Abstract
A wounded gene WI12 was used as a marker to examine the interaction between biotic stress (wounding) and abiotic stress (high salt) in the facultative halophyte ice plant (Mesembryanthemum crystallinum). The deduced WI12 amino acid sequence has 68% similarity to WUN1, a known potato (Solanum tuberosum) wound-induced protein. Wounding, methyl jasmonate, and pathogen infection induced local WI12 expression. Upon wounding, the expression of WI12 reached a maximum level after 3 h in 4-week-old juvenile leaves, whereas the maximum expression was after 24 h in 8-week-old adult leaves. The temporal expression of WI12 in salt-stressed juvenile leaves was similar to that of adult leaves. The result suggests that a salt-induced switch from C3 to Crassulacean acid metabolism has a great influence on the ice plant's response to wounding. The expression of WI12 and the accumulation of WI12 protein were constitutively found in phloem and in wounded mesophyll cells. At the reproductive stage, WI12 was constitutively found in petals and styles, and developmentally regulated in the placenta and developing seeds. The histochemical analysis showed that the appearance of WI12 is controlled by both environmental and developmental factors. Immunogold labeling showed WI12 preferentially accumulates in the cell wall, suggesting its role in the reinforcement of cell wall composition after wounding and during plant development.
The plant used in this study is a facultative halophyte, the ice plant (Mesembryanthemum crystallinum). It has been used as a model system for studying plant responses to high salinity and related osmotic stresses (Bohnert et al., 1988). The ice plant possesses a set of unique salt-tolerant mechanisms (Bohnert et al., 1995). According to its growth characteristics and stress perception, the pattern of growth was divided into five stages: germinating, juvenile, adult, flowering, and seed set (Adams et al., 1998). During normal development and/or under osmotic-type abiotic stresses, one well-known physiological change during the late juvenile stage is a change in the carbon assimilation mode from C3 to Crassulacean acid metabolism (CAM; Edwards et al., 1996). Developmental programming (Cushman et al., 1990), the hormone abscisic acid (ABA; Chu et al., 1990), and cytokinin (Thomas and Bohnert, 1993; Dai et al., 1994) were shown to be involved in CAM induction. There is a 9-fold increase in the ABA content in salt-stressed young ice plants; the induction of CAM by ABA and cytokinin was believed to be due to an acceleration of the process for developmental maturity in young ice plants (Thomas and Bohnert, 1993). Although the physiological responses to osmotic-related abiotic stresses in the ice plant have been extensively studied, the responses to other environmental stresses, especially biotic stresses, are relatively unknown.
We previously isolated a highly abundant cDNA from the light-grown ice plant callus. Databank search showed high homology to a potato (Solanum tuberosum) wound-induced gene WUN1 (Yen et al., 1999). Mechanical wounding to simulate herbivore or pathogen attacks causes rapid changes of gene expression at the injured site. These gene products, called wound-induced proteins, are involved in plant-defense responses to herbivore attack (Bowles, 1990). Jasmonic acid (JA) and its methyl ester, methyl jasmonate (MeJA), are key signal compounds in the expression of wound-induced proteinase inhibitor genes (Farmer and Ryan, 1990). Many reports also suggest that the JA-induced expressions of wound-induced genes can be coordinated with other hormones such as ABA (Peña-Cortés et al., 1991), ethylene (O'Donnell et al., 1996), and cytokinin (Sano et al., 1996). The JA-mediated signaling pathway for defense gene expression has been proposed (Koiwa et al., 1997) and the timing of expression is correlated with its role in the pathway (Ryan, 2000). Genes involved in the signal transduction pathway, such as MAP kinase DS22 (Seo et al., 1995) and JA biosynthesis enzyme LOXH3 (Royo et al., 1999), reached maximum expression within an hour. Defense genes, such as proteinase inhibitor pin1 and pin2, reached a maximum expression after 10 h (Graham et al., 1986). The wound-induced expression of WUN1 was detectable in 30 min and reached a maximum level in 10 h (Logemann et al., 1988). Therefore, according to the time course of the wound-induced progression of gene expression, WUN1 should be classified as a defense gene. The histochemical analysis showed the tissue-specific, wound-induced expression of WUN1 in the epidermis and phloem of the leaves and stem. The cell-specific expression of WUN1 has been correlated to the cell-specific production of callose, a polysaccharide involved in wound healing after mechanical wounding or pathogen attack (Logemann and Schell, 1989). Because WI12 is the first wound-induced gene found in the ice plant, it is interesting to compare the pattern of WI12 expression as well as the role in defense mechanism to its homolog WUN1 in glycophytes.
The ways in which plants respond to various environmental stresses including biotic and abiotic stresses are often interrelated. For example, the expression of genes encoding soybean (Glycine max) vegetative storage proteins was increased by water deficit stress and wounding (Mason and Mullet, 1990). Roles of calcium, activated oxygen, ABA, and ethylene in communicating the signaling network have been suggested (Bowler and Fluhr, 2000). Although it is known that both salt stress and wounding elicited the same signals, such as ABA, the physiological evidences for this connection have not been presented in the ice plant. In this paper, we first examine the effects of factors that induce the expression of wound-induced genes on the expression of WI12. Comparison was focused on the differential responses to wounding between two developmental stages of ice plants (C3 juvenile and CAM adult). The juvenile stage was studied to determine if there was any change in the temporal expression of WI12 under salt stress. Finally, histochemical techniques were used to examine the tissue specificity and the cellular localization of WI12 to assess the role of WI12 under environmental stresses and during development.
RESULTS
Time Course Progression of WI12 Induction by Wounding and MeJA in Two Developmental Stages
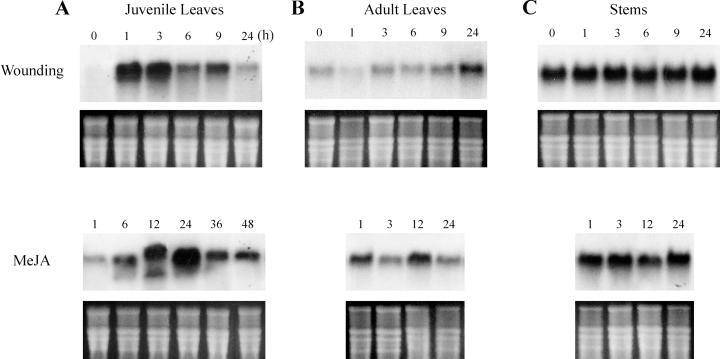
To test the effects of wounding and MeJA on WI12 induction, two stages of ice plant were mechanically injured or sprayed with MeJA and collected at different time intervals. As shown in Figure 1A, the expression of WI12 was not detectable in healthy plants and was rapidly induced by wounding in 1-month-old juvenile leaves. The peak expression was reached at 1 h and lasted to 3 h, and declined down after then. As for the JA treatment, northern analysis detected WI12 expression 1 h after MeJA spraying, with the maximum accumulation of steady-state mRNA occurring after 24 h and dropping 36 h after treatment. The result showed that wounding triggered a faster response of WI12 expression than the application of MeJA in juvenile leaves. To examine if air-borne JA could cause different response kinetics, an additional set of MeJA treatments were performed by placing ice plants and 0.5 μL L−1 pure MeJA together in an airtight Plexiglas container. The temporal expression of WI12 was similar to the result obtained by direct spraying but the level of WI12 expression was much lower compared with direct spraying (data not shown).
Figure 1.
Time course of WI12 induction by wounding and MeJA. Juvenile leaves (A), adult leaves (B), and stem from the side branches (C) of healthy ice plants were cut into 5-mm pieces (wounding) or sprayed with 200 μL L−1 MeJA. Samples from each treatment were collected at different time interval (nos. on the top) from 0 to 48 h. Ten micrograms of total RNA isolated from each treatment was separated by agarose-formaldehyde gel. The RNA gel blot was hybridized against a 32P-labeled WI12 probe. Bottom figure on each RNA gel blot is an ethidium bromide (EtBr)-stained agarose gel to ensure equal loading of each lane.
The onset of secondary growth, i.e. adult leaves and stems appearing as side shoots, is the most prominent morphological change in the transition from juvenile C3 to adult CAM stages. We also examined the expression of WI12 by wounding and MeJA in leaves and stems of the adult ice plant. In 2-month-old adult leaves, a low level of constitutive expression was detected in healthy plants. Wounding caused a much lower level of induction and the response kinetics to wounding in adult leaves was much slower than that of juvenile leaves. The transcript level increased gradually and reached the maximum level 24 h after wounding. JA treatment triggered a faster response in adult leaves when compared with the wounding treatment. The maximum expression of WI12 occurred after 12 h and decreased 24 h after MeJA spraying (Fig. 1B). The level of WI12 was constitutively high in the stems, and wounding and MeJA treatment did not cause a significant increase in WI12 expression within 24 h (Fig. 1C).
Effects of Systemic Response, Pathogen Infection, and SA at Juvenile Stage of Leaves
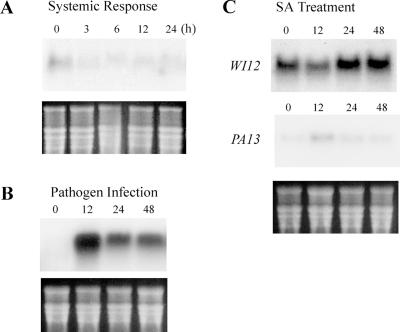
Because 1-month-old leaves have the highest level of WI12 expression by wounding and MeJA treatment, they were used to examine other factors that commonly affect the expression of wound-induced genes. Lower parts of leaves were wounded and upper parts of leaves were sampled to examine the systemic response of WI12. Twenty-four hours after wounding the lower part, WI12 expression was not induced in the upper part of the young leaves, suggesting that the expression of WI12 is restricted to the locally damaged tissues (Fig. 2A). When leaves were inoculated with the spawn of Pestalotia sp., a pathogen known to infect ice plants (Farr et al., 1989), lesions appeared 12 h after inoculation, rapidly enlarged after 24 h, and were restricted after 48 h of infection. At the same time, the expression of WI12 increased to the maximum level at 12 h and continued to express 48 h after inoculation (Fig. 2B). The expression of WI12 was parallel to the development and restriction of necrosis spots. SA is another important signal transduction compound in plant defense mechanisms; therefore, we tested the effect of SA on the WI12 induction. The expression of WI12 in SA-treated leaves was so low that a large RNA sample (40 μg) and a prolonged exposure time (3 d) were required to detect the signal. During the 48 h of SA treatment, the expression of WI12 decreased after 12 h and returned to a steady level afterward (Fig. 2C). When the same blot was probed with PA13, an SA-inducible osmotin-like gene, there was an increase in PA13 expression after 12 h and then decreased afterward (Fig. 2C). The results show induction by pathogen infection and transient suppression by SA, whereas there is no apparent systemic response to wounding of WI12.
Figure 2.
Effects of systemic response, pathogen infection, and salicylic acid (SA) treatment on expression of WI12 in leaves of juvenile plants. A, For systemic response, basal leaves were injured and upper unwounded leaves of the same plant were collected from 0 to 24 h (nos. on the top). B, For pathogen infection, leaves were infected with pathogenic fungus Pestalotia sp. for 0 to 48 h. Ten micrograms of total RNA was separated by agarose-formaldehyde gel and the RNA gel blot was hybridized against a 32P-labeled WI12 probe. C, For SA treatment, 200 μm SA was soil applied for 0 to 48 h. Forty micrograms of total leaf RNA was separated by agarose-formaldehyde gel and the RNA gel blot was hybridized against a 32P-labeled WI12 or PA13 probe. A 3-d film exposure time was required to get a detectable signal on the blot. Bottom figure on each RNA gel blot is an EtBr-stained agarose gel to ensure equal loading of each lane.
Salt Stress Causes Dramatic Change of WI12 Expression at Juvenile Stage of Leaves
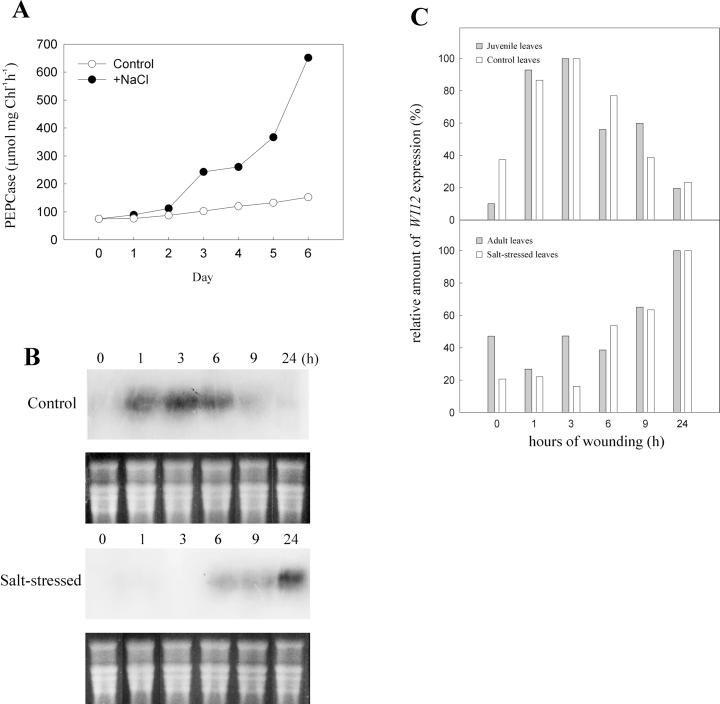
One-month-old ice plants are at the stage of competence for CAM induction; the addition of a high concentration of salt accelerates the transition. To test the effect of salt stress on WI12 expression, 1-month-old ice plants were irrigated with water (control) or 200 mm NaCl for a week. The induction of CAM was indicated by the increased activity of phosphoenolpyruvate carboxylase (PEPCase), the major carboxylation enzyme in the CAM cycle. When 1-month-old plants were irrigated with NaCl, the extractable activities of PEPCase increased, whereas in the control plants there was less increase of the PEPCase activity (Fig. 3A). The increase in PEPcase activity over a 1-week period was about 8-fold. Leaves from the control and 6-d-stressed plants were wounded and samples were collected at different time intervals. As shown in Figure 3B, although these leaves were at the same age, the response kinetics to wounding was different. The maximum expression of WI12 occurred after 3 h in the control leaves and was delayed to 24 h in salt-stressed leaves (Fig. 3B). Quantification of hybridization signals (Fig. 3C) showed the level of expression in the control treatment was very similar to that of the juvenile leaves shown in Figure 1A, whereas the level of expression in salt-stressed leaves was parallel to that of the adult leaves, shown in Figure 1B. The similarity of the temporal expression between adult leaves and stressed juvenile leaves indicates that it is the internal ontogenetic programming, not the actual age, that determines the time course induction of WI12 expression.
Figure 3.
Effect of salt stress on CAM induction and WI12 expression. Juvenile ice plants were irrigated with water (control) or 200 mm NaCl (salt stressed) for 1 week. A, For CAM induction, plants were treated with water (white circle) or 200 mm NaCl (black circle), leaf discs were collected every day, and the activity of PEPcase was measured (μmol mg Chl−1 h−1). B, For wounding treatment, leaves were collected from control or 7-d-stressed unwounded plants and wound-treated for 0 to 24 h (nos. on the top). Ten micrograms of total RNA isolated from each treatment was separated by agarose-formaldehyde gel. The RNA gel blot was hybridized against a 32P-labeled WI12 probe. Bottom figure on each RNA gel blot is an EtBr-stained agarose gel to ensure equal loading of each lane. C, The quantitative comparison of wound-induced WI12 mRNA level between adult leaves and salt-stressed juvenile leaves. Gray bars were relative amount of WI12 expression of juvenile and adult leaves shown in Figure 1, A and B. White bars were relative amount of WI12 mRNA of control and salt-stressed juvenile leaves shown in B. The density of each signal that appeared on the autoradiogram was measured and the highest value of each blot was set as 100%.
Tissue-Specific Expression, Accumulation, and Subcellular Localization of WI12
To facilitate the interpretation of the developmental regulation of WI12, the tissue-specific and stage-dependent expression of WI12 and localization of WI12 proteins were examined. Paraffin sections were taken from healthy, wounded, or MeJA-treated leaves, stems, and flowers. To verify whether the expression of the WI12 gene coincided with an accumulation of WI12 protein, in situ hybridization and immunostaining experiments were carried out on similar sets of sections. Sense and antisense probes for in situ hybridization were amplified from the open reading frame of WI12. The anti-WI12 antiserum used as the primary antibodies for immunostaining specifically recognized WI12 protein (Yen, 2000).
In the control experiments, very low background signals were detected in wounded leaves and stems when probed with sense RNA or pre-immune serum (Fig. 4, A–C). In unwounded leaves, when an antisense probe (Fig. 4D) or anti-WI12 serum (Fig. 4F) was used, low signals were detected in mesophyll and epidermal cells. A slight purple color appeared in the phloem cells. In unwounded stems, a strong constitutive expression of WI12 was associated with the phloem area (Fig. 4E). The result was consistent with the result from RNA gel blots that the expression of WI12 was low in healthy leaves and constitutive in the stems.
Figure 4.
Tissue-specific expression and protein accumulation of WI12 by wounding and MeJA in vegetative organs. Sections prepared from 1-month-old leaves and stems were wounded or MeJA treated as indicated in the bottom left of each figure. The pattern of WI12 mRNA was detected by in situ hybridization (left two panels) using digoxigenin-labeled sense WI12 RNA probe (A and B) or antisense WI12 RNA probe (D, E, G, H, J, and K), and detected by alkaline phosphatase-conjugated anti-DIG antibodies. The accumulation of WI12 protein was detected by immunostaining (right) using pre-immune serum (C) or anti-WI12 antiserum (F, I, and L), followed by alkaline phosphatase-conjugated anti-rabbit antibodies. Purple color developed, marked by small arrowheads, indicating the positions where WI12 expressed or accumulated. A similar pattern of WI12 expression was observed in wounded and MeJA-sprayed leaves. Ph, Phloem; Xy, xylem; M, mesophyll; Pi, pith; C, cortex; E, epidermis. Bars represent 200 μm in all figures.
When juvenile leaves were wounded for 6 h (Fig. 4G) or sprayed with MeJA for 12 h (Fig. 4J), a high level of WI12 expression was detected in all leaf tissues including the mesophyll, epidermal, and phloem cells. There is no apparent difference in the distribution of WI12 by wounding and MeJA. As for wound-induced WI12 protein accumulation, it accumulated in all parts of the wounded leaves, especially in the phloem region and in the discrete area of the epidermis (Fig. 4I). Phloem-specific expression of WI12 was observed in wounded (Fig. 4H) and MeJA-treated (Fig. 4K) stems and a low level of WI12 was detected in the epidermal cells or cortex parenchyma. Phloem-specific WI12 protein accumulation was also observed in wounded stems (Fig. 4L).
Tissue- and stage-specific distribution of WI12 in the floral organs of unwounded ice plants was also examined. There was a high level of WI12 expression in the epidermal cells of the petals (Fig. 5A) and in the transmitting tissues of the styles (Fig. 5C). The pattern of WI12 protein accumulation exactly matched its expression in petals (Fig. 5B) and styles (Fig. 5D). In developing ovaries, both WI12 gene expression (Fig. 5E) and protein accumulation (Fig. 5F) were detected in the placenta, the tissue to which the ovules are attached. In mature ovaries, the expression of WI12 in the placenta was even stronger (Fig. 5G) and consistent with the highest accumulation of WI12 protein, especially in the palisade-like cells located at the outermost layer of placenta (Fig. 5H). The results show that WI12 gradually accumulates in the placenta during development. Cell-specific expression of WI12 was also found in the formation of seeds. The expression of WI12 was detected in all parts of the developing seed (DS in Fig. 5E) and became stronger in the seed coat as the seeds became mature (MS in Fig. 5G). A “mature seed” is defined here by the appearance of a prominent cotyledon inside the seed prior to dehydration. The accumulation of WI12 protein in the developing seeds was also found in all parts of the seed (DS in Fig. 5F), and as the cotyledon developed, WI12 was preferentially accumulated in the cells surrounding the cotyledon (MS of Fig. 5H).
Figure 5.
Tissue-specific expression and protein accumulation of WI12 in floral organs of healthy ice plants. Sections of floral organs at defined stages were hybridized with DIG-labeled antisense WI12 RNA probe (left) or with anti-WI12 antiserum (right) and then detected by alkaline phosphatase-conjugated anti-DIG or anti-rabbit antibodies, respectively. A and B, Cross-sections of petals (Pt) and color developed in the epidermal cells are indicated by arrowheads. C and D, Sliver sections of styles (St) and color developed in the transmitting tissues are indicated by arrowheads. E and F, Cross-sections of developing ovary and color developed in the placenta (P) and developing seeds (DS) are indicated by arrowheads. G and H, Cross-sections of mature ovary with cotyledons developed in seeds. Signals are detected in the outer layer of placenta (P) and in mature seeds (MS). Bars represent 100 μm in all figures.
The cell-specific expression and accumulation of WI12 during development is shown in Figures 4 and 5, yet it is still unclear where WI12 proteins are located at the subcellular level. We prepared sections of placenta collected from a mature ovary, the stage with the highest amount of WI12, and used anti-WI12 serum for the subcellular localization of WI12. Along the palisade cells of the mature placenta, immunogold particles appeared mostly in the middle lamella of the cell wall and in the intercellular space (Fig. 6A). Some gold particles were also found inside the cells. The gold particles in the cytoplasm appeared to be randomly distributed and we were not able to judge if they were specifically associated with any organelles or endomembrane system (Fig. 6A). When similar sections were incubated with pre-immune serum, a low background of gold particle binding was detected (Fig. 6B). Gold particles were also observed in the primary cell wall of wounded mesophyll cells and phloem parenchyma cells, consistent with the results carried out by immunostaining (data not shown). Protein structure analyses and motif search predicted WI12 was a soluble protein with two potential glycosylation sites and a hydrophobic N-terminal signal peptide (Yen, 2000). This, combined with the results obtained by immunogold labeling, suggests that WI12 is synthesized and processed through the endoplasmic reticulum-Golgi system and eventually secreted into the apoplastic compartment.
Figure 6.
Subcellular localization of WI12 protein in placenta of the mature ovary by immunolocalization. Ultrathin sections obtained from placenta were labeled with anti-WI12 antiserum (A) or with pre-immune serum (B) and detected by gold-conjugated anti-rabbit antibodies. Arrowheads indicate accumulation of WI12 in the primary cell wall. Bars represent 1 μm in all figures. W, Cell wall; V, vacuole; N, nucleus.
DISCUSSION
CAM Induction Alters JA Signaling Pathway
WI12 and WUN1 not only have high similarity (68%) at the deduced amino acid level but also share similar expression patterns. WI12 cDNA was originally isolated from cultured ice plant cells (Yen et al., 1999), whereas WUN1 is expressed in suspension cells and isolated protoplasts of potato (Logemann et al., 1989). The response to wounding follows a similar trend in that the WI12 and WUN1 transcripts start to accumulate right after the tissues are injured and level off 24 h after wounding (Logemann et al., 1988). According to the time course progression of signal transduction pathway in response to wounding (Ryan, 2000) and the response kinetics of WI12 expression, WI12 should belong to the class of “defense gene” but is not involved in the signal pathway. Based on the model proposed by Farmer and Ryan (1992) and further extended by Creelman and Mullet (1995), JA acts as a critical signal molecule in the wound signal transduction pathways. Because both wounding and JA trigger WI12 expression at the same cellular locations (Fig. 4, G and J), we might expect that JA would mediate the wound signal in the expression of this defense gene. In other words, direct JA spraying should trigger WI12 expression more quickly than wounding. However, the temporal expression indicated that the wound-induced expression of WI12 in 1-month-old juvenile leaves was by far faster than JA treatment (Fig. 1A). The juvenile stage of ice plant used in Figure 1A was undergoing C3-CAM transition. Several lines of evidence revealed cross talk between the signaling pathway for CAM induction and the wound-induced JA-mediated pathway that caused the abnormal temporal expression of WI12.
First, the young ice plant switches its photosynthetic mode from C3 to CAM in response to developmental and environmental signals as well as to hormones ABA and cytokinins (Edwards et al., 1996). In the review written by Creelman and Mullet (1997), JA synthesis is affected by a list of developmental and environmental factors. Wounding and pathogen attacks produce transient increases in the concentration of endogenous JA, and as a result, many wound-induced genes are turned on. Exogenous application of JA regulates these genes in a similar way (Sembdner and Parthier, 1993). In addition to wound-related biotic stresses, abiotic stresses such as drought (Mason and Mullet, 1990) and metabolic alteration such as depodding (Tranbarger et al., 1991) induce JA accumulation and JA-related gene expression. The interaction between biotic and abiotic stresses is thought to be mediated by ABA, ethylene, and cytokinin (Seo et al., 1997; Wasternack and Parthier, 1997). Therefore, JA-induced expression of WI12 might be complex, with certain environmental and developmental signals for CAM induction at the juvenile ice plant stage. The threshold concentrations for ABA, cytokinin, or other growth factors at the convergent point for CAM induction might cause an alteration of wound-signaling cascades.
Second, if the above-mentioned statement were correct, what would be the pattern of WI12 expression when CAM induction is complete? In 2-month-old adult leaves, in which CAM is fully operating, the induction of WI12 by MeJA treatment was faster than by wounding (Fig. 1B). The statement was further elucidated by using same-age plants with two photosynthetic modes (C3 or CAM). In 1-month-old juvenile leaves, in which CAM was induced by salt stress, the response kinetics of WI12 to wounding is similar to that of adult leaves (Fig. 3). Therefore, it is the transition that occurs at a specific stage of ice plant development that causes the alteration of the wound signal pathway.
In addition to the acceleration of developmental competence from juvenile to adult, high level of Na+ may have a direct effect on the expression of this cell wall protein. Salt-induced changes in cell wall composition in salt-adapted tobacco (Nicotiana tabacum L.) suspension cells have been reported (Iraki et al., 1989). We have tried to use cultured ice plant cells to clarify these two possibilities because the salt-induced changes of cell wall protein accumulation has been reported in these cultured cells (Yen et al., 1994, 1997). When increased concentrations of NaCl were added to the culture medium, there was not any significant change of WI12 expression (Yen et al., 1999). These halophytic cells were unable to perform CAM in response to salinity or ABA (Yen et al., 1995) even though they grew well in NaCl medium. The direct salt effect on WI12 expression may require an organized tissue to be displayed, just like the operation of CAM (Adams et al., 1992).
Another possible interpretation for the differences in response kinetics is that wounding and MeJA might indicate two separate pathways for inducing WI12 in ice plants. Several reports recently showed that certain JA-independent wound-induced genes were related to defense mechanisms (Titarenko et al., 1997; Leon et al., 1998; Royo et al., 1999). Royo et al. (1999) reported that exogenous JA failed to recover pin2 mRNA levels in LOX-H3-deficient plants, indicating that another signal transduction pathway triggered pin2 expression. Therefore, wounding might induce an alternative signaling pathway for WI12 gene expression at certain developmental stages. SA, another wound signal compound (Seo et al., 1997), was unable to induce WI12 gene expression (Fig. 2C). More experiments must be performed, especially involving the relationship between JA and other growth regulators such as ABA, ethylene, and cytokinin, to support this point of view.
Suggested Roles for WI12
The results of northern analysis and tissue-specific localization showed WI12 genes were expressed and accumulated in the epidermis and phloem of leaves by environmental factors including wounding, MeJA, and fungal pathogens. The expression of WI12 (Fig. 1) and WUN1 (Logemann and Schell, 1989) in unwounded mature leaves is much higher than in young leaves, suggesting both genes are also developmentally regulated. Furthermore, WI12 was constitutively expressed in the reproductive organs of ice plants (Fig. 5), whereas the expression of WUN1 was also noticed in the anthers and pollen grains (Siebertz et al., 1989). A similar pattern has been demonstrated in pin2, gene for proteinase inhibitor II, one of the best studied defense genes (Peña-Cortés et al., 1991; Wingate et al., 1991). The expression of the proteinase inhibitor II gene family is both developmentally and environmentally regulated and different signals are involved in its regulation (Lorberth et al., 1992). Other wound-induced defense genes, such as hmg (Choi et al., 1992) and Aco (Titarenko et al., 1997), were also constitutively expressed in the reproductive organs. It has been proposed that these genes can play a protective role against pest and pathogen infection in the reproductive organs (Peña-Cortés et al., 1991).
Three WI12-like expressed sequence tag sequences of ice plant were reported recently (accession nos. BE035598, BE036967, and AI861017). The deduced amino acid sequences all have high identity (95%–99%) to WI12, but the 5′- or 3′-untranslated sequences vary. Southern analysis also showed that there are at least two WI12-like genes in the ice plant genome (Yen, 2000). Using gene-specific probe designed from the 3′-untranslated region of WI12, we have found that the wound-induced pattern of expression exactly matches the result showed in Figure 1A. However, opposite from the constitutive expression in stem (Fig. 1C), very weak signal was detected in stem using WI12-specific probe (S.-K. Yen and H.E. Yen, unpublished data). Therefore, various environmental stimuli and developmental factors might differentially affect the expression of different members of WI12 gene family in an organ- or cell-specific manner. Future work will be directed toward the understanding of the expression pattern of each member of this WI12 gene family.
Overall, the results of in situ hybridization were mostly consistent with the results obtained by immunostaining, with one exception. At the later stage of seed development, the different distribution of WI12 mRNA (Fig. 5G) and protein (Fig. 5H), i.e. seed coat versus cotyledon, was evident. This result suggested that the WI12 gene is regulated at translational and/or posttranslational levels during seed development. The different distribution of mRNA and protein may also be explained by the extracellular localization of WI12. At the later stage of the seed development, the cells around the seed coat synthesized WI12, then transported apoplastically to the lumen surrounding the cotyledon to protect the young seedlings once germinated.
Most known wound-induced defense systems respond to systemic signals (Peña-Cortés et al., 1988); however, the expression of WI12 was restricted to the wounded site. Only two mechanisms were known to be involved in the local defense systems and interestingly, both of them operated in the cell wall region. First, the accumulation of Hyp-rich glycoproteins was found to reinforce the structure of cell wall after wounding and pathogen attack (Lawton and Lamb, 1987; Kawalleck et al., 1995). WI12 is similar to Hyp-rich glycoproteins in that both of them are cell wall localized and have phloem-specific induction by wounding. Second, wound-induced callose formation has been suggested as a healing mechanism by producing physical barriers at the injured site (Hahlbrock and Scheel, 1987). In wounded tobacco leaves, callose was accumulated, locally but not systemically, in the epidermal cells and vascular tissues. Logemann and Schell (1989) pointed out that the wound-induced cell-specific expression of WUN1- GUS in transgenic tobacco fits the localization of wound-induced callose. We also observed a massive accumulation of callose in the mesophyll cells and the phloem tissue upon wounding (Yen, 2000). The possible involvement of WI12 in the change of cell wall structure and formation of callose is under investigation.
In conclusion, the expression of WI12, the first wound-induced gene found in ice plants, is controlled by both environmental (wounding stimuli) and developmental (stage- and organ-specific) factors. The wound-induced JA-signaled WI12 gene expression is complicated by the onset of CAM induction in juvenile leaves of ice plants. The extracellular localization and epidermis- and phloem-specific accumulation suggests that WI12 is actively involved in the modification of the cell wall structure at the site of injury.
MATERIALS AND METHODS
Plant Growth Conditions and Treatments
Ice plants (Mesembransemum crystallinum) were grown in mixed soil (humus:vermiculite:sand, 3:1:1, v/v) in a growth chamber with a 16-h-light (800 μmol quanta m−2 s−1)/8-h-dark period at temperature of 30°C/18°C. One-month-old ice plants that are on set of CAM transition are referred as “juvenile” plants and 2-month-old CAM form plants are referred as “adult” plants in the text. Healthy ice plants were wounded mechanically by cutting to 5-mm pieces and laying on a few layers of damp paper towel under continuous illumination (100 μmol quanta m−2 s−1) at room temperature. To examine the systemic response, the lower part of a young leaf was scratched with a toothpick and the unwounded upper-part leaves in the same plant were sampled. For pathogen infection, actively growing hyphae of Pestalotia sp. maintained in potato dextrose agar were cut into 1-cm2 agar blocks, directly contacted with leaf surface, and kept at 100% (v/v) relative humidity until the end of the experiment. MeJA treatments were done by foliar spraying with 200 μL L−1 MeJA emulsified in 0.02% (v/v) Tween 20 by sonication. SA treatments were done by applying 200 μm SA (adjusted to pH 7) directly to soil. For MeJA and SA treatments, each plant was sampled for only one time point to avoid induction of WI12 upon cutting off the leaves for sampling. At each time point, samples were collected from three or four separated plants and directly frozen in liquid nitrogen.
RNA Gel-Blot Analysis
RNA was extracted from different parts of plants by the phenol-LiCl method (Logemann et al., 1987). Ten micrograms of total RNA from different treatments was loaded on a formaldehyde-agarose gel and blotted to a nylon membrane (Genescreen Plus, NEN Life Science Products, Inc., Boston) according to Sambrook et al. (1989). Blots were hybridized with 32P-labeled WI12 probes (by rediprime II, Amersham Pharmacia Biotech, Buckinghamshire, UK) at 65°C overnight, sequentially washed twice in 2× SSC for 15 min at room temperature, 2× SSC, 1% (w/v) SDS for 30 min at 65°C, and 0.1× SSC for 15 min at room temperature. Finally, the hybridization signals were detected by x-ray film exposure (BioMax film, Eastman Kodak Co., New Haven, CT). RNA gel blotting data presented in this article were all established using at least two separate sets of RNA samples and only one representative of the patterns was shown.
DNA and Protein Sequences Analysis
The amino acid sequence of WI12 was predicted and analyzed using the DNASTAR software. The sequence comparison was done using the BLAST network service (National Institutes of Health, Bethesda, MD) and GCG program (Genetic Computer Group, Madison, WI). Analyses of protein structure and signal peptides were performed by the Motif Search program on the GenomeNet World Wide Web server on the Internet.
Sample Preparation for Cytochemical Analyses
Tissue of ice plant was cut into small pieces and immediately fixed in 4% (w/v) paraformaldehyde, 0.25% (w/v) glutaraldehyde, and 0.01% (w/v) Tween 20 in 0.1 m sodium phosphate buffer, pH 7.0, at 4°C for 16 h under vacuum. The tissues were then dehydrated in a graded alcohol series (30%–100%, w/v) and embedded in paraffin for light microscopic observation or in LR White (London Resin Company, Berkshire, UK) for transmission electron microscope. The embedded tissues were sectioned in microtones and affixed to poly-l-Lys-coated slides (6–10 μm) or formvar-coated nickel grids (70 nm). The open reading frame of WI12 was amplified by PCR and cloned to a pGEM-T EazyVector system (Promega, Madison, WI). Antisense and sense RNA probes were synthesized by a SP6/T7 digoxigenin RNA labeling kit (Boehringer Mannheim, Mannheim, Germany). Recombinant WI12 protein was produced by overexpression in E. coli using pET29a vector (Novagen, Madison, WI) as described by Yen (2000). WI12 was first purified using His-tagged resin (Novagen), separated by SDS-PAGE, and the gel slices containing WI12 were collected. Rabbit polyclonal antiserum was prepared against purified recombinant WI12 protein by the Institute of Botany, Academia Sinica.
In Situ Hybridization
Procedure for in situ hybridization was based on Coen et al. (1990) with certain modifications. Hybridization was performed in a humid chamber at 37°C overnight in 1× salt (0.3 m NaCl, 10 mm Tris-HCl [pH 6.8], 10 mm NaH2PO4/Na2HPO4, and 5 mm EDTA), 50% (w/v) formamide, 10% (w/v) dextran sulfate, 1× Denhardt's solution (10 mg mL−1 bovine serum albumin [BSA], 0.02% [w/v] Ficoll 400, and 0.02% [w/v] polyvinylpyrrolidone), 1 mg mL−1 tRNA (Boehringer Mannheim), and 1 ng μL−1 probes. After hybridization, sections were first treated with RNase (20 μg mL−1) at 37°C for 30 min and washed with 2× SSC for 30 min at 25°C, then a stringency wash in 0.1× SSC for 1 h at 57°C. Hybrids were detected using the digoxigenin nucleic acid detection kit (Boehringer Mannheim) at 25°C for 24 h. Slides were air dried, mounted, and photographed by a light microscope (BX 50, Olympus Optical Co., LTD, Tokyo).
Immunostaining
For immunostaining, 6- to 10-μm paraffin sections were first blocked in 0.5% (w/v) blocking reagent (Boehringer Mannheim) 37°C for 1 h, followed by hybridizing with either pre-immune or immune anti-WI12 serum at a 1:250 dilution at 37°C for 1 h. After four times in a 10-min wash in 150 mm NaCl, 100 mm Tris-HCl, pH 7.5, sections were then reacted 1 h with alkaline-phosphatase-conjugated goat anti-rabbit IgG secondary antibody (1:500 dilution, Jackson Immumoresearch, West Grove, PA). The signal was detected by nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate solution (Boehringer Mannheim) for 10 min to 4 h in the dark.
Immunolocalization
For immunolocalization, ultrathin sections (70 nm) on formvar-coated nickel grid were floated with the tissue-containing side downward on droplet of blocking solution (3% [w/v] n-goat serum, 0.2% [w/v] gelatin, 0.5% [w/v] BSA, and 0.05% [w/v] Tween 20 in phosphate-buffered saline) for 10 min. The sections were then incubated for 1 h with anti-WI12 antiserum (1:100 dilution) in a diluted blocking solution (1% [w/v] n-goat serum, 0.1% [w/v] gelatin, 0.3% [w/v] BSA, and 0.05% [w/v] Tween 20 in phosphate-buffered saline). After washing five times in distilled, deionized water, sections were incubated for 30 min with a protein A-gold conjugate (12 nm) diluted 1:40. The sections were post-stained for 20 min with 2.5% (w/v) uranyl acetate and lead citrate (80.3 mm lead nitrate, 136.4 mm sodium citrate, and 0.16 n NaOH) for 10 min. Sections were examined using transmission electron microscopy (CM100, Philips Electron Optics, Eindhoven, The Netherlands).
ACKNOWLEDGMENTS
We thank Dr. Kuo-Chin Tzeng (Department of Plant Pathology, National Chung-Hsing University, Taichung, Taiwan) for providing various fungal pathogens. We are also grateful to Dr. Tony H.H. Chen (Department of Horticulture, Oregon State University, Corvallis) for providing the potato PA13 cDNA clone.
Footnotes
This work was supported by the National Science Council of Taiwan (grant no. NSC 89–2311–B005–023 to H.E.Y.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010205.
LITERATURE CITED
- Adams P, Nelson DE, Yamada S, Chmara W, Jensen RG, Bohnert HJ, Griffiths H. Growth and development of Mesembryanthemum crystallinum(Aizoaceae) New Phytol. 1998;138:171–190. doi: 10.1046/j.1469-8137.1998.00111.x. [DOI] [PubMed] [Google Scholar]
- Adams P, Thomas JC, Vernon DM, Bohnert HJ, Jensen RG. Distinct cellular and organismic responses to salt stress. Plant Cell Physiol. 1992;33:1215–1223. [Google Scholar]
- Bohnert HJ, Nelson DE, Jensen RG. Adaptations to environmental stresses. Plant Cell. 1995;7:1099–1111. doi: 10.1105/tpc.7.7.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert HJ, Ostrem JA, Cushman JC, Michalowski CB, Rickers J, Meyer G, DeRocher EJ, Vernon DM, Krueger M, Vazquez-Moreno L. Mesembryanthemum crystallinum, a higher plant model for the study of environmentally induced changes in gene expression. Plant Mol Biol Rep. 1988;6:10–28. [Google Scholar]
- Bowler C, Fluhr R. The role of calcium and activated oxygens as signals for controlling cross-tolerance. Trends Plant Sci. 2000;5:241–246. doi: 10.1016/s1360-1385(00)01628-9. [DOI] [PubMed] [Google Scholar]
- Bowles DJ. Defense-related proteins in higher plants. Annu Rev Biochem. 1990;59:873–907. doi: 10.1146/annurev.bi.59.070190.004301. [DOI] [PubMed] [Google Scholar]
- Choi D, Ward BL, Bostock RM. Differential induction and suppression of potato 3-hydroxy-3-methylglutaryl coenzyme A reductase genes in response to Phytophthora infestansand to its elicitor arachidonic acid. Plant Cell. 1992;4:1333–1344. doi: 10.1105/tpc.4.10.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C, Dai Z, Ku MSB, Edwards GE. Induction of Crassulacean acid metabolism in the facultative halophyte Mesembryanthemum crystallinumby abscisic acid. Plant Physiol. 1990;93:1253–1260. doi: 10.1104/pp.93.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen ES, Romero JM, Doyle S, Elliot R, Murphy G, Carpenter R. Floricaula: a homeotic gene required for flower development in Antirrhinum majus. Cell. 1990;63:1311–1322. doi: 10.1016/0092-8674(90)90426-f. [DOI] [PubMed] [Google Scholar]
- Creelman RA, Mullet JE. Jasmonic acid distribution and action in plants: regulation during development and response to biotic and abiotic stress. Proc Natl Acad Sci USA. 1995;92:4114–4119. doi: 10.1073/pnas.92.10.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman RA, Mullet JE. Biosynthesis and action of jasmonates in plants. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:355–381. doi: 10.1146/annurev.arplant.48.1.355. [DOI] [PubMed] [Google Scholar]
- Cushman JC, Michalowaki CB, Bohnert HJ. Developmental control of Crassulacean acid metabolism inducibility by salt stress in the common ice plant. Plant Physiol. 1990;94:1137–1142. doi: 10.1104/pp.94.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z, Ku MSB, Zhang D, Edwards GE. Effects of growth regulators on the induction of Crassulacean acid metabolism in the facultative halophyte Mesembryanthemum crystallinumL. Planta. 1994;192:287–294. [Google Scholar]
- Edwards GE, Dai Z, Cheng S-H, Ku MSB. Factors effecting the induction of Crassulacean acid metabolism in Mesembryanthemum crystallinum. In: Winter K, Smith JAC, editors. Crassulacean Acid Metabolism: Biochemistry, Ecophysiology and Evolution: Ecological Studies. Vol. 114. Berlin: Springer-Verlag; 1996. pp. 119–134. [Google Scholar]
- Farmer EE, Ryan CA. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci USA. 1990;87:7713–7716. doi: 10.1073/pnas.87.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer EE, Ryan CA. Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors. Plant Cell. 1992;4:129–134. doi: 10.1105/tpc.4.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr DF, Bills GF, Chamuris GP, Rossman AY. Fungi: On Plants and Plant Products in the United States. APS Press, American Phytopathological Society, St. Paul, Minnesota. 1989. p. 20. [Google Scholar]
- Graham JS, Hall G, Pearce G, Ryan CA. Regulation of synthesis of proteinase inhibitors I and II mRNAs in leaves of wounded tomato plants. Planta. 1986;169:399–405. doi: 10.1007/BF00392137. [DOI] [PubMed] [Google Scholar]
- Hahlbrock K, Scheel D. Biochemical response of plants to pathogens. In: Chet I, editor. Offprints from Innovative Approaches to Plant Disease Control. New York: John Wiley and Sons; 1987. pp. 229–254. [Google Scholar]
- Iraki NM, Bressan RA, Carpita NC. Extracellular polysacchrides and proteins of tobacco cell cultures and changes in composition associated with growth-limiting adaptation to water and saline stress. Plant Physiol. 1989;91:54–61. doi: 10.1104/pp.91.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawalleck P, Schmelzer E, Hahlbrock K, Somssich IE. Two pathogen-responsive genes in parsley encode a tyrosine-rich hydroxyproline-rich glycoprotein (HRGP) and an anionic peroxidase. Mol Gen Genet. 1995;247:444–452. doi: 10.1007/BF00293146. [DOI] [PubMed] [Google Scholar]
- Koiwa H, Bressan RA, Hasegawa PM. Regulation of protease inhibitors and plant defense. Trends Plant Sci. 1997;2:379–384. [Google Scholar]
- Lawton MA, Lamb CJ. Transcriptional activation of plant defense genes by fungal elicitor, wounding, and infection. Mol Cell Biol. 1987;7:335–341. doi: 10.1128/mcb.7.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon J, Rojo E, Titarenko E, Sánchez-Serrano JJ. Jasmonic acid-dependent and -independent wound signal transduction pathways are differentially regulated by Ca2+/calmodulin in Arabidopsis thaliana. Mol Gen Genet. 1998;258:412–419. doi: 10.1007/s004380050749. [DOI] [PubMed] [Google Scholar]
- Logemann J, Lipphardt S, Lörz H, Häuser I, Willmitzer L, Schell J. 5′ upstream sequences from the wun1gene are responsible for gene activation by wounding in transgenic plants. Plant Cell. 1989;1:151–158. doi: 10.1105/tpc.1.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logemann J, Mayer JE, Schell J, Willmitzer L. Differential expression of genes in potato tubers after wounding. Proc Natl Acad Sci USA. 1988;85:1136–1140. doi: 10.1073/pnas.85.4.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logemann J, Schell J. Nucleotide sequence and regulated expression of a wound-induced potato gene (wun1) Mol Gen Genet. 1989;219:81–88. doi: 10.1007/BF00261161. [DOI] [PubMed] [Google Scholar]
- Logemann J, Schell J, Willmitzer L. Improved method for the isolation of RNA from plant tissues. Anal Biochem. 1987;163:16–20. doi: 10.1016/0003-2697(87)90086-8. [DOI] [PubMed] [Google Scholar]
- Lorberth R, Dammann C, Ebneth M, Amati S, Sánchez-Serrano JJ. Promoter elements involved in environmental and developmental control of potato proteinase inhibitor II expression. Plant J. 1992;2:477–486. [PubMed] [Google Scholar]
- Mason HS, Mullet JE. Expression of two soybean vegetative storage protein genes during development and in response to water deficit, wounding and jasmonic acid. Plant Cell. 1990;2:569–579. doi: 10.1105/tpc.2.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell PJ, Calvert C, Atzorn R, Wasternack C, Leyser HMO, Bowles DJ. Ethylene as a signal mediating the wound response of tomato plants. Science. 1996;274:1914–1917. doi: 10.1126/science.274.5294.1914. [DOI] [PubMed] [Google Scholar]
- Peña-Cortés H, Sánchez-Serrano JJ, Rocha-Soca M, Willmitzer L. Systemic induction of proteinase inhibitor II gene expression in potato plants by wounding. Planta. 1988;174:84–89. doi: 10.1007/BF00394877. [DOI] [PubMed] [Google Scholar]
- Peña-Cortés H, Willmiter L, Sánchez-Serrano JJ. Abscisic acid mediates wound induction but not developmental-specific expression of the proteinase inhibitor II gene family. Plant Cell. 1991;3:963–972. doi: 10.1105/tpc.3.9.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royo J, Leon J, Vancanneyt G, Albar JP, Rosahl S, Ortego F, Castanera P, Sánchez-Serrano JJ. Antisense-mediated depletion of a potato lipoxygenase reduces wound induction of proteinase inhibitors and increases weight gain of insect pests. Proc Natl Acad Sci USA. 1999;96:1146–1151. doi: 10.1073/pnas.96.3.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan CA. The systemic signaling pathway: differential activation of plant defensive genes. Biochim Biophys Acta. 2000;1477:112–121. doi: 10.1016/s0167-4838(99)00269-1. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. New York: Cold Spring Harbor; 1989. [Google Scholar]
- Sano H, Seo S, Koizumi N, Niki T, Iwamura H, Ohashi Y. Regulation by cytokinins of endogenous levels of jasmonic and salicylic acids in mechanically wounded tobacco plants. Plant Cell Physiol. 1996;37:762–769. [Google Scholar]
- Sembdner G, Parthier B. The biochemistry and the physiological and molecular actions of jasmonates. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:569–589. [Google Scholar]
- Seo S, Okamoto M, Seto H, Ishizuka K, Sano H, Ohashi Y. Tobacco MAP kinase: a possible mediator in wound signal transduction pathways. Science. 1995;270:1988–1992. doi: 10.1126/science.270.5244.1988. [DOI] [PubMed] [Google Scholar]
- Seo S, Sano H, Ohashi Y. Jasmonic acid in wound signal transduction pathways. Physiol Plant. 1997;101:740–745. [Google Scholar]
- Siebertz B, Logemann J, Willmitzer L, Schell J. cis-analysis of the wound-inducible promoter WUN1in transgenic tobacco plants and histochemical localization of its expression. Plant Cell. 1989;1:961–968. doi: 10.1105/tpc.1.10.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JC, Bohnert HJ. Salt stress perception and plant growth regulators in the halophyte Mesembryanthemum crystallinum. Plant Physiol. 1993;103:1299–1304. doi: 10.1104/pp.103.4.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titarenko E, Rojo E, León J, Sánchez-Serrano JJ. Jasmonic acid-dependent and -independent signaling pathways control wound-induced gene activation in Arabidopsis thaliana. Plant Physiol. 1997;115:817–826. doi: 10.1104/pp.115.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranbarger TJ, Franceschi VR, Hildbrand DF, Grimes HD. The soybean 94-kilodalton vegetative storage protein is a lipoxygenase that is localized in paraveinal mesophyll cell vacuoles. Plant Cell. 1991;3:973–987. doi: 10.1105/tpc.3.9.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C, Parthier B. Jasmonate-signaled plant gene expression. Trends Plant Sci. 1997;2:302–307. [Google Scholar]
- Wingate VPM, Franceschi VR, Ryan CA. Tissue and cellular localization of proteinase inhibitors I and II on the fruit of the wild tomato Lycopersicon peruvianum(L.) Mill. Plant Physiol. 1991;97:490–495. doi: 10.1104/pp.97.2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen HE, Edwards GE, Grimes HD. Characterization of a salt-responsive 24-kilodalton glycoprotein in Mesembryanthemum crystallinum. Plant Physiol. 1994;105:1179–1187. doi: 10.1104/pp.105.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen HE, Grimes HD, Edwards GE. The effects of high salinity, water-deficit, and abscisic acid on phosphoenolpyruvate carboxylase activity and proline accumulation in Mesembryanthemum crystallinumcell cultures. J Plant Physiol. 1995;145:557–564. [Google Scholar]
- Yen HE, Zhang D, Lin J-H, Edwards GE, Ku MSB. Salt-induced changes in protein composition in light-grown callus of M. crystallinum. Physiol Plant. 1997;101:526–532. [Google Scholar]
- Yen S-K. Tissue-specific characterization of a wound-induced protein WI12 in Mesembryanthemum crystallinum. PhD thesis. Taichung, Taiwan: National Chung-Hsing University; 2000. [Google Scholar]
- Yen S-K, Chen P-C, Yen HE. Cloning of a wound-induced gene WI12 from Mesembryanthemum crystallinum (PGR 99-030) Plant Physiol. 1999;119:1147. [Google Scholar]