the model that K+in channels contribute to K+ uptake during light-induced stomatal opening. Furthermore, transpirational water loss from leaves was reduced in the K+in channel depressor lines. Comparisons of guard cell K+in current magnitudes among four different transgenic lines with different K+in current magnitudes show the range of activities of K+in channels required for guard cell K+ uptake during light-induced stomatal opening.
Inward-rectifying K+ (K+in) channels in the plasma membrane of plant cells have been suggested to play important roles for diverse cellular functions. K+in channels have been proposed to function in K+ uptake and membrane potential regulation in roots (Findlay et al., 1994; Gassmann and Schroeder, 1994; Maathuis and Sanders, 1995; Roberts and Tester, 1995; Hirsch et al., 1998), coleoptiles (Thiel et al., 1996; Philippar et al., 1999), during leaf movements (Moran and Satter, 1989), in vascular tissues (Wegner and Raschke, 1994), and during stomatal movements (Schroeder et al., 1984, 1987; for review, see Schroeder et al., 1994; MacRobbie, 1998; Czempinski et al., 1999). Analyses of insertional disruption mutants in the Arabidopsis K+in channel genes AKT1 (Sentenac et al., 1992) and SKOR1 provide molecular evidence for roles of K+ channels in K+ uptake in roots (Hirsch et al., 1998) and in K+ release into the xylem sap (Gaymard et al., 1998). However, molecular physiological analyses of other plant K+ channels are important for assessing various proposed K+in channel functions.
The Arabidopsis K+in channel cDNA, KAT1, was isolated by complementation of a yeast (Saccharomyces cerevisiae) mutant defective in K+ uptake (Anderson et al., 1992). KAT1 and the potato (Solanum tuberosum) orthologue KST1 have been shown to be expressed predominantly in guard cells (Müller-Röber et al., 1995; Nakamura et al., 1995). Heterologous expression of KAT1 cRNA in Xenopus laevis oocytes resulted in ion currents that displayed properties typical of plant K+in currents (Schachtman et al., 1992).
Classical studies showed that light-induced stomatal opening is mediated by K+ and anion accumulation in guard cells (Imamura, 1943; Humble and Raschke, 1971). Biophysical and physiological studies have suggested that K+in channels provide a pathway for K+ uptake into guard cells during stomatal opening (Schroeder et al., 1987; for review, see Schroeder et al., 1994; Czempinski et al., 1999). Transgenic expression of KAT1 in tobacco (Nicotiana tabacum) mesophyll cells resulted in plasma membrane K+in channel activity (Bei and Luan, 1998). Furthermore, transgenic Arabidopsis that express KAT1 mutants with a reduced sensitivity to Cs+ block exhibit partial light-induced stomatal opening in the presence of Cs+ concentrations that ordinarily inhibit stomatal opening in wild type (Ichida et al., 1997). These studies provide molecular evidence that guard cell transgenic K+in channels constitute a mechanism for light-induced stomatal opening in the presence of Cs+ and that KAT1 functions as a plasma membrane K+in channel in planta (Ichida et al., 1997; Bei and Luan, 1998).
However, physiological assays in previous studies required the use of pharmacological blockers, which can be problematic. For example, 10 mm Ba2+ blocks 90% of K+in channel currents in fava bean (Vicia faba) guard cells (Schroeder et al., 1987), and reduced the rate of stomatal opening (Kelly et al., 1995). However, Ba2+ also blocks outward-rectifying K+ channels (Schroeder et al., 1987). Furthermore, several genetic loci in Arabidopsis affect Cs+ sensitivity (Sheahan et al., 1993). Moreover, electrophysiological studies on guard cell K+ channels have not been accompanied by measurements of K+ contents in guard cells to verify the proposed functions of second messenger K+in channel regulators for K+ uptake.
Patch clamp studies on Arabidopsis guard cells showed that K+in channel properties differ from KAT1 expressed in X. laevis oocytes, suggesting that additional subunits or regulators contribute to K+in channel function in vivo (Ichida et al., 1997; Dietrich et al., 1998). Together with KAT1, an Arabidopsis K+ channel β-subunit homolog (Tang et al., 1996) and other K+in channel α subunits may constitute heteromultimeric K+in channels in guard cells. Based on physiological functions of K+in channels in guard cells and because there are seven KAT/AKT homologs in the Arabidopsis genome (Mäser et al., 2001), some of which are expressed in guard cells, redundancy appears to exist for K+in channel α subunits (J. Kwak and J.I. Schroeder, unpublished data). Therefore, an approach using a dominant negative K+in channel mutant was used to address the physiologically relevant question of whether K+in channels contribute to K+ uptake during light-induced stomatal opening.
In the present study, we addressed the following questions: Can dominant negative K+in channel mutants depress K+in channel activity in vivo? Does reduction in K+in channel activity in guard cells, without the use of pharmacological blockers, affect light-induced stomatal opening, K+ uptake and transpiration from leaves? What is the quantitative level of K+in channel depression required for physiological effects on light-induced stomatal opening to be manifested?
To directly address these questions, we have generated transgenic Arabidopsis plants expressing a dominant negative KAT1 mutant containing a point mutation in the pore region of KAT1. A previous study has shown that two-point mutations in the pore region of KAT1 (T256R and G262K) function as dominant negative KAT1 mutations in X. laevis oocytes (Baizabal-Aguirre et al., 1999). Furthermore, these dominant negative KAT1 channel subunits were also capable of repressing AKT2 currents, indicating that they can form heteromultimeric channels with K+ channel subunits in vivo (Baizabal-Aguirre et al., 1999). A recent study has suggested that plant K+in channels do not indiscriminately form heteromultimers, suggesting that non-physiological multimers are less likely to form in vivo with dominant negative KAT1 mutants (Urbach et al., 2000). Here, we show that expression of a dominant negative KAT1 mutant in Arabidopsis reduces K+in currents in guard cell protoplasts, stomatal opening, K+ uptake, and transpirational water loss from leaves.
RESULTS
Overexpression of Dominant Negative KAT1 Mutant Inhibits Light-Induced Stomatal Opening
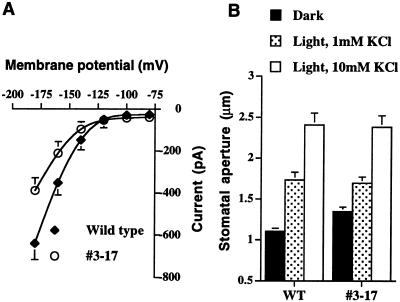
To investigate the suggested role of K+in channels in stomatal opening in vivo, we initially generated transgenic Arabidopsis plants that express the kat1-T256R mutant under control of a single cauliflower mosaic virus (CaMV) 35S promoter. Transgenic guard cells from three independent lines showed similar reduction in K+in currents. As shown in Figure 1A, the magnitude of K+in currents was reduced in guard cells of transgenic line number 3-17 by 39% (n = 8) at −180 mV compared with wild-type controls (n = 8). Northern-blot analyses confirmed the expression of the kat1-T256R transgene (data not shown). However, light-induced stomatal opening was not affected in transgenic guard cells when epidermal peels were incubated for 2 h in white light (Fig. 1B), suggesting that the reduction in K+in currents in these transgenic guard cells did not affect light-induced stomatal opening (P > 0.99, 10 mm KCl in the stomatal opening solution; Fig. 1B, white bars). Moreover, when extracellular K+ was reduced to 1 mm, K+in channel depression also did not affect light-induced stomatal opening (Fig. 1B, dotted bars, P > 0.95).
Figure 1.
Light-induced stomatal opening and reduced K+in channel current magnitudes in transgenic line number 3-17 in which dominant negative KAT1 mutant is expressed under the control of a single CaMV 35S promoter. A, Current voltage curves plotted from whole-cell recordings of guard cells show reduction in inward K+ currents in transgenic guard cells (n = 8, line no. 3-17) compared with wild-type controls (n = 8). Membrane potentials were stepped from a holding potential of −40 to −180 mV in −20-mV increments. K+in channel currents are shown from −80 to −180 mV. Data are mean ± se B, Solid bars indicate stomatal apertures before light exposure. Dotted bars and white bars show stomatal apertures after 2 h of light exposure in 1 mm KCl or 10 mm KCl, respectively. n = 20 stomata for each condition. Error bars show se in all figures.
Because the single 35S promoter mediates moderate gene expression levels in Arabidopsis guard cells (Ichida et al., 1997; Allen et al., 1999), we subsequently used a plant expression vector containing a tandem repeat of the CaMV 35S promoter to increase the level of expression of the dominant negative KAT1 mutant, kat1-T256R. Light-induced stomatal opening was examined following transformation of all lines. Preliminary stomatal aperture measurements on the 19 transgenic lines (two plants analyzed per line) led to identification of nine transgenic depressor lines with a single T-DNA insertion that showed a Mendelian segregation ratio of 3:1 for kanamycin resistance in the T2 generation. Of these lines, two transgenic K+in depressor lines (kat1-T256R line nos. 15-4 and 22-6) were selected because both lines showed a reproducible and significant inhibition of light-induced stomatal opening. Homozygous offspring (T3 and T4 generations) of these two lines that showed 100% kanamycin resistance in the T3 generation were used for further detailed analyses. We also chose another homozygous transgenic line (kat1-T256R no. 23-4) that did not show inhibition of light-induced stomatal opening for quantitative comparisons.
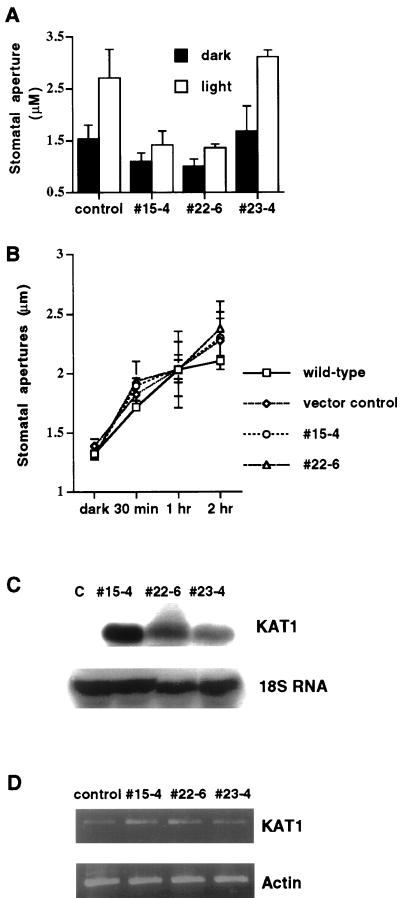
As shown in Figure 2A, stomata of the two transgenic lines 15-4 and 22-6 did not open widely in response to white light compared with vector-transformed controls (P < 0.001, no. 15-4; P < 0.001, no. 22-6) and the transgenic control line number 23-4 (P < 0.001, no. 15-4; P < 0.001, no. 22-6). It is interesting that prior to light exposure, stomatal apertures were significantly smaller in the number 15-4 line (P < 0.001) and the number 22-6 line (P < 0.001) compared with vector-transformed controls (Fig. 2A). Stomatal apertures in vector-transformed control plants were enhanced by light by an average of 77% (Fig. 2A, left). Furthermore, stomatal apertures increased by an average of 85% in the control line number 23-4 (Fig. 2A, right). In contrast, stomatal apertures increased in the kat1-T256R line number 15-4 in the light by an average of only 29% and in line number 22-6 by an average of 35%. It is interesting that stomatal apertures after 3 h light exposure in both depressor lines barely reached levels of those from dark-treated control plants (Fig. 2A).
Figure 2.
Inhibition of light-induced stomatal opening in transgenic K+in channel depressor lines numbers 15-4 and 22-6 in which expression of dominant negative KAT1 mutant is driven by a tandem-repeat of the 35S promoter, and detection of transgenic dominant negative KAT1 transcripts in the guard cells of depressor lines. A, Stomatal aperture before treatment with stomatal opening conditions (black bars). Stomata were exposed to light for 3 h in 30 mm KCl (white bars; vector-transformed control, n = 5 [100 stomates]; line no. 15-4, n = 3 [60 stomates]; line no. 22-6, n = 3 [60 stomates]; line no. 23-4, n = 2 [40 stomates]). Error bars show se B, Time course measurements of stomatal apertures at high extracellular (100 mm) K+. Stomata were incubated in 100 mm KCl and measured at 30 min, 1 and 2 h after light exposure (n = 2 experiments, 40 stomates were measured at each time point). Error bars show se C, RNA-blot analysis of transgenic dominant negative KAT1 transcripts in wild-type controls (c) and in transgenic line numbers 15-4, 22-6, and 23-4. Total cellular RNA was extracted from leaves of plants, separated on a 1.2% (w/v) denaturing agarose gel, and then transferred onto a nylon membrane. The blot was probed with 32P-radiolabeled KAT1 cDNA. The same blot was hybridized with 32P-radiolabeled 18S rDNA to show relative amounts of RNA samples loaded. D, Reverse transcriptase (RT)-PCR analysis of endogenous and transgenic KAT1 transcripts in guard cells of wild-type controls and in transgenic line numbers 15-4, 22-6, and 23-4. The same amount of cDNA was used to amplify actin, to determine relative amounts of cDNA used for KAT1 amplification. The average from three independent pooled PCR amplifications of actin and KAT1 transcripts is shown.
To test whether a high K+ concentration could recover stomatal opening in transgenic lines, time course measurements of stomatal aperture were performed with a stomatal opening solution containing 100 mm KCl. As shown in Figure 2B, stomatal apertures of the two transgenic lines (nos. 15-4 and 22-6) opened as wide as vector-transformed controls, possibly due to rescue of reduced K+ uptake (P > 0.765 for no. 15-4 at 2 h; P > 0.229 for no. 22-6 at 2 h).
To examine KAT1 mutant expression in transgenic Arabidopsis plants, northern-blot analyses were performed. As shown in Figure 2C, the transgenic KAT1 transcript was detected in all three transgenic K+in suppressor lines. In contrast, KAT1 transcript was not detected in vector-transformed control plants as previously reported (Cao et al., 1995; Ichida et al., 1997) due to preferential expression of KAT1 in guard cells (Nakamura et al., 1995). To determine the relative amounts of RNA samples loaded, the blot was hybridized with an 18S rDNA probe (Fig. 2C, bottom). Furthermore, RT-PCR analyses were performed to compare KAT1 mutant expression level in transgenic Arabidopsis guard cells. KAT1 transcript was detected in transgenic guard cells of all three lines and in vector controls (Fig. 2D). However, amplification of KAT1 in transgenic lines was stronger, most likely due to expression of dominant negative kat1-T256R mutant transcripts in guard cells. To determine the relative amounts of cDNA used in KAT1 amplification, the same amount of cDNA was used for amplification of actin (Fig. 2D, bottom; Choi et al., 2000). Compared with vector controls, KAT1 expression in transgenic lines was increased by 90% in line numbers 15-4 and 22-6, and 30% in line number 23-4 when KAT1 amplification was normalized to actin mRNA levels.
Inhibition of Light-Induced Stomatal Opening Correlates with Reduced K+in Currents
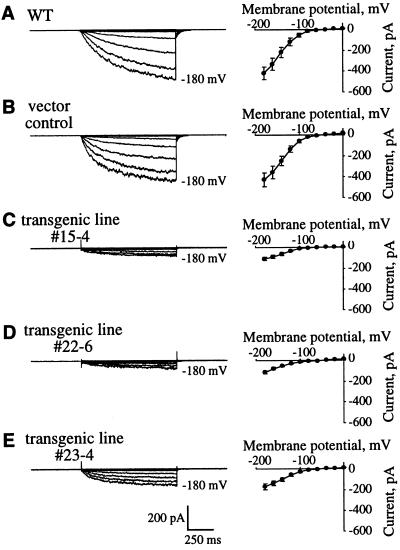
The differences in light-induced stomatal opening prompted us to examine activities of Arabidopsis guard cell K+in channels (Ichida et al., 1997; Roelfsema and Prins, 1997) of wild-type, vector-transformed control plants and the three transgenic K+in depressor lines. As shown in Figure 3, A and B, hyperpolarization of Arabidopsis guard cells activated K+in currents in wild-type and vector-transformed control plants. The average steady-state whole-cell currents at −180 mV under the imposed conditions for wild type (−427.6 ± 67.1 pA, n = 13) were similar to vector-transformed control plants (−417.3 ± 62.0 pA, n = 10; P > 0.92; Fig. 3, A and B). The magnitudes of K+in currents in the number 15-4 and number 22-6 depressor lines were significantly reduced compared with wild-type and control plants (Fig. 3, C and D; P < 0.002). The average steady-state whole-cell current at −180 mV in line number 15-4 was −106.9 ± 16.2 pA (Fig. 3C; 74% reduction compared with controls, n = 14) and −103.7 ± 16.7 pA in line number 22-6 (Fig. 3D; 76% reduction compared with controls, n = 10). In the transgenic line number 23-4 that did not show a clear reduction in light-induced stomatal opening (Fig. 2A, right), K+in currents were reduced by 60% (Fig. 3E; −168.0 ± 27.6 pA at −180 mV, n = 5). Together with data shown in Figure 1 (line no. 3-17), these results suggest that reduction in K+in channel activity by 60% or less in guard cells still allows light-induced stomatal opening under the imposed conditions but a reduction by 75% reduces light-induced stomatal opening.
Figure 3.
Guard cells from the two strong transgenic K+in channel depressor lines showing inhibition of light-induced stomatal opening have significantly reduced K+in currents compared with wild-type and vector-transformed control plants. Inward K+ currents (left) recorded in the presence of 30 mm KCl and current-voltage relationships (right) in wild type (A, n = 13), vector-transformed control (B, n = 10), and transgenic line numbers 15-4 (C, n = 14), 22-6 (D, n = 10), and 23-4 (E, n = 5) guard cells. Membrane potentials were stepped from a holding potential of −40 mV to pulse potentials of 0 to −180 mV in −20-mV increments.
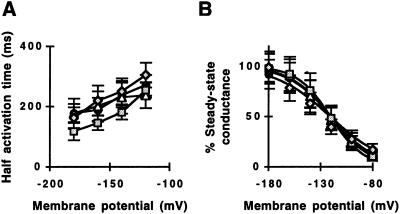
We analyzed half activation times and chord conductances of K+in channel currents to determine whether physiological properties of K+in channels are changed in the strong transgenic K+in channel depressor lines. As shown in Figure 4A, half activation times showed only a weak voltage dependence at membrane potentials from −120 to −160 mV in all plant lines examined and there were no significant differences (P > 0.19) among plant lines. The steady-state chord conductance was analyzed as a function of membrane potential (Fig. 4B) to determine whether inward-rectifying current activation is affected by dominant negative KAT1 mutations. Half-maximal activation potentials were similar among plant lines (Fig. 4B; −120 to −121 mV). The slope conductance of line number 15-4 was 16% smaller than vector-transformed controls and that of line number 22-6 was 34% larger than the slope conductance of controls, indicating a variation in this parameter that showed no consistent trend in K+in channel depressor lines and that may be due to the reduced signal to noise ratios in K+in channel depressor lines.
Figure 4.
Comparison of half activation times and steady-state chord conductance of inward K+ currents in the transgenic K+in channel depressor lines, vector-transformed control plants, and wild-type plants. A, Half activation times of inward K+ currents plotted as a function of the membrane potential. B, Steady-state chord conductances were determined relative to the K+ equilibrium potential and were plotted as a function of the membrane potential. Solid lines represent Boltzman fits. Wild type (black circles, n = 13), vector-transformed controls (white triangles, n = 10) and transgenic K+in channel depressor line numbers 15-4 (gray rectangles, n = 14) and 22-6 (gray diamonds, n = 10). Half activation voltage: −120.0 (wild type), −120.8 (vector-transformed control), −120.7 (line no. 15-4), and −120.9 (line no. 22-6). Slope factor: 19.4 (wild type), 19.2 (vector-transformed controls), 16.1 (line no. 15-4), and 25.8 (line no. 22-6). Slope factor correlation coefficient: 0.998 (wild type), 0.996 (vector-transformed controls), 0.999 (line no. 15-4), and 0.959 (line no. 22-6).
K+in Channel Activity Depression Reduces Transpirational Water Loss
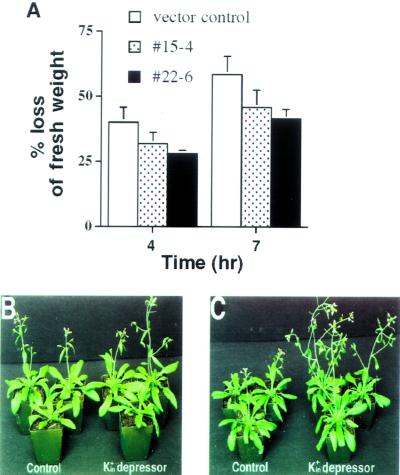
To determine whether reduction in light-induced stomatal opening affects transpirational water loss from leaves, we measured water loss rates of detached leaves of transgenic K+in channel depressor lines (Meyer et al., 1994; Leung et al., 1997; Parcy and Giraudat, 1997). During 7-h measurements, as shown in Figure 5A, detached leaves of both of the K+in channel depressor line numbers 15-4 and 22-6 consistently lost less water than vector-transformed control leaves (P < 0.02 for no. 15-4 and P < 0.01 for no. 22-6 at 4 h; P < 0.05 for no. 15-4 and P < 0.03 for no. 22-6 at 7 h, in four experiments). After 7 h, control leaves lost approximately 60% (58.5% ± 7%) of fresh weight (Fig. 5A). In contrast, only approximately 45% (45.9% ± 6.6% loss in no. 15–4 and 41.5% ± 3.7% loss in no. 22–6) of fresh weight was lost in leaves from both of the two transgenic K+in depressor lines. These data show that expression of the dominant negative KAT1 mutant causes reduced water loss from leaves of transgenic K+in depressor lines, which correlates with the observed reduction in stomatal apertures in both light- and dark-treated leaves (Fig. 2A).
Figure 5.
Effects of dominant negative KAT1 mutant expression on leaf water loss and leaf fresh weights. A, Measurements of transpirational water loss 4 and 7 h after leaf detachment show that depression of K+in channel activity causes less water loss in detached leaves of transgenic K+in channel depressor line numbers 15-4 and 22-6 than in vector-transformed controls. Data represent results of four independent measurements. Error for line number 22-6 after 4 h was 1.3%. In each experiment, fresh weights of three leaves per plant were measured under 10% (v/v) humidity conditions. Error bars show se. B and C, Both vector-transformed controls (left in B and C) and transgenic K+in channel depressor line number 15-4 (right in B and C) were grown under well-watered (B) and reduced watering conditions (C). Pictures were taken 34 d after germination. Well-watered corresponds to watering every 3 d and reduced watering to every 7 to 8 d (see “Materials and Methods”).
Experiments were pursued to determine whether reduction in K+in current activity affected whole plants. Plant growth of both controls and the transgenic K+in depressor lines was similar under well-watered growth chamber conditions (Fig. 5B). Plants were subjected to reduced watering conditions (see “Materials and Methods”). Thirty-four days after germination when water supply was limited, the two transgenic K+in depressor lines showed a subtle but reproducible enhancement of fresh weights of excised leaves (average leaf fresh weight [n = 12], 136.8 ± 19.2 mg for no. 15-4 and 149.9 ± 27.8 mg for no. 22-6) compared with vector-transformed control plants (average leaf fresh weight [n = 12], 101.8 ± 9.1 mg; P < 0.05 for no. 15-4 and no. 22-6). Furthermore, fresh weights of excised leaves of the intermediate depressor transgenic line no. 23-4 (118.6 ± 15.8 mg) were statistically similar to those of controls (101.8 ± 9.1 mg). Note that transgenic depressor lines consistently showed earlier flowering compared with controls under reduced watering conditions (Fig. 5C) but not in well-watered plants (Fig. 5B). When plants were watered sufficiently, no clear difference in fresh weights of excised leaves of the transgenic K+in depressor lines was found (average leaf fresh weight [n = 12], 131.2 ± 19.6 mg for no. 15-4 and 154.9 ± 12.0 mg for no. 22-6) compared with controls (average leaf fresh weight [n = 12], 146.9 ± 7.6 mg for vector controls, and 143.3 ± 14.8 mg for no. 23-4). At reduced watering, fresh weights of total aerial tissue including inflorescences were also increased by 42% in line no. 15-4 (P < 0.05) and 39% in line no. 22-6 (P < 0.05) 41 d after germination compared with vector controls. We measured dry weights of total aerial tissue and found no significant differences among the vector control and K+in depressors lines (data not shown). These data are consistent with the hypothesis that expression of dominant negative KAT1 mutant primarily affects water content and thus fresh weight of transgenic leaves.
Reduced K+ Uptake in Transgenic Guard Cells
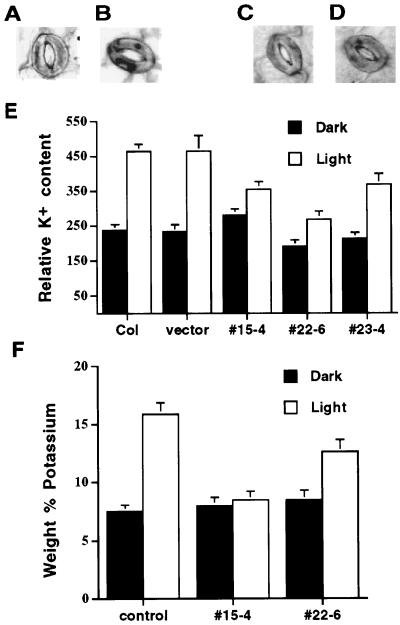
To determine whether the dominant negative kat1-T256R mutants reduce K+ uptake during light-induced stomatal opening, epidermal strips were stained with sodium hexanitrocobaltate (III) (SHC), which is a K+-specific stain that produces K+ granules in guard cells (Green et al., 1990). Figure 6 shows K+ staining analyzed in wild-type Arabidopsis guard cells before (Fig. 6A) and after (Fig. 6B) exposure to light. Before light exposure, the cells had a low K+ content as demonstrated by a lack of substantial staining (Fig. 6, A and C). After 30 min of light exposure, dark K+ granules were observed that label cellular K+ in wild-type guard cells (Fig. 6B; Green et al., 1990). In transgenic K+in depressor lines (Fig. 6D), K+ granules were consistently less pronounced after 30 min light exposure than in wild-type controls. Note that the relative K+ content of line no. 15-4 in dark-treated leaves was similar to controls, although apertures were smaller. This may be due to the qualitative nature and resolution of SHC stain measurements (Green et al., 1990). Furthermore, clear differences in stomatal apertures were not observed following staining because acetic acid treatment during the staining procedure reduces apertures (Fig. 6, B and D; Green et al., 1990). Pooling of data from all experiments showed that light-induced stomatal opening caused a 2-fold increase in the relative K+-dependent staining in vector-transformed control (n = 61 stomata before and after light incubation) and wild-type guard cells (n = 60 stomata before and 35 stomata after light incubation; Fig. 6E). In contrast, the relative intracellular K+ content was increased by only 26.5% in guard cells of transgenic line number 15-4 (n = 61 stomata before and 71 stomata after light incubation) and by 41% in guard cells of line number 22-6 (n = 49 stomata before and after light incubation, Fig. 6E). Accumulation of K+ in transgenic guard cells of line number 23-4 was increased by 73% (n = 40 stomata before and after light incubation). These data show that K+ accumulation in guard cells of transgenic lines numbers 15-4 and 22-6 was significantly reduced compared with wild-type and vector control guard cells (P < 0.01).
Figure 6.
Analysis of relative intracellular K+ content shows that depression of K+in channel activity reduces K+ uptake in guard cells of transgenic K+in channel depressor line numbers 15-4 and 22-6 during light-induced stomatal opening. Examples of SHC-stained Arabidopsis stomatal complexes from wild-type Arabidopsis before (A) and after (B) light incubation for 30 min. Examples of SHC-stained stomatal complexes from transgenic K+in channel depressor line number 22-6 before (C) and after (D) light incubation for 30 min. E, Relative intracellular K+ content in guard cells of wild type, vector-transformed control, and transgenic K+in channel depressor line numbers 15-4, 22-6, and 23-4. Black bar and white bar represent before and after light incubation, respectively. Data from three independent experiments. F, Elemental x-ray microanalysis shows change in K+ as a weight percent of seven elements (K, Ca, Cl, Mg, Na, O, and P) in guard cells of vector controls and transgenic K+in channel depressor line numbers 15-4 and 22-6. Leaves were harvested 10 min prior to illumination and at 30 min after onset of illumination. Black bar and white bar represent before and after light incubation, respectively. Each value is the mean ± se from 30 guard cells, 10 each from three separate leaves.
Additional elemental x-ray microanalyses (Blanke et al., 1999) were performed to measure K+ contents in guard cells and to independently test the results from SHC staining. Elemental x-ray analyses were performed under different growth conditions at a different site (see “Materials and Methods”). Therefore, these analyses also served to monitor the robustness of differential K+ contents under different conditions. After light exposure, the percent weight of potassium in guard cells of vector controls was increased by 111% (n = 30 guard cells before and after light exposure), whereas the percent weight of K+ was increased by only 6% in guard cells of transgenic line number 15-4 (n = 30 guard cells before and after light incubation) and by 48% in guard cells of line number 22-6 (n = 30 guard cells before and after light incubation, Fig. 6F). These data correlate with results obtained from K+ staining with SHC and show that K+ accumulation in guard cells of transgenic lines numbers 15-4 and 22-6 was reduced compared with control guard cells (P < 0.01).
DISCUSSION
Stomatal opening requires K+ uptake into guard cells (Imamura, 1943; Humble and Raschke, 1971; MacRobbie, 1983), which mediate turgor and volume increases. K+in channels were proposed to contribute to K+ uptake during stomatal opening (Schroeder et al., 1984, 1987; Schroeder, 1988). A study with KAT1 knockout mutants allows investigation of the in vivo role of KAT1 in guard cells. By screening all publicly available T-DNA pools during the time of this work, we found only one line with a T-DNA insertion in the 3′-untranslated region of KAT1, which did not affect KAT1 expression (J. Kwak and J. I. Schroeder, unpublished data; T-DNA-tagged genomic DNA collections donated to the Arabodopsis Biological Resource Center [Columbus, OH] by Drs. K. Feldmann and T. Jack, and T-DNA- and transposon-tagged genomic DNA collections obtained from Drs. S. Dellaporta, J. Ward, and A. Pereira).
An Arabidopsis mutant with a maize (Zea mays) En-1 transposon insertion in the KAT1 gene was recently reported (Szyroki et al., 2001). Stomatal aperture measurements revealed that light-induced stomatal opening was not inhibited in the mutant, which is supported by results obtained from guard cell impalement measurements that showed similar inward ionic current amplitudes in wild type and the mutant, suggesting redundancy (Szyroki et al., 2001). However, in patch-clamp experiments, 79% of the mutant guard cell protoplasts showed −4-pA steady-state K+in currents. An average of approximately −55-pA steady-state K+in currents at −189 mV were obtained in the remaining 21% of guard cell protoplasts (Szyroki et al., 2001). The K+in currents observed in the mutant might be due to the resolution of guard cell expression of low levels (approximately 6% of wild type) of endogenous KAT1 mRNA. Other K+in channel genes were detected including KAT2, AKT1, AKT2, and AtKC1 (KAT3) in the mutant as revealed by RT-PCR (Szyroki et al., 2001). A low level of endogenous KAT1 mRNA was found in the mutant and was proposed to result from somatic conversion of En-1 (removal of transposon insertion; Szyroki et al., 2001). Loss of En-1 transposon insertions is predicted (Wisman et al., 1998) and can interfere with the molecular and cellular analysis of mutants because chimeric plants are likely being analyzed (Wisman et al., 1998). To overcome cellular excission, the isolation of stable footprint alleles, complementation of stable alleles or isolation of additional alleles is advantageous for functional analysis of En-1-tagged loci (Wisman et al., 1998; Yephremov et al., 1999).
In the present study, we have pursued a complementary approach using dominant negative K+in channel mutants. This approach has an advantage over K+in channel knockout mutants, circumventing redundancy problems (Ichida et al., 1997; Dietrich et al., 1998; Szyroki et al., 2001) because the dominant negative K+in channel mutants used here can form nonfunctional heteromultimeric channel proteins with K+ channel α subunits in X. laevis oocytes (Baizabal-Aguirre et al., 1999). Note that the present study examines the underlying molecular physiology of guard cell K+in channel function rather than solely the KAT1 gene. Furthermore, this approach has allowed “titration” of K+in channel activity in vivo to analyze effects of partial K+in current reduction on stomatal and whole-plant responses. A recent study has suggested that plant K+in channels do not indiscriminately form heteromultimers, suggesting that transgenic KAT1 point mutants may only or largely form heteromultimers with true in vivo partners in plants (Urbach et al., 2000). The present study shows that regardless of subunit composition and channel redundancy, the dominant negative point mutant kat1-T256R is able to depress K+in channel activity in guard cells in vivo.
The CaMV 35S promoter used in our study would lead to expression of the dominant negative KAT1 mutant in other tissues, which may affect K+in channel activity by forming heteromultimeric K+in channels. We did not observe any clear differences at the whole-plant level between controls and trangenic plants under well-watered conditions (Fig. 5B). It is interesting, however, that transgenic K+in channel depressor lines showed early flowering under reduced watering conditions (Fig. 5C). Expression of KAT1 and the potato homolog KST1 have been reported in flowers (Müller-Röber et al., 1995; Lacombe et al., 2000). Furthermore, KAT2, AKT2, AKT5, and AKT6 are expressed in flowers (Lacombe et al., 2000). Our data point to a possible role of K+in channels in flowering time.
To date, no single point mutations that depress wild-type ion channel activity have been reported in plants. In mammals, a few examples of dominant negative K+ channel depression have been reported. In humans, the genetic disease, long QT syndrome, an inherited cardiac disorder which causes arrhythmia and sudden death, is associated with dominant missense mutations in cardiac K+ channel genes (Curran et al., 1995; Sanguinetti et al., 1996; Doyle and Stubbs, 1998) that reduce K+ current magnitudes by up to 79% (Sanguinetti et al., 1996).
K+in Channel Activity Required for K+ Uptake during Light-Induced Stomatal Opening
Functional analyses of individual K+in channel depression lines were achieved through patch clamping of Arabidopsis guard cells, combined with stomatal movement measurements, leaf water loss assays, and analyses of K+ uptake in guard cells. Together, the data provide molecular evidence that K+in channels contribute to K+ uptake during stomatal opening in response to light. Note that additional partially redundant K+ transporters contributing to K+ uptake in guard cells have not been excluded in the present study and previous studies (Ichida et al., 1997; Szyroki et al., 2001). Patch-clamp studies have suggested that additional cation influx channels exist in guard cells (Wu, 1995; Henriksen et al., 1996; Véry et al., 1998). Furthermore, in addition to K+ salts, Suc accumulation in guard cells functions as an alternative osmoticum for stomatal opening depending on growth conditions (Talbott and Zeiger, 1998).
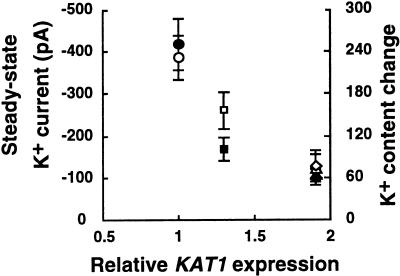
Reduction of K+in currents by 75% reduced stomatal opening by 38% to 45% (Fig. 2 and 3). Because K+in currents depend on the membrane potential, hyperpolarization by proton pumps could partially compensate for reduction of K+in currents. Reduced stomatal apertures (Fig. 2), leaf water loss (Fig. 5A), and fresh weights (Fig. 5C) at reduced watering suggest that in vivo hyperpolarization by proton pumps are not sufficient to fully compensate for reduced K+in channel activity in intact plants. This is most likely due to parallel depolarizing activities including anion channels, Ca2+ and Mg2+ influx pathways, proton-coupled substrate uptake transporters, and other depolarizing conductances. Figure 7 shows the relationship between KAT1 expression (endogenous plus transgene expression), steady-state K+in currents, and relative K+ accumulation among lines analyzed here.
Figure 7.
Correlation between KAT1 expression, steady-state K+ currents, and relative K+ accumulation. White symbols indicate steady-state K+in currents at −180 mV. Black symbols indicate changes in K+ content in response to 30 min of light incubation (relative intracellular K+ content after light incubation minus relative intracellular K+ content before light incubation) in guard cells. K+ content determined by SHC stain is presented. Averages from all cells analyzed in Figures 3 and 6 are included. KAT1 expression includes endogenous and transgenic KAT1 expression. Circles, Vector-transformed controls; squares, line no. 23-4; triangles, line no. 15-4; diamonds, line no. 22-6.
Implications for K+in Channel Regulation during Signal Transduction
When wild-type KAT1 was overexpressed in Arabidopsis, light-induced stomatal opening was not affected in transgenic plants (data not shown) as previously reported (Ichida et al., 1997). These results are consistent with early estimates that K+ channel activity in guard cells is much larger than physiologically required (Schroeder et al., 1984, 1987). For example, the average K+ uptake rate during stomatal opening in fava bean corresponds to an absolute K+ channel current of approximately 8 pA (Outlaw, 1983), whereas K+in current magnitudes are substantially larger (Schroeder et al., 1987). Ion channel activities in animal cells also often are substantially larger (up to 1,000-fold) than physiologically required (Hille, 1992). Previous pharmacological blocker studies on fava bean and Arabidopsis guard cells resulted in estimates suggesting that reduction in K+in channel activity by 50% would not affect stomatal opening and that K+in channel activity needs to be depressed by approximately 90% to obtain a substantial effect on stomatal opening (Kelly et al., 1995; Ichida et al., 1997). The present study supports but also refines this hypothesis demonstrating that approximately 60% of K+in channel inhibition did not greatly affect light-induced stomatal opening under the imposed conditions. However, reduction in K+in channel activity by 75% impaired light-induced stomatal opening (Figs. 2A and 3). These data show a nonlinearity that is expected from a complex system in which multiple active and passive transporters' function in parallel, thus determining the membrane potential, which in turn drives K+ uptake via K+in channels.
Previous studies have shown that cytosolic second messengers such as Ca2+, inositol-1,4,5-trisphosphate, guanosine-5′-0-(3-thiotriphosphate), inositol hexakisphosphate, and phosphorylation events inhibit K+in channels in guard cells (Schroeder and Hagiwara, 1989; Blatt et al., 1990; Fairley-Grenot and Assmann, 1991; Luan et al., 1993; Li et al., 1994; Thiel and Blatt, 1994; Hoshi, 1995; Lemtiri-Chlieh et al., 2000). Down-regulation of K+in channels in fava bean by the physiological stimulus abscisic acid causes approximately 57% to 75% reduction in K+in channel activity (Blatt, 1990; Lemtiri-Chlieh and MacRobbie, 1994; Schwartz et al., 1994), which alone would be close to the threshold for reducing K+ uptake into guard cells and stomatal opening (Figs. 1–3). The presented data illustrate the importance of even partial K+in channel down-regulation because parallel modulation of H+ pumps (Kinoshita et al., 1995) and other mechanisms (e.g. anion channel regulation; Schwartz et al., 1995; Pei et al., 1997), can synergistically regulate K+ uptake via K+in channels.
Reduction in K+in Channel Activity Reduces Leaf Water Loss
The reduction of K+in currents in transgenic guard cells resulted in less water loss in detached leaves of the depressor lines compared with vector-transformed controls (Fig. 5A). Moreover, this reduction of K+in currents maintains the fresh weight in whole plants under limited watering conditions (Fig. 5C). Fresh water is increasingly becoming a scarce and threatened resource due in large part to agricultural production (Serageldin, 1995; Postel et al., 1996). Some studies have suggested that partial reduction in stomatal apertures could optimize CO2 and H2O exchange for horticultural plants or when water availability is marginal, particularly in light of rising atmospheric CO2 levels (Morison, 1987). Titration of K+in channel activity in guard cells by using dominant negative KAT1 mutants as shown here might provide an approach to fine tune the degree of stomatal opening of certain plants, leading to reduced CO2 influx while reducing transpirational water loss.
In conclusion, our data provide molecular evidence for the model that K+in channels contribute to K+ uptake in guard cells under physiological conditions and show that partial reduction in K+in channel reduces transpirational water loss from leaves.
MATERIALS AND METHODS
Plant Transformation
A dominant negative KAT1 mutant that has a point mutation at amino acid 256 (T256R; Baizabal-Aguirre et al., 1999) was subcloned into plant expression vectors (pBIN-JIT) containing a tandem repeat of CaMV 35S promoter (gift from Dr. Cathie Martin, John Innes Center, Norwich, UK) and the pMON530 vector (Monsanto, St. Louis). Two vectors and the two resulting constructs, pKAT1-T256R, either in the pBIN-JIT or in the pMON530 were introduced into Agrobacterium tumefaciens C58 strain and the resulting strains were used to transform Arabidopsis ecotype Columbia by the vacuum infiltration method (Bechtold et al., 1993).
RNA-Blot Analysis
Total RNA was extracted from Arabidopsis leaves using Trizol reagent (Life Technologies, Rockville, MD). Twenty micrograms of RNA was separated on a denaturing 1.2% (w/v) agarose gel and then transferred onto a Hybond-N nylon membrane (Amersham Pharmacia Biotech, Piscataway, NJ). The blot was hybridized and washed as described (Kwak et al., 1997). RNA loading was assessed by probing the blot with an 18S rRNA gene of Brassica napus (Park et al., 1993).
RT-PCR Analysis
Total cellular RNA was extracted from Arabidopsis guard cell-enriched strips prepared from vector-transformed controls, and transgenic lines using Trizol reagent (Life Technologies). After estimation of RNA concentration of each sample, approximately 500 ng of total RNA was converted to first strand cDNA using the first-strand cDNA synthesis kit (Amersham Pharmacia Biotech). Similar amounts (<10 ng) of each cDNA were used to amplify KAT1 and actin (Choi et al., 2000) in each PCR reaction, which was prepared in a 50-μL mixture (primers [KAT1-5 and KAT1-3, actin 2-5 and actin 2-3] at 200 nm, 1× Extaq polymerase buffer, each dNTP at 200 μm, and 2.5 units of ExTaq polymerase [Takara, Kusatsu, Japan]). The PCR mixture was denatured at 94°C for 4 min and followed by 25 cycles of amplification (94°C, 30 s; 58°C, 30 s; and 72°C, 3 min). PCR was repeated three times and 10 μL of each reaction was pooled. Five microliters of the pooled reactions was loaded in an agarose gel for quantification of transcripts. Intensity of the bands was measured three times using the IPLab Gel program (Signal Analytics, Vienna, VA). Primers used for PCR were the following: KAT1-5, 5′-cgttacagagcgtgggagatgtggctag-3′; KAT1-3, 5′-gttgcagcctccaaacttctcacttgc-3′; actin2-5, 5′-ggccgatggtgaggatattcagccacttg-3′; and actin2-3, tcgatggacctgactcatcgtactcactc-3′.
Stomatal Aperture, Leaf Water Loss Measurements, and Whole-Plant Analyses
Plants were grown at 25°C under constant light (with a fluence rate of 50 μmol m−2 s−1). Stomatal aperture measurements were performed as described (Ichida et al., 1997) with slight modifications. Fully expanded rosette leaves from 4-week-old plants were detached and floated in water in darkness overnight to induce stomatal closing. One or two leaves were then transferred and submerged in stomatal opening solution (30 mm KCl, 0.1 mm CaCl2, and 10 mm MES [2-(N-morpholino)-ethanesulfonic acid]-KOH, pH 6.15) and incubated under white light with a fluence rate of 150 μmol m−2 s−1 for 3 h. Transgenic lines that showed inhibition of light-induced stomatal opening in the T1 generation were selected by measuring 16 to 20 stomata from each independent plant. Stomatal apertures were measured in the focal plane of outer edges of guard cells in epidermal strips. In the T2 generation, two plants per transgenic line were chosen and 20 stomata were assayed for each plant. Transgenic depressor lines with a single T-DNA insertion were selected that showed a Mendelian segregation ratio of 3:1 for kanamycin resistance in the T2 generation. The homozygosity of plants was determined by 100% kanamycin resistance in T3 generation. Twenty stomata of homozygous transgenic K+in depressor lines and control plants were measured for each time point and measurements were repeated several times for each line. se of mean is represented relative to the square root of the number of epidermal peel experiments. Measurements of transpirational water loss in detached rosette leaves of transgenic K+in depressor lines and vector-transformed control plants were performed as described (Hong et al., 1997). In each experiment, three leaves per plant were used and four independent experiments were conducted. To analyze plant fresh weight under reduced watering conditions, plants were watered every 7 or 8 d with approximately 30 mL per pot (5.5 × 5.5 × 6.0 cm) from 12 d after germination, whereas under normal watering conditions, plants were watered every 3 d with approximately 30 mL per pot. At 26 and 34 d after germination, fresh weights of sixth and seventh leaves were measured. In each experiment, three plants were used and two independent experiments were performed. Three 6-week-old plants were used to measure fresh weights of total aerial tissue including inflorescences and two independent experiments were performed.
Patch-Clamp Analyses
Arabidopsis guard cell protoplasts were enzymatically isolated from rosette leaves of 4- to 6-week-old plants using 1% (w/v) cellulase R10 and 0.5% (w/v) macerozyme R10 (Yakult Honsha, Tokyo), as previously described (Pei et al., 1997). Whole-cell recordings of Arabidopsis guard cells were performed as described (Ichida et al., 1997). The pipette solution was composed of 30 mm KCl, 70 mm K-Glu, 2 mm MgCl2, 6.7 mm EGTA, 3.35 mm CaCl2, 5 mm ATP, and 10 mm HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid]-Tris, pH 7.1 (Ichida et al., 1997). The bath solution contained 30 mm KCl, 40 mm CaCl2, 2 mm MgCl2, and 10 mm MES-Tris, pH 5.5 (Ichida et al., 1997). Leak currents were not subtracted.
Determination of Relative Intracellular Potassium Ion Content
Rosette leaves detached from 4- to 6-week-old plants were incubated in a solution containing 5 mm KCl and 1 mm CaCl2 overnight at 22°C in the dark to close stomata at the time experiments were begun. Then, to induce stomatal opening, the leaves were floated in the same solution for 30 min at 22°C in white light. Before and after light incubation, epidermal segments were peeled under deionized water and the epidermal peels were stained with freshly prepared 0.5 m SHC in 10% (v/v) acetic acid as described (Green et al., 1990). Images of intracellular K+ precipitate granules in stomata produced by SHC treatment were observed with a Diaphot 300 microscope (Nikon, Melville, NY) and stored with image software (NIH, Bethesda, MD). Then, captured images of stomata were converted to pixels by a fixed brightness threshold and scoring of the dark granules was done by measuring pixels after manual removal of noise pixels that were not within the outline of guard cells. Data analyses were performed using Excel software (version 98, Microsoft Corporation, Redwood, CA). The statistical significance of data was determined using Student's t test, SigmaStat, version 2.03 (SPSS Inc., Chicago).
Elemental X-Ray Microanalyses of Potassium in Guard Cells
Plants were grown at 28°C under white fluorescent light on a 16-h-light/8-h-dark regime (with a fluence rate of 150 μmol m−2 s−1) at Willamette University. Ten minutes prior to light incubation, and at 30 min after the light incubation (150 μmol m−2 s−1), mature rosette leaves were detached from 4- to 5-week-old plants. Leaves were washed in deionized water, frozen immediately in liquid nitrogen, and placed at −20°C for a minimum of 72 h to dry. After dehydration, leaves were removed, placed in a dessicator under vacuum, and allowed to warm to room temperature for 1 h. Conductive carbon tabs (12 mm, Ted Pella, Inc., Redding, CA) were mounted onto aluminum stubs (one-eighth-inch pin, one-half-inch slotted head, Ted Pella, Inc.) and then each leaf was mounted adaxial side down on a carbon tab. Mounted specimens were carbon-coated using an SPI-Module Carbon Coater (model 1142B, Structure Probe, Inc., West Chester, PA). Coated specimens were scanned at 4,000× at 10.0 kV in an XL 20 scanning electron microscope (Phillips Electronics N.V., Eindhoven, The Netherlands). After instrument calibration, guard cells were analyzed for elemental composition using an EDAX DX-4 (Edax International, Mahwah, NJ) x-ray microanalysis system attached to the scanning electron microscope. Using the reduced area scan mode, a box was drawn inside a guard cell and its contents were scanned for 100 live s. X-ray spectra were captured and quantified using EDAX software to determine the weight percent of Ca, Cl, K, Mg, Na, O, and P present in each cell. At 10. 0 kV with a 200-Å carbon layer on cellulose, 95% of the beam energy is confined to 1.8-μm depth penetration, and 90% is confined to a 0.75-μm lateral distance. Potassium contents were measured from 30 guard cells (10 each from three different mounted specimens for each treatment).
ACKNOWLEDGMENTS
We thank David Waner and Gethyn Allen for comments on the manuscript and Nathalie Leonhardt and Kelly Turner for discussions.
Footnotes
This work was supported by the Department of Energy (grant no. De–FG03–94–ER20148 to J.I.S.), by the National Science Foundation (grant nos. MCB–9506191 and MCB–00–77791 to J.I.S., grant no. MCB–9900525 to G.T., and REU supplement to J.I.S.), by the Human Frontier Science Program Organization (fellowship to J.M.K.), by the Pew Foundation (fellowship to V.M.B.-A.), and by the Ministry of Education, Science, Sports and Culture of Japan (fellowship to Y.M.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010428.
LITERATURE CITED
- Allen GJ, Kwak JM, Chu SP, Llopis J, Tsien RY, Harper JF, Schroeder JI. Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant J. 1999;19:735–747. doi: 10.1046/j.1365-313x.1999.00574.x. [DOI] [PubMed] [Google Scholar]
- Anderson JA, Huprikar SS, Kochian LV, Lucas WJ, Gaber RF. Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1992;89:3736–3740. doi: 10.1073/pnas.89.9.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baizabal-Aguirre VM, Clemens S, Uozumi N, Schroeder JI. Suppression of inward-rectifying K+ channels KAT1 and AKT2 by dominant negative point mutations in KAT1. J Membr Biol. 1999;167:119–125. doi: 10.1007/s002329900476. [DOI] [PubMed] [Google Scholar]
- Bechtold N, Ellis J, Pelletier G. In planta agrobacterium-mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C R Acad Sci. 1993;316:1194–1199. [Google Scholar]
- Bei Q, Luan S. Functional expression and characterization of a plant K+ channel gene in a plant cell model. Plant J. 1998;13:857–865. doi: 10.1046/j.1365-313x.1998.00084.x. [DOI] [PubMed] [Google Scholar]
- Blanke MM, Pring RJ, Baker EA. Structure and elemental composition of grape berry stomata. J Plant Physiol. 1999;154:477–481. [Google Scholar]
- Blatt MR. Potassium channel currents in intact stomatal guard cells: rapid enhancement by abscisic acid. Planta. 1990;180:445–455. doi: 10.1007/BF00198799. [DOI] [PubMed] [Google Scholar]
- Blatt MR, Thiel G, Trentham DR. Reversible inactivation of K+ channels of Vicia stomatal guard cells following the photolysis of caged 1,4,5-trisphosphate. Nature. 1990;346:766–769. doi: 10.1038/346766a0. [DOI] [PubMed] [Google Scholar]
- Cao Y, Ward JM, Kelly WB, Ichida AM, Gaber RF, Anderson JA, Uozumi N, Schroeder JI, Crawford NM. Multiple genes, tissue specificity, and expression-dependent modulation contribute to the functional diversity of potassium channels in Arabidopsis thaliana. Plant Physiol. 1995;109:1093–1106. doi: 10.1104/pp.109.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Hong J, Ha J, Kang J, Kim S. ABFs, a family of ABA-responsive element binding factors. J Biol Chem. 2000;275:1723–1730. doi: 10.1074/jbc.275.3.1723. [DOI] [PubMed] [Google Scholar]
- Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- Czempinski K, Gaedeke N, Zimmermann S, Müller-Röber B. Molecular mechanisms and regulation of plant ion channels. J Exp Bot. 1999;50:955–966. [Google Scholar]
- Dietrich P, Dreyer I, Wiesner P, Hedrich R. Cation sensitivity and kinetics of guard-cell potassium channels differ among species. Planta. 1998;205:277–287. [Google Scholar]
- Doyle JL, Stubbs L. Ataxia, arrhythmia and ion-channel gene defects. Trends Genet. 1998;14:92–98. doi: 10.1016/s0168-9525(97)01370-x. [DOI] [PubMed] [Google Scholar]
- Fairley-Grenot K, Assmann SM. Evidence for G-protein regulation of inward K+ channel current in guard cells of fava bean. Plant Cell. 1991;3:1037–1044. doi: 10.1105/tpc.3.9.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay GP, Tyerman SD, Garrill A, Skerrett M. Pump and K+ inward rectifiers in the plasmalemma of wheat root protoplasts. J Membr Biol. 1994;139:103–116. doi: 10.1007/BF00232429. [DOI] [PubMed] [Google Scholar]
- Gassmann W, Schroeder JI. Inward-rectifying K+ channel currents in root hairs of wheat: a mechanism for aluminum-sensitive low-affinity K+ uptake and membrane potential control. Plant Physiol. 1994;105:1399–1408. doi: 10.1104/pp.105.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaymard F, Pilot G, Lacombe B, Bouchez D, Bruneau D, Boucherez J, Michaux-Ferriere N, Thibaud J, Sentenac H. Identification and disruption of a plant shaker-like outward channel involved in K+ release into the xylem sap. Cell. 1998;94:647–655. doi: 10.1016/s0092-8674(00)81606-2. [DOI] [PubMed] [Google Scholar]
- Green D, Dodge S, Lee J, Tallman G. Effect of sodium hexanitrocobaltate (III) decomposition on its staining of intracellular potassium ions. Stain Technol. 1990;65:647–655. doi: 10.3109/10520299009105603. [DOI] [PubMed] [Google Scholar]
- Henriksen GH, Taylor AR, Brownlee C, Assmann SM. Laser microsurgery of higher plant cell walls permits patch-clamp access. Plant Physiol. 1996;110:1063–1068. doi: 10.1104/pp.110.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer Associates, Inc.; 1992. [Google Scholar]
- Hirsch RE, Lewis BD, Spalding EP, Sussman MR. A role for the AKT1 potassium channel in plant nutrition. Science. 1998;280:918–921. doi: 10.1126/science.280.5365.918. [DOI] [PubMed] [Google Scholar]
- Hong SW, Jon JH, Kwak JM, Nam HG. Identification of a receptor-like protein kinase gene rapidly induced by abscisic acid, dehydration, high salt, and cold treatments in Arabidopsis thaliana. Plant Physiol. 1997;113:1203–1212. doi: 10.1104/pp.113.4.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T. Regulation of voltage-dependence of the KAT1 channel by intracellular factors. J Gen Physiol. 1995;105:309–328. doi: 10.1085/jgp.105.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humble GD, Raschke K. Stomatal opening quantitatively related to potassium transport. Evidence from electron probe analysis. Plant Physiol. 1971;48:447–453. doi: 10.1104/pp.48.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichida AM, Pei Z-M, Baizabal-Aguirre V, Turner KJ, Schroeder JI. Expression of a Cs+ resistant guard cell K + channel confers Cs +-resistant light-induced stomatal opening in transgenic Arabidopsis. Plant Cell. 1997;9:1843–1857. doi: 10.1105/tpc.9.10.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura S. Untersuchungen über den Mechanismus der Turgorschwankung der Spaltoffnungs-Schliesszellen. Jap J Bot. 1943;12:251–346. [Google Scholar]
- Kelly WK, Esser JE, Schroeder JI. Effects of cytosolic calcium and limited, possible dual, effects of G protein modulators on guard cell inward potassium channels. Plant J. 1995;8:479–489. [Google Scholar]
- Kinoshita T, Nishimura M, Shimazaki K-I. Cytosolic concentration of Ca2+ regulates the plasma membrane H+-ATPase in guard cells of fava bean. Plant Cell. 1995;7:1333–1342. doi: 10.1105/tpc.7.8.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Kim SA, Hong SW, Nam HG. Evaluation of 515 expressed sequence tags obtained from guard cells of Brassica campestris. Planta. 1997;202:9–17. doi: 10.1007/s004250050097. [DOI] [PubMed] [Google Scholar]
- Lacombe B, Pilot G, Michard E, Gaymard F, Sentenac H, Thibaud J-B. A shaker-like K+ channel with weak rectification is expressed in both source and sink phloem tissues of Arabidopsis. Plant Cell. 2000;12:837–851. doi: 10.1105/tpc.12.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemtiri-Chlieh F, MacRobbie EAC. Role of calcium in the modulation of Vicia guard cell potassium channels by abscisic acid: a patch-clamp study. J Membr Biol. 1994;137:99–107. doi: 10.1007/BF00233479. [DOI] [PubMed] [Google Scholar]
- Lemtiri-Chlieh F, MacRobbie EAC, Brealey CA. Inositol hexakisphosphate is a physiological signal regulating the K+-inward rectifying conductance in guard cells. Proc Natl Acad Sci USA. 2000;97:8687–8692. doi: 10.1073/pnas.140217497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J, Merlot S, Giraudat J. The Arabidopsis abscisic acid-insensitive 2 (ABI2) and ABI1 genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction. Plant Cell. 1997;9:759–771. doi: 10.1105/tpc.9.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Luan S, Schreiber S, Assmann S. Evidence for protein phosphatase 1 and 2A regulation of K+ channels in two types of leaf cells. Plant Physiol. 1994;106:963–970. doi: 10.1104/pp.106.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan S, Li W, Rusnak F, Assmann SM, Schreiber SL. Immunosuppressants implicate protein phosphatase regulation of K+ channels in guard cells. Proc Natl Acad Sci USA. 1993;90:2202–2206. doi: 10.1073/pnas.90.6.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maathuis FJM, Sanders D. Contrasting roles in ion transport of two K+ channel types in root cells of Arabidopsis thaliana. Planta. 1995;197:456–464. doi: 10.1007/BF00196667. [DOI] [PubMed] [Google Scholar]
- MacRobbie EAC. Effects of light/dark on cation fluxes in guard cells of Commelina communis L. J Exp Bot. 1983;34:1695–1710. [Google Scholar]
- MacRobbie EAC. Signal transduction and ion channels in guard cells. Philos Trans R Soc Lond. 1998;1374:1475–1488. doi: 10.1098/rstb.1998.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäser P, Thomine S, Schroeder JI, Ward J, Hirschi K, Sze H, Talke IN, Amtmann A, Maathuis FJM, Sanders D. Phylogentic relationships within cation transporter families of Arabidopsis. Plant Physiol. 2001;126:1646–1667. doi: 10.1104/pp.126.4.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K, Leube MP, Grill E. A protein phosphatase 2C involved in ABA signal transduction in Arabidopsis thaliana. Science. 1994;264:1452–1455. doi: 10.1126/science.8197457. [DOI] [PubMed] [Google Scholar]
- Moran N, Satter RL. K+ channels in plasmalemma of motor cells of Samanea saman. In: Dainty J, Michelis MI, Marré E, Rasi-Coldogno F, editors. Plant Membrane Transport. Amsterdam: Elsevier; 1989. pp. 529–530. [Google Scholar]
- Morison JIL. Intercellular CO2 concentration and stomatal response to CO2. In: Zeiger E, Farquhar GD, Cowan IR, editors. Stomatal Function. Stanford, CA: Stanford University; 1987. pp. 229–251. [Google Scholar]
- Müller-Röber B, Ellenberg N, Provart N, Willmitzer L, Busch H, Becker D, Dietrich P, Hoth S, Hedrich R. Cloning and electrophysiological analysis of KST1, an inward rectifying K+ channel expressed in potato guard cells. EMBO J. 1995;14:2409–2416. doi: 10.1002/j.1460-2075.1995.tb07238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura RL, McKendree WL, Hirsch RE, Sedbrook JC, Gaber RF, Sussman MR. Expression of an Arabidopsis potassium channel gene in guard cells. Plant Physiol. 1995;109:371–374. doi: 10.1104/pp.109.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw WH. Current concepts on the role of potassium in stomatal movements. Physiol Plant. 1983;59:302–311. [Google Scholar]
- Parcy F, Giraudat J. Interactions between the ABI1 and the ectopically expressed ABI3 genes in controlling abscisic acid responses in Arabidopsis vegetative tissues. Plant J. 1997;11:693–702. doi: 10.1046/j.1365-313x.1997.11040693.x. [DOI] [PubMed] [Google Scholar]
- Park YS, Kwak JM, Kwon O-Y, Kim YS, Lee DS, Cho MJ, Lee HH, Nam HG. Generation of expressed sequence tags of random root cDNA clones of Brassica napus by single-run partial sequencing. Plant Physiol. 1993;103:359–370. doi: 10.1104/pp.103.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Z-M, Kuchitsu K, Ward JM, Schwarz M, Schroeder JI. Differential abscisic acid regulation of guard cell slow anion channels in Arabdiopsis wild-type and abi1 and abi2 mutants. Plant Cell. 1997;9:409–423. doi: 10.1105/tpc.9.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippar K, Fuchs I, Luethen H, Hoth S, Bauer CS, Haga K, Thiel G, Ljung K, Sandberg G, Boettger M. Auxin-induced K+ channel expression represents an essential step in coleoptile growth and gravitropism. Proc Natl Acad Sci USA. 1999;96:12186–12191. doi: 10.1073/pnas.96.21.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postel SL, Daily GC, Ehrlich PR. Human appropriation of renewable fresh water. Science. 1996;271:785–788. [Google Scholar]
- Roberts K, Tester M. Inward and outward K+-selective currents in the plasma membrane of protoplasts from maize root cortex and stele. Plant J. 1995;8:811–825. [Google Scholar]
- Roelfsema MRG, Prins HBA. Ion channels in guard cells of Arabidopsis thaliana (L) Heynh. Planta. 1997;202:18–27. doi: 10.1007/s004250050098. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Curran ME, Spector PS, Keating MT. Spectrum of HERG K+ channel dysfunction in an inherited cardiac arrhythmia. Proc Natl Acad Sci USA. 1996;93:2208–2212. doi: 10.1073/pnas.93.5.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman D, Schroeder JI, Lucas WJ, Anderson JA, Gaber RF. Expression of an inward-rectifying potassium channel by the Arabidopsis KAT1 cDNA. Science. 1992;258:1654–1658. doi: 10.1126/science.8966547. [DOI] [PubMed] [Google Scholar]
- Schroeder JI. K+ transport properties of K+ channels in the plasma membrane of Vicia faba guard cells. J Gen Physiol. 1988;92:667–683. doi: 10.1085/jgp.92.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Hagiwara S. Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature. 1989;338:427–430. [Google Scholar]
- Schroeder JI, Hedrich R, Fernandez JM. Potassium-selective single channels in guard cell protoplasts of Vicia faba. Nature. 1984;312:361–362. [Google Scholar]
- Schroeder JI, Raschke K, Neher E. Voltage dependence of K+ channels in guard cell protoplasts. Proc Natl Acad Sci USA. 1987;84:4108–4112. doi: 10.1073/pnas.84.12.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Ward JM, Gassmann W. Perspectives on the physiology and structure of inward-rectifying K+ channels in higher plants: biophysical implications for K+ uptake. Annu Rev Biophys Biomol Struct. 1994;23:441–471. doi: 10.1146/annurev.bb.23.060194.002301. [DOI] [PubMed] [Google Scholar]
- Schwartz A, Ilan N, Schwarz M, Scheaffer J, Assmann SM, Schroeder JI. Anion-channel blockers inhibit S-type anion channels and abscisic acid responses in guard cells. Plant Physiol. 1995;109:651–658. doi: 10.1104/pp.109.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A, Wu W-H, Tucker EB, Assmann SM. Inhibition of inward K+ channels and stomatal response by abscisic acid: an intracellular locus of phytohormone action. Proc Natl Acad Sci USA. 1994;91:4019–4023. doi: 10.1073/pnas.91.9.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentenac H, Bonneaud N, Minet M, Lacroute F, Salmon JM, Gaymard F, Grignon C. Cloning and expression in yeast of a plant potassium ion transport system. Science. 1992;256:663–665. doi: 10.1126/science.1585180. [DOI] [PubMed] [Google Scholar]
- Serageldin I. Toward sustainable management of water resources. Washington, DC: World Bank; 1995. pp. 1–33. [Google Scholar]
- Sheahan JJ, Ribeiro-Neto L, Sussman MR. Cesium-insensitive mutants of Arabidopsis thaliana. Plant J. 1993;3:647–656. [Google Scholar]
- Szyroki A, Ivashikina N, Dietrich P, Roelfsema MRG, Ache P, Reintranz B, Deeken R, Godde M, Felle H, Steinmeyer R. KAT1 is not essential for stomatal opening. Proc Natl Acad of Sci USA. 2001;98:2917–2921. doi: 10.1073/pnas.051616698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott LD, Zeiger E. The role of sucrose in guard cell osmoregulation. J Exp Bot. 1998;49:329–337. [Google Scholar]
- Tang HX, Vasconcelos AC, Berkowitz GA. Physical association of KAB1 with plant K+ channel alpha subunits. Plant Cell. 1996;8:1545–1553. doi: 10.1105/tpc.8.9.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel G, Blatt MR. Phosphatase antagonist okadaic acid inhibits steady-state K+ currents in guard cells of Vicia faba. Plant J. 1994;5:727–733. [Google Scholar]
- Thiel G, Brudern A, Gradmann D. Small inward rectifying K+ channels in coleoptiles: inhibition by external Ca2+ and function of cell elongation. J Membr Biol. 1996;149:9–20. doi: 10.1007/s002329900002. [DOI] [PubMed] [Google Scholar]
- Urbach S, Cherel I, Sentenac H, Gaymard F. Biochemical characterization of the Arabidopsis K+ channels KAT1 and AKT1 expressed or co-expressed in insect cells. Plant J. 2000;23:527–538. doi: 10.1046/j.1365-313x.2000.00828.x. [DOI] [PubMed] [Google Scholar]
- Véry A-A, Robinson MF, Mansfield TA, Sanders D. Guard cell cation channels are involved in Na+-induced stomatal closure in a halophyte. Plant J. 1998;14:509–521. [Google Scholar]
- Wegner LH, Raschke K. Ion channels in the xylem parenchyma cells of barley roots: a procedure to isolate protoplasts from this tissue and a patch-clamp exploration of salt passageways into xylem vessels. Plant Physiol. 1994;105:799–813. doi: 10.1104/pp.105.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisman E, Hartmann U, Sagasser M, Baumann E. Knock-out mutants from an En-1 mutagenized Arabidopsis thaliana population generate phenylpropanoid biosynthesis phenotypes. Proc Natl Acad Sci USA. 1998;95:12432–12437. doi: 10.1073/pnas.95.21.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W-H. A novel cation channel in Vicia faba guard cell plasma membrane. Acta Phytol Sin. 1995;21:347–354. [Google Scholar]
- Yephremov A, Wisman E, Huijser P, Huijser C, Wellesen K, Saedler H. Characterization of the FIDDLEHEAD gene of Arabidopsis reveals a link between adhesion response and cell differentiation in the epidermis. Plant Cell. 1999;11:2187–2201. doi: 10.1105/tpc.11.11.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]