Abstract
In the fern Adiantum capillus-veneris, chloroplast movement is induced by mechanical stimulation as well as by light stimulation. Directional movement of both types depends on an actin-based motile system. To investigate the physiological relationship between mechanical and light signaling in the regulation of chloroplast movement, we examined the mechano-response of chloroplasts whose motility had been already restricted after photo-relocation. Chloroplast mechano-avoidance movement was induced under all of the photo-relocation conditions tested, indicating that mechano-specific signals generated by mechanical stimulation dominate over the light signals and reactivate the motility of chloroplasts. When the effects of external Ca2+ on the induction of mechano- and light responses were examined, strikingly different requirements of external Ca2+ were found for each. In medium without Ca2+, the mechano-response was suppressed but no effects were observed on photo-response. Mechano-relocation movement of chloroplasts was inhibited by 100 μm lanthanum (La3+), a plasma membrane calcium channel blocker, and by 10 μm gadolinium (Gd3+), a stretch-activated channel blocker. However, the same concentrations of these drugs did not affect the photo-relocation movement at all. These results suggest that the influx of external Ca2+ is crucial for the early signaling step of chloroplast mechano-relocation but not for that of photo-relocation. This is the first report showing the separation of signaling pathways in mechano- and photo-relocation of chloroplasts.
Plants are non-motile and must thus suffer and cope with environmental changes to survive, whereas animals can move away from hazardous conditions. Among many environmental factors, light and mechanical stimulation are the most significant stresses to plants. Plants use these stimuli as signals for the regulation of development and morphology to adapt to the environment. Responses caused by these two environmental factors are known as photomorphogenesis and thigmomorphogenesis, respectively (Jaffe, 1973; Kendrick and Kronenberg, 1994). In addition to macroscopic responses, such as the morphology of individuals, plants also respond rapidly and reversibly at microscopic level to these fluctuating signals. One of the most prominent responses at this level is an intracellular relocation of chloroplasts.
Chloroplast movement directed by light has been known over 100 years and was comprehensively described in the first decade of the 20th century (Senn, 1908). Chloroplasts move to a position where they can most effectively absorb light of moderate fluence rate for efficient photosynthesis, but they move away from regions of potentially hazardous light conditions where the fluence rate is too high. This photo-relocation movement of chloroplasts is well known in various plant cells and the effective wavelength is limited to the blue light (BL) region in many plants (Zurzycki, 1980). In some lower plants, however, phytochrome as well as a BL receptor also mediate chloroplast movement (Haupt and Scheuerlein, 1990; Wada et al., 1993; Kadota et al., 2000). In particular, in Adiantum capillus-veneris, similar effects of polarized light on chloroplast relocation are observed for both red light (RL) and BL, which are mediated by dichroic phytochrome and a dichroic BL receptor, respectively (Yatsuhashi et al., 1985).
Chloroplast movement in A. capillus-veneris is an excellent model system for studying the signal transduction pathways downstream of both light and mechano-perception. We recently discovered a mechano-relocation movement of chloroplasts in protonemal cells of the fern; that is, chloroplasts move away from the mechanically stimulated site (Sato et al., 1999). In this system, we can easily observe the response under the microscope and mechanical stimulation is readily applied with a capillary to portions of intact cells, which become the perception site of mechanical stimulation. Even brief stimulation can induce mechano-relocation of chloroplasts. The motile system of chloroplast mechano-relocation is found to be dependent on actin microfilaments, as is the case for photo-relocation movement (Kadota and Wada, 1992; Sato et al., 1999).
Until now, no studies have been performed on the relationship between light and mechano-signaling at the cellular level. In the present study, we show the utility of the fern protonemal cells in this regard. We first demonstrate the occurrence of mechano-relocation movement in photo-relocated chloroplasts, indicating that signaling after mechanical stimulus affects the actin-based motility of chloroplasts independent from light signaling. Examining the effects of external Ca2+ on mechano- and photo-relocation of chloroplasts, we report different requirement of external Ca2+ in signaling of mechano- and photo-relocation movement.
RESULTS
Tracking of Chloroplasts during Mechano- Relocation Movement
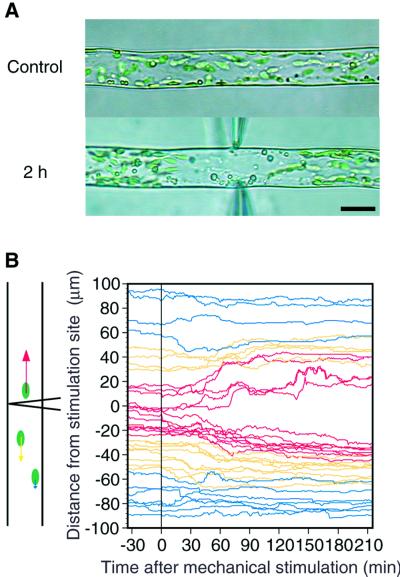
The avoidance response of chloroplasts induced by mechanical stimulation was analyzed using infrared video microscopy in the dark to exclude any light effects on chloroplast movement. When mechanical stimulation was continuously applied to part of a protonema from both sides with the flanks of bent capillaries, the chloroplasts moved away from the stimulated site, but oil drops kept moving constantly regardless of the stimulation site (Fig. 1A; see also video supplement). Brief stimulation periods shorter than 1 min were enough to induce the avoidance response to the maximum level (Sato et al., 1999). Therefore, the paths of individual chloroplasts along the long axis of the cell were analyzed by tracking each chloroplast at 1-min intervals before and after a 1-min mechanical stimulation (Fig. 1B). Directional movement of chloroplasts away from the mechanically stimulated site was apparent in an area within 25 μm of the stimulation site, whereas the chloroplasts located further than 50 μm from the site did not show any avoidance response. Thus, the avoidance signal generated by mechanical stimulation could be diffusible but may not reach beyond 50 μm from the stimulation point.
Figure 1.
Mechano-avoidance movement of chloroplasts in the dark. A, A protonemal cell was continuously stimulated from both sides of the cell with the flank of bent microcapillaries. The avoidance movement of chloroplasts can be seen in a video supplement at www.plantphysiol.org. B, Time course of avoidance movement of individual chloroplast induced by short-term (1 min) mechano-stimulation from one side (vertically) with the flank of a capillary. Bar, 20 μm.
Mechano-Relocation Movement of Photo- Relocated Chloroplasts
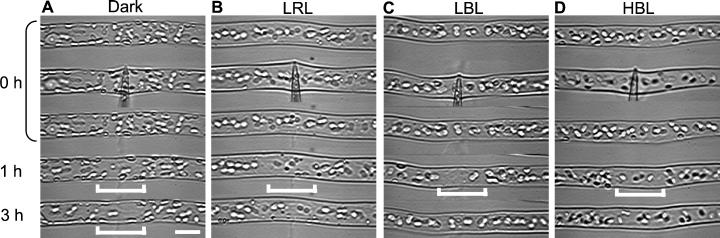
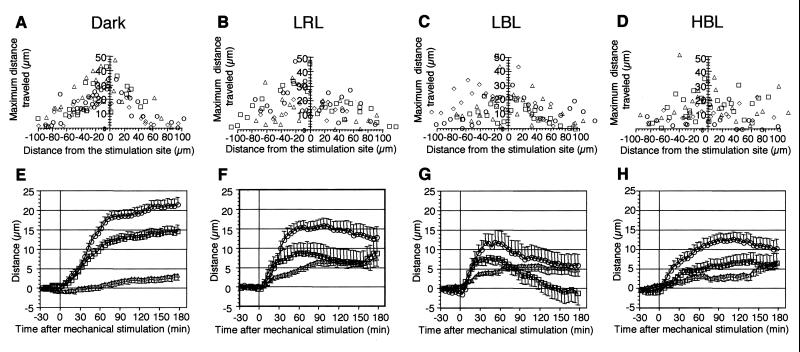
To investigate whether mechano-relocation movement was evoked in chloroplasts that have already had certain intracellular positions after photo-relocation movement, we applied mechanical stimulation to cells under light irradiation. Photo-relocation of chloroplasts was induced by continuous irradiation from the tip of protonemata with polarized light. For low fluence rate responses, horizontally polarized light of low fluence rate RL (LRL, 0.44 W m−2) and BL (LBL, 0.79 W m−2) were used and vertically polarized light of high fluence rate BL (HBL, about 10 W m−2) was employed for the high fluence rate response. As shown in Figure 2, chloroplasts accumulated in a line along the upper or lower surface of the cells, and movement at the relocation site ceased after 3 h of polarized light treatment. While maintaining irradiation with polarized light, we also applied mechanical stimulation to part of the cell using the flank of a capillary for 1 min. Mechano-avoidance responses of chloroplasts were observed 1 h after mechanical stimulation in all the light condition tested (Fig. 2, B–D). The relationship between chloroplast location from the stimulation site and the maximum distance traveled after mechanical stimulation was investigated (Fig. 3, A–D). There was a common feature. Regardless of the light conditions used, the nearer the chloroplasts were to the stimulation site, the further they moved away. Time courses of chloroplast mechano-relocation movement showed that under polarized light conditions, the chloroplasts moved away for about 1 h. However, they tended to relocate to the stimulated area in the next 1 to 2 h, particularly in LBL, whereas in cells stimulated in the dark, this recovery movement was not observed within 3 h of mechanical stimulation (Figs. 2 and 3, E–H). Thus, the avoidance response in the dark-adapted cells was the largest among all light conditions tested (Fig. 3, E–H).
Figure 2.
Serial images of mechano-avoidance response of photo-relocated chloroplasts. Mechanical stimulation was applied vertically with the flank of a capillary for 1 min. A, Dark control; B through D, cells were continuously pre-irradiated with polarized light for 3 h before mechanical stimulation and the light was kept on throughout the experiments. B, LRL (0.44 W m−2); C, LBL (0.79 W m−2); D, HBL (about 10 W m−2). Bar, 20 μm.
Figure 3.
Mechano-avoidance response of photo-relocated chloroplasts. A through D, Relationship between the initial location of chloroplasts and the maximum distance traveled. Different symbols indicate different cells analyzed. E through H, Time course of chloroplast avoidance response under various light conditions. Chloroplasts were divided into three groups with respect to their initial location before mechanical stimulation: those located with in 25 μm (○), 25to 50 μm (□), and over 50 μm (▵). Each point represents the mean ± se. A and E, Dark control; B and F, LRL; C and G, LBL; D and H, HBL.
Lag Period in Mechano-Relocation Response and Velocity of Chloroplast Movement
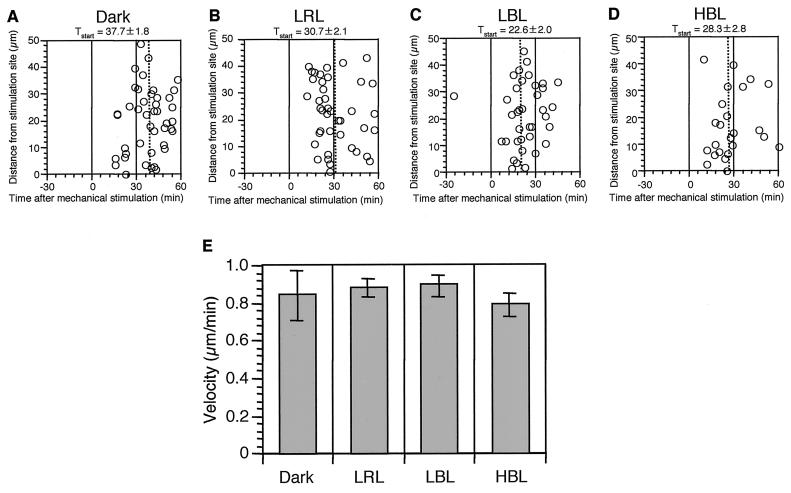
Next, we measured how long it took for chloroplasts to start to move away after mechanical stimulation. We found that there was no relationship between the initial intracellular location of the chloroplasts and the lag period before chloroplasts started to move away under any of the light conditions tested (Fig. 4, A–D). When the lag periods are compared for different light treatments, they are longest in cells stimulated in the dark. We also calculated the velocity of chloroplast movement during the avoidance response (Fig. 4E). No apparent difference in chloroplast velocities was found between any of the light conditions, and the average value was about 0.8 to 0.9 μm min−1.
Figure 4.
Time lag and velocity of chloroplasts during avoidance response. A through D, Time lag between the mechanical stimulation and the start of chloroplast relocation movement. A, Dark control; B, LRL; C, LBL; D, HBL. Means and se are also presented on the top of each figure and the mean is also indicated with dotted line in each graph. E, Velocity of chloroplasts during avoidance movement. Each bar represents the mean ± se derived from analyses of 24 to 42 chloroplasts from four protonemata. Data were obtained from the same cells used in Figure 3.
Effects of External Ca2+ on the Mechano- and Photo-Relocation of Chloroplasts
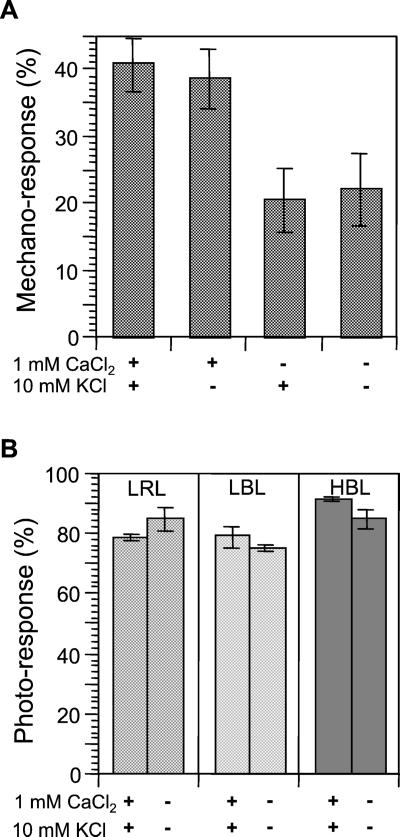
The data for mechano-induced avoidance movement in photo-relocated chloroplasts indicate that mechanical stimulation dominates over the light signal in directing chloroplasts, and that further, mechano-relocation may be regulated differently from the actin-based chloroplast motility associated with photo-relocation. The actin cytoskeleton is known to be regulated by Ca2+ in many aspects of cell function. Thus, we tested the effects of external Ca2+ on mechano- and photo-relocation. Both mechano- and photo-relocation of chloroplasts was evident when cells were kept in 10 mm MES [2-(N-morpholino)-ethanesulfonic acid] buffer (pH 6.0) supplemented with 1 mm CaCl2 and 10 mm KCl. Chloroplast mechano-relocation caused by 1 min of stimulation was inhibited by omitting CaCl2 from the medium, whereas no effect was observed when KCl was excluded (Fig. 5A). In contrast, chloroplast photo-relocation responses induced by continuous irradiation with polarized light were not affected at all by omission of both CaCl2 and KCl from the medium (Fig. 5B).
Figure 5.
External Ca2+ dependency of mechano- and photo-relocation movement. Cells were pre-incubated for 2 h in 10 mm MES with or without supplement of 1 mm CaCl2 and/or 10 mm KCl. A, Chloroplast mechano-relocation was inhibited in the medium without Ca2+. Each bar represents the mean ± se obtained from 10 protonemata. B, Chloroplast photo-relocation was not affected by the external ions. Each bar represents the mean ± se derived from analysis of 150 or more protonemata from three dishes.
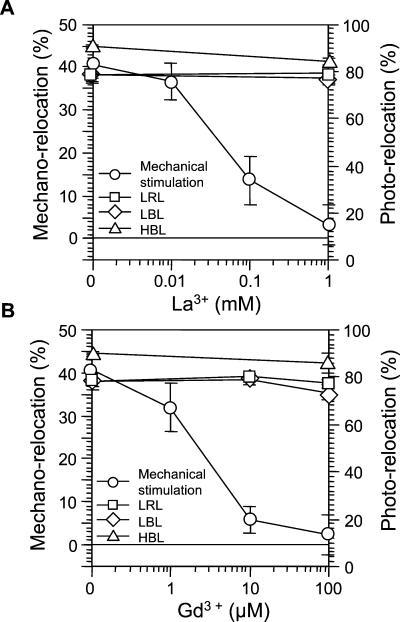
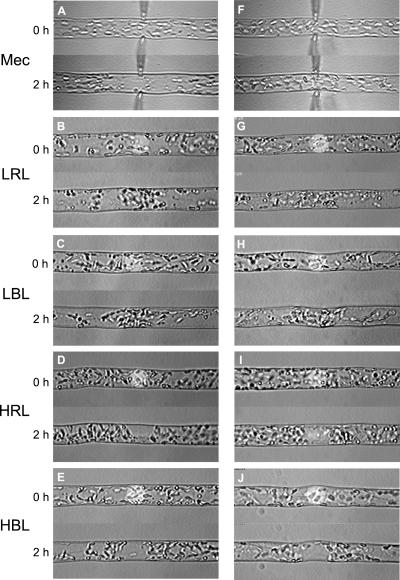
Next, we examined the effects of the calcium channel blocker, lanthanum (La3+), and the stretch channel inhibitor, gadolinium (Gd3+), in MES buffer containing 1 mm CaCl2 and 10 mm KCl (Fig. 6). Mechano-relocation of chloroplasts was inhibited by these ions in a dose-dependent manner. La3+ was inhibitory at a concentration of less than 100 μm (Fig. 6A). The inhibitory effects of Gd3+ were stronger than La3+ and mechano-relocation was abolished at a concentration of less than 10 μm (Fig. 6B). In contrast, these reagents did not inhibit chloroplast photo-relocation. These results suggest that external Ca2+ is crucial for induction of mechano-relocation of chloroplasts. To further confirm the differences in the effects of these drugs between mechano- and photo-relocation, mechanical stimulation was continuously applied while photo-relocation was induced by microbeam irradiation. With the latter, we could induce an avoidance response with RL because we could obtain a fluence rate of 600 W m−2, which is necessary to induce the high fluence rate response with RL. Figure 7 shows representative images of mechano- and photo-relocation of chloroplasts. Clear mechano- and photo-relocation were observed in medium containing 1 mm CaCl2 (Fig. 7, A–E). When 100 μm Gd3+ was used in the medium containing 1 mm CaCl2, mechano-relocation of chloroplasts was largely inhibited, but the inhibition was incomplete (data not shown). Complete abolition of mechano-relocation was observed when Gd3+ at 100 μm added to the medium without supplementing with CaCl2 (Fig. 7F). We also examined photo-relocation movement under these conditions; namely, the least favorable for mechano-relocation (Fig. 7, G–J). There was no effect on photo-relocation movement in response to light quality, either via accumulation or avoidance. We obtained almost the same results when La3+ was used instead of Gd3+ (data not shown). Together, these data suggest that an influx of Ca2+ from extracellular sites is essential for induction of chloroplast mechano-relocation, but not for photo-relocation.
Figure 6.
Effects of Ca2+ channel blockers on mechano- and photo-relocation of chloroplasts. Cells were pretreated with each drug in 10 mm MES containing 1 mm CaCl2 and 10 mm KCl for 2 h. A, Effects of La3+ on mechano- and photo-relocation. B, Effects of Gd3+ on mechano- and photo-relocation. Each point represents the mean ± se. Other details are the same as in Figure 5.
Figure 7.
Representative images of chloroplast mechano- and photo- relocation. Mechanical and microbeam light stimulation was continuously applied for 2 h. A through E, Mechano- and photo-relocation of chloroplasts in 10 mm MES containing 1 mm CaCl2 and 10 mm KCl. F through J, Mechano- and photo-relocation of chloroplasts in 10 mm MES containing 10 mm KCl and 100 μm Gd3+. A and F, Mechanical stimulation (Mec); B and G, LRL (1 W m−2); C and H, LBL (1 W m−2); D and I, HRL (600 W m−2); E and J, HBL (10 W m−2). A through J, Each panel shows the response in the same cell.
DISCUSSION
Chloroplast Behavior after Mechanical Stimulation
In this study, we first analyzed the behavior of individual chloroplasts during mechano-relocation using computer recording systems, which enable us to analyze at a higher time resolution than in our previous study (Sato et al., 1999). The kinetics of mechano-relocation movement showed similar features to those of photo-relocation. The directional movement of chloroplasts was clearly seen in mechano-relocation photo-relocation. The velocities of both movements were close to each other (Kagawa and Wada, 1996, 1999). Furthermore, both stimulation movements depend on the acto-myosin system (Kadota and Wada, 1992, Sato et al., 1999). Therefore, a common motile system may be shared by both responses, although the molecular motor responsible has yet to be identified.
Mechano-Relocation Movement of Photo- Relocated Chloroplasts
In the previous study, when chloroplast photo-relocation response attained the stationary state, the photo-relocated chloroplasts were surrounded by actin filaments and their motility was reduced, indicating that they were mechanically anchored to the location by actin cytoskeleton (Kadota and Wada, 1989, 1992). This notion is also supported by studies showing that photo-relocated chloroplasts become resistant to centrifugal force in the epidermal cells of Vallisneria gigantea (Takagi et al., 1991; Dong et al., 1998). In this study, we clearly showed that the mechano-avoidance response occurred in these anchored chloroplasts under all the photo-relocation conditions tested (Fig. 2), suggesting that different signal transduction pathways are activated and regulate chloroplasts in mechano- and photo-relocation. It is surprising that the lag time before avoidance movement occurs was longer in the dark than that under any of the photo-relocation conditions tested, whereas no significant difference in the velocity of chloroplast avoidance movement was observed among the conditions (Fig. 4). These findings may indicate that reactivation of a motor apparatus by the mechano-signal leads to avoidance of chloroplasts using the actin tracks already arranged around them by photo-relocation, rather than the availability of energy for movement under photosynthetic conditions.
Roles of Ca2+ in Mechano- and Photo- Relocation of Chloroplasts
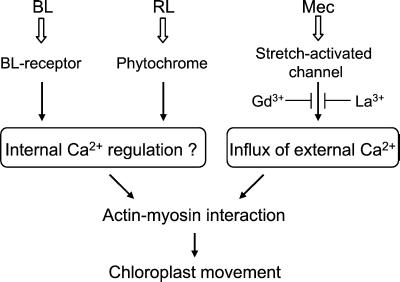
Many pharmacological studies have been performed to investigate the role of Ca2+ on chloroplast movement induced exclusively by light (Haupt and Scheuerlein, 1990; Nagai, 1993; Wada et al., 1993, and references therein). The presence of Ca2+ is essential for chloroplast photo-movement, but whether it functions in the light-signaling processes remains unclear because of the problem on the specificity of drugs used to disturb Ca2+ level. Our fern protonemal cell experimental system has the advantage that actin-based directional movement of chloroplasts can be induced with different stimuli, which allowed us to demonstrate the distinct contribution of external Ca2+ in the mechano- and photo-relocation of chloroplasts. A model for Ca2+ contribtion in the signaling events of photo- and mechano-relocation of chloroplast is shown in Figure 8. Extracellular Ca2+ is necessary for mechano-relocation but not for photo-relocation. We propose that the entry of external Ca2+, possibly through the stretch-activated channels (Morris, 1990), in response to localized deformation of a cell is essential for directional chloroplast movement in an early step of mechano-signaling. Because Ca2+ mobility in the cytoplasm is much lower than that in free solution, the result that only chloroplasts near the stimulation site respond may indicate that a localized stimulus establishes a localized [Ca2+]i gradient in the cell.
Figure 8.
A model for the signaling events of photo- and mechano-relocation of chloroplast in A. capillus-veneris.
Our finding that external Ca2+ plays important roles in the mechano-response in A. capillus-veneris does not support the hypothesis based on work with transgenic plants expressing apoaequorin that Ca2+ influx from apoplastic sites is not required for the mechano-response (Knight et al., 1992; Haley et al., 1995). This difference between the two studies may occur because a plant is constructed of many types of differentiated cells, and the response in specialized cells may be occluded by responses in other cells. We currently cannot detect the response of the minor cells in the analysis of a whole plant or of cell masses. Another reason may be the different ways in which mechanical stimulation was applied in the two studies: Direct touch on a cell that caused localized deformation of the cell was applied in this study, whereas wind or application of isotonic medium was used as the mechanical stimulation in the transgenic plant studies. Light-induced elevation of [Ca2+]i, on the other hand, was reported in transformed plants with an aequorin gene (Russell et al., 1998; Baum et al., 1999). The elevation in these studies was completely abolished by La3+ at concentrations close to those having no effects on chloroplast photo-relocation in the present study. This suggests that [Ca2+]i elevation is not involved in early signaling of photo-relocation, or the increase of [Ca2+]i in signaling for photo-relocation of chloroplasts was too small to detect in the tissue level analysis. An experimental system enabling us to detect the spatial information of [Ca2+]i at the subcellular level would be required at this point.
Finally, chloroplast movement induced by an artificial increase of [Ca2+]i via the Ca2+ ionophore A23187 has been reported in Mougeotia sp. and Lemna trisulca (Serin and Roux, 1984; Tlalka and Fricker, 1999). In these plants, however, the external Ca2+ does not play a major role in chloroplast photo-movement (Schönbohm et al., 1990a, 1990b; Tlalka and Gabryś, 1993; Tlalka and Fricker, 1999). Therefore, we can suggest the possibility that Ca2+ ionophore-induced chloroplast movement may result from mimicry of the signaling pathway for mechano-relocation rather than that for photo-relocation.
MATERIALS AND METHODS
Plant Material and Aseptic Culture
Two-celled protonemata of Adiantum capillus-veneris were cultured using the same procedure as described previously (Sato et al., 1999). In brief, spores of A. capillus-veneris, sterilized with a sodium hypochlorite, were sown in a line between two layers of agar-gelatin film on a coverslip. They were submerged in modified Murashige and Skoog liquid medium. Spores were cultured under continuous RL (0.5 W m−2) for 9 d. The protonemata were irradiated with white light (4.5 W m−2) for 6 h for induction of cell division and then kept in the dark for 2 d. The resultant two-celled protonemata were composed of a short apical cell and long basal cell. The basal cells of the protonemata were used for the present study.
Light Sources
Fluorescent lamps (FL40SD or FL10D, Toshiba Lighting and Technology Corp., Tokyo) were used as the source of white light. A halogen lamp (15 V, 150 W; Philips Japan, Tokyo) was also used as a light source for the experiments on the high-fluence rate response of chloroplasts. Colored light was obtained from the lamps through a red plastic filter (Shinkolite A, no. 102; Mitsubishi Rayon Co., Ltd., Tokyo) or a blue plastic film (Ryutate no. 63; Ryudensha, Tokyo). The colored lights were polarized through a linear polarizer (Polaroid HN 32 or HN38; Polaroid Corporation of Japan, Tokyo).
Chloroplast Photo-Relocation Induced by Polarized Light
Protonemata were irradiated horizontally from the tip with either vertically or horizontally vibrating polarized light. The response of chloroplast photo-relocation was observed 3 h after the onset of polarized light irradiation in the apical 100-μm region of the basal cell. The photo-relocation response was quantified as the percentage of protonemata in which all chloroplasts within the 100-μm region gathered at the central zone when seen from above.
Chloroplast Photo-Relocation Induced by Microbeam Irradiation
Microbeam irradiation was performed on a custom-made microbeam irradiator as previously described (Kadota et al., 2000). Monochromatic RL and BL were obtained through interference filters, and neutral density filters were used to attenuate the fluence rate.
Mechanical Stimulation
Mechanical stimulation was applied to individual cells as described by Sato et al. (1999). In brief, cells were pressed with the flank of a capillary until deformation of the cell was observed under the microscope. For continuous stimulation, individual cells were stimulated with bent capillaries from both sides. The avoidance response was determined as the percentage of chloroplasts that had moved away from the stimulus region, as previously described (Sato et al., 1999). For detailed analysis of the movement of individual chloroplasts, we used the computer recording system described by Sato et al. (2001). Lag periods in chloroplast avoidance were determined as the time when they moved more than 10 μm following the mechanical stimulation.
Inhibitor Treatments
Lanthanum chloride and gadolinium chloride (both from Sigma, St. Louis) were dissolved in a deionized, distilled water as stock solutions of 10 mm and 100 mm, respectively. After diluting to an appropriate concentration, cells were incubated with the solution for 2 h before light and mechanical stimulation.
Supplementary Material
ACKNOWLEDGMENT
We are grateful to Prof. Jane Silverthore (University of California, Santa Cruz) for critical reading of the manuscript.
Footnotes
This work was supported by the National Institute for Basic Biology Cooperative Research Program (grant no. 1–120), and in part by the Japan Society for the Promotion of Science (Grant-in-Aid for Scientific Research [C] no. 11640651 to A.K. and Grant-in-Aid for Scientific Research [B] no. 09440270 to M.W.), by the Program for Promotion of Basic Research Activities for Innovative Biosciences (to M.W.), by the Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists (grant no. 12740202 to Y.S.), and by the Japan Science Society (Sasagawa Scientific Research Grant to Y.S.).
Indicates Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010405.
LITERATURE CITED
- Baum G, Long JC, Jenkins GI, Trewavas AJ. Stimulation of the blue light phototropic receptor NPH1 causes a transient increase in cytosolic Ca2+ Proc Natl Acad Sci USA. 1999;96:13554–13559. doi: 10.1073/pnas.96.23.13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X-J, Nagai R, Takagi S. Microfilaments anchor chloroplasts along the outer periclinal wall in Vallisneria epidermal cells through cooperation of PFR and photosynthesis. Plant Cell Physiol. 1998;39:1299–1306. [Google Scholar]
- Haley A, Russell AJ, Wood N, Allan AC, Knight M, Campbell AK, Trewavas AJ. Effects of mechanical signaling on plant cell cytosolic calcium. Proc Natl Acad Sci USA. 1995;92:4124–4128. doi: 10.1073/pnas.92.10.4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupt W, Scheuerlein R. Chloroplast movement. Plant Cell Environ. 1990;13:595–614. [Google Scholar]
- Jaffe MJ. Thigmomorphogenesis: the response of plant growth and development to mechanical stimulation: with special reference to Bryonia dioca. Planta. 1973;114:143–157. doi: 10.1007/BF00387472. [DOI] [PubMed] [Google Scholar]
- Kadota A, Sato Y, Wada M. Intracellular chloroplast photorelocation in the moss Physcomitrella patens is mediated by phytochrome as well as by a blue-light receptor. Planta. 2000;210:932–937. doi: 10.1007/s004250050700. [DOI] [PubMed] [Google Scholar]
- Kadota A, Wada M. Photoinduction of circular F-actin on chloroplast in a fern protonemal cell. Protoplasma. 1989;151:171–174. [Google Scholar]
- Kadota A, Wada M. Photoorientation of chloroplasts in protonemal cells of the fern Adiantum as analyzed by use of a video-tracking system. Bot Mag Tokyo. 1992;105:265–279. [Google Scholar]
- Kagawa T, Wada M. Phytochrome- and blue-light-absorbing pigment-mediated directional movement of chloroplasts in dark-adapted prothallial cells of fern Adiantum as analyzed by microbeam irradiation. Planta. 1996;198:488–493. [Google Scholar]
- Kagawa T, Wada M. Chloroplast-avoidance response induced by high-fluence blue light in prothallial cells of the fern Adiantum capillus-veneris as analyzed by microbeam irradiation. Plant Physiol. 1999;119:917–923. doi: 10.1104/pp.119.3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick RE, Kronenberg GHM. Photomorphogenesis in Plants. Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. [Google Scholar]
- Knight MR, Smith SM, Trewavas AJ. Wind-induced plant motion immediately increases cytosolic calcium. Proc Natl Acad Sci USA. 1992;89:4967–4971. doi: 10.1073/pnas.89.11.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CE. Mechanosensitive ion channels. J Membr Biol. 1990;113:93–107. doi: 10.1007/BF01872883. [DOI] [PubMed] [Google Scholar]
- Nagai R. Regulation of intracellular movements in plant cells by environmental stimuli. Int Rev Cytol. 1993;145:251–310. [Google Scholar]
- Russell AJ, Cove DJ, Trewavas AJ, Wang TL. Blue light but not red light induces a calcium transient in the moss Physcomitrella patens (Hedw.) B., S. & G. Planta. 1998;206:278–283. [Google Scholar]
- Sato Y, Kadota A, Wada M. Mechanically induced avoidance response of chloroplasts in fern protonemal cells. Plant Physiol. 1999;121:37–44. doi: 10.1104/pp.121.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Wada M, Kadota A. Choice of tracks, microtubules and/or actin filaments for chloroplast photo-movement is differentially controlled by phytochrome and a blue light receptor. J Cell Sci. 2001;114:269–279. doi: 10.1242/jcs.114.2.269. [DOI] [PubMed] [Google Scholar]
- Schönbohm E, Meyer-Wegener J, Schönbohm E. No evidence for Ca2+ influx as an essential link in the signal transduction chains of either light-oriented chloroplast movements or Pfr-mediated chloroplast anchorage in Mougeotia. J Photochem Photobiol B: Biol. 1990a;5:331–341. [Google Scholar]
- Schönbohm E, Schönbohm E, Meyer-Wegener J. On the signal-transduction chains of two Pfr-mediated short-term processes: increase of anchorage and movement of Mougeotia chloroplasts. Photochem Photobiol. 1990b;52:203–209. [Google Scholar]
- Senn G. Die Gestalts– und Lageveränderung der Pflanzen-Chromatophoren. Leipzig, Germany: Wilhem Engelmann; 1908. [Google Scholar]
- Serin BS, Roux SJ. Modulation of chloroplast movement in the green alga Mougeotia by the Ca2+ ionophore A23187 and by calmodulin antagonists. Proc Natl Acad Sci USA. 1984;81:6368–6372. doi: 10.1073/pnas.81.20.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi S, Kamitsubo E, Nagai R. Light-induced changes in the behavior of chloroplasts under centrifugation in Vallisneria epidermal cells. J Plant Physiol. 1991;138:257–262. [Google Scholar]
- Tlalka M, Fricker M. The role of calcium in blue-light-dependent chloroplast movement in Lemna trisulca L. Plant J. 1999;20:461–473. doi: 10.1046/j.1365-313x.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Tlalka M, Gabryś H. Influence of calcium on blue-light-induced chloroplast movement in Lemna trisulca L. Planta. 1993;189:491–498. [Google Scholar]
- Wada M, Grolig F, Haupt W. Light-oriented chloroplast positioning: contribution to progress in photobiology. J Photochem Photobiol B: Biol. 1993;17:3–25. [Google Scholar]
- Yatsuhashi H, Kadota A, Wada M. Blue- and red-light action in photoorientation of chloroplasts in Adiantum protonemata. Planta. 1985;165:43–50. doi: 10.1007/BF00392210. [DOI] [PubMed] [Google Scholar]
- Zurzycki J. Blue-light-induced intracellular movements. In: Senger H, editor. The Blue Light Syndrome. Berlin: Springer; 1980. pp. 50–68. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.