Abstract
Background
Social determinants of health (SDOHs) may impact outcomes of patients with high-grade glioma (HGG). We examined the effect of rurality and socioeconomic status on treatment and survival of patients with newly diagnosed HGG.
Methods
This retrospective analysis used 2 cohorts from the Utah Cancer Registry, including all patients diagnosed with HGG (2000-2019) (cohort 1) and all HGG patients who underwent surgery (2000-2020) (cohort 2). Patient demographics were evaluated descriptively. Kaplan-Meier curves, log-rank tests, and multivariable Cox regression analysis were used for survival analyses.
Results
Patients from cohort 1 living in Frontier (n = 60), Rural (n = 363), and Urban (n = 1502) Utah traveled mean distances of 95.9 ± 69.7, 50.0 ± 66.5, and 10.7 ± 11.3 miles, respectively, for treatment (P < .001). Urban patients were diagnosed at a younger age (54.2 ± 20.2 years) than patients from Frontier (56.8 ± 20.3 years) and Rural (57.3 ± 17.6 years) areas (P < .019). Frontier patients were more likely to have lower income than Urban patients (30.7% vs 65.5%, P < .001). Rural patients (13.0 months [95% CI 11.2-14.8]) experienced shorter median survival than Urban patients (16 months [95% CI 14.3-17.7]) (P = .049). Among patients undergoing surgery, those with private insurance (54.2%, P < .001) and in high-income (60.2%, P = .007) and socioeconomic (60.4%, P = .005) quartiles received adjuvant chemotherapy and radiation more frequently.
Conclusion
HGG patients from Frontier and Rural counties in Utah encountered greater SDOH barriers without experiencing delays in resection. Rural patients had shorter survival than Urban patients. Further investigation is needed to determine whether additional SDOHs (income and insurance status) may intersect and contribute to shorter survival and less access to adjuvant therapy.
Keywords: healthcare disparities, high-grade glioma, social determinants of health, socioeconomic factors, tumor
Key Points.
HGG patients from Frontier Utah experience greater socioeconomic barriers without experiencing poorer overall survival.
HGG patients of lower socioeconomic status experienced significantly lower access to adjuvant therapies after resection.
Importance of the Study.
This study explores the impact of several social determinants of health (SDOHs), namely rurality, poverty, and insurance status, on high-grade glioma (HGG) outcomes in Utah. By examining how factors like geographic location and socioeconomic status affect treatment accessibility and survival, the research highlights disparities in healthcare delivery for Rural populations. Importantly, although the study found no significant differences in overall survival across population densities, it did reveal evidence that patients who reside in lower socioeconomic areas, which are often Frontier and Rural regions, face significant challenges in accessing adjuvant therapies, which indeed may affect long-term survival. These insights underscore the need for improved healthcare access and tailored interventions for underserved communities to ensure equitable care for all HGG patients.
Social determinants of health (SDOHs) are the social, economic, and political factors (eg, income, education level, employment status, and neighborhood conditions) that contribute to a person’s overall state of well-being.1 The unequal distribution of these determinants may be a product of racial, ethnic, and socioeconomic factors that lead to unfair but avoidable health disparities.
Several SDOHs have been shown to affect outcomes in adult patients with high-grade glioma (HGG) (eg, insurance type, age, and socioeconomic status).2,3 The literature has established that non-white patients and those from lower socioeconomic backgrounds experience longer hospital stays, lower rates of home discharge, and reduced overall survival (OS).2,4,5 Sociodemographic factors such as education and income influence a patient’s treatment course both before and after their diagnosis.6
Rurality (how populated and how far a patient’s primary residence is from metropolitan areas) is a potential prognostic factor in primary central nervous system (CNS) tumors, because population density significantly affects outcomes for patients with HGG.7,8 Stockdill et al7 suggested that patients in Urban areas with primary brain tumors experience shorter symptom duration before diagnosis and faster treatment initiation compared with patients in Rural or Frontier settings. Best et al8 also observed longer survival among patients with glioma who were from metropolitan communities compared with patients from non-metropolitan communities. Our previous research found that there are disparities in clinical trial enrollment for patients with glioma in Utah—patients from Rural and Frontier counties were underrepresented but had survival equivalent with patients from Urban counties across nearly all glioma types, including glioblastomas (GBMs).9 These results highlight the importance of improving recruitment in underrepresented regions to deliver equitable care for all patients.9
We aimed to examine the association of population density, particularly rurality, with glioma treatment accessibility and adjuvant therapy patterns in Utah. Understanding the nuances in SDOHs at statewide or region-wide levels is crucial to developing actionable solutions to equitable care, because predominant SDOH characteristics vary across communities and, therefore, solutions to health equity should vary accordingly. Our analysis may provide patients with a better understanding of how SDOHs affect not only their operative course but also their long-term postoperative treatment needs. This is of critical importance because adjuvant therapy contributes to long-term patient care in HGGs and should not be overlooked when considering access to surgery. We hypothesized that patients experiencing SDOH barriers, such as living far from surgical centers, lacking high-quality insurance, or facing poverty, may have challenges in undergoing timely resection and receiving long-term postoperative treatment, which may impact overall outcomes.
Methods
Patient Populations
We performed a retrospective analysis of the Utah Cancer Registry (UCR) to evaluate all patients with newly diagnosed HGG in Utah from 2000 through 2020. The UCR collects and organizes surveillance and epidemiologic data for all patients diagnosed with cancer in Utah.9 The UCR subsequently submits these data to the Surveillance, Epidemiology, and End Results Database, a resource from the National Cancer Institute (NCI) that provides information on cancer incidence and survival in the United States.10
Two cohorts of patients were defined in the study. Cohort 1 included all patients diagnosed with HGG in Utah from January 2000 through December 2019 (Figure 1). Patients were identified by International Classification of Disease Oncology codes (9400/3, 9420/3, 9410/3, 9411/3, 9450/3, 9382/3, 9401/3, 9440/3, 9441/3, 9442/3, 9451/3, and 9380/3). We excluded patients without Utah zip codes as their primary residence. This cohort included a total of 1925 patients. We received Institutional Review Board approval for cohort 1 with a waiver of informed consent through the University of Utah (IRB #00176754). Cohort 2 included an update of all patients who underwent primary resection for HGG in Utah from January 2000 through December 2020 with additional variables of interest, namely 2 different measures of SDOHs (Yost Index and Rural-Urban Communicating Area (RUCA) code) (IRB #00166210 including waiver of consent). This cohort comprised 2264 patients. Cohort 2 includes an extra year of data because this cohort was accessed after 2020 data were submitted to the UCR (vs before, as was the case in cohort 1). Although duplicate patient records naturally exist, patients are not cross-referenced across datasets, and the cohorts are treated as mutually exclusive.
Figure 1.
Utah county population classifications. Utah counties classified by rurality using county population density estimates (July 1, 2021) and total county area.11 Frontier counties are indicated in light shades (<6 people/mi2); Rural counties are indicated in middle shades (6-99 people/mi2); and Urban counties are indicated in dark shades (≥100 people/mi2).12 Star indicates University of Utah, Salt Lake City, Utah, USA.9
Geographic Classification
Patients in cohort 1 were separated into 3 groups—Frontier (<6 people/mi2), Rural (6-99 people/mi2), and Urban (≥100 people/mi2)—using the Utah Department of Health and Human Services population density classification system (Figure 1).12 Approximately 80% of Utah’s population resides in 5 Urban counties.11 Patients in cohort 2 were delineated using RUCA codes, which are defined by the US Department of Agriculture and US Department of Health and Human Services as a combination of population density, urbanization, and daily commuting.
Treatment and Outcomes
Patient demographics including age, sex, race, ethnicity, marital status, insurance status, poverty status (ie, the percentage of the population living below the poverty designation in the patient’s home community at the time of diagnosis), median household income, socioeconomic status (quantified with the Yost Index), and population density of home county were assessed.13
Statistics
Descriptive analyses were performed using IBM SPSS Statistics (V27.0) (Armonk, NY, USA), with P < .05 indicating significance. Chi-square and Fisher-Freeman-Halton exact tests were used to describe associations between categorical variables, and ANOVA was used to describe group differences. Kaplan-Meier curves and log-rank tests were used to illustrate and describe differences among the survival distributions from the date of diagnosis to the date of last known follow-up. Multivariable Cox regression was used to determine hazard ratios. Geographic maps were created using Microsoft Excel maps.
Results
HGG Patient Demographics
Of the 1925 patients diagnosed with HGG (Table 1), 60 (3%) resided in Frontier counties, 363 (19%) in Rural counties, and 1502 (78%) in Urban counties (Cohort 1). Overall, 57.6% of patients identified as male (P = .671), 97.6% identified as white (P = .459), and 93.5% identified as non-Hispanic (P = .675), with no differences among county designations. Patients diagnosed with HGG from Urban areas were diagnosed at a younger age (54.2 ± 20.2 years) than patients from Frontier (56.8 ± 20.3 years) and Rural (57.3 ± 17.6 years) areas (P = .019). Marital status did not differ among the county designations (P = .119). There was a significant difference in insurance status seen among counties, with 43.3% and 44.1% of patients using Medicare/Medicaid in Frontier and Rural counties, respectively, and 45.9% of patients using private insurance in Urban counties (P = .036).
Table 1.
Cohort 1: patient demographics
| Variable | Total (%) N = 1925 |
Frontier (%) N = 60 |
Rural (%) N = 363 |
Urban (%) N = 1502 |
P-value |
|---|---|---|---|---|---|
| Sex, male | 1108 (57.6) | 33 (55.0) | 221 (60.9) | 854 (56.9) | .671 |
| Race | .459b | ||||
| American Indian/Alaska Native | <11 | 0 | <11 | <11 | |
| Asian | 17 (0.9) | 0 | <11 | 16 (1.1) | |
| Pacific Islander | 11 (0.6) | <11 | <11 | <11 | |
| Black | <11 | 0 | <11 | <11 | |
| White | 1878 (97.6) | 59 (98.3) | 356 (98.1) | 1463 (97.4) | |
| More than one race | <11 | 0 | 0 | <11 | |
| Not listed | <11 | 0 | <11 | <11 | |
| Ethnicity | .675 | ||||
| Non-Hispanic | 1799 (93.5) | 56 (93.3) | 343 (94.5) | 1400 (93.2) | |
| Age at diagnosis (SD) | 54.9 (19.7) | 56.8 (20.3) | 57.3 (17.6) | 54.2 (20.2) | .019 |
| Marital status | .119b | ||||
| Single, never married | 310 (16.1) | 11 (18.3) | 43 (11.8) | 256 (17.0) | |
| Married | 1295 (67.3) | 41 (68.3) | 257 (70.8) | 997 (66.4) | |
| Single, previously married | 291 (15.1) | <11 | 54 (14.9) | 230 (15.3) | |
| Not listed | 29 (1.5) | <11 | <11 | 19 (1.3) | |
| Insurance status | .036 b | ||||
| Uninsured | 85 (4.4) | <11 | 15 (4.1) | 65 (4.3) | |
| Insurance, NOS | 63 (3.3) | <11 | 17 (4.7) | 45 (3.0) | |
| Private insurance | 844 (43.8) | 20 (33.3) | 134 (36.9) | 690 (45.9) | |
| Medicare and Medicaid | 766 (39.8) | 26 (43.3) | 160 (44.1) | 580 (38.6) | |
| Other government program | 49 (2.5) | <11 | 11 (3.0) | 35 (2.3) | |
| Not listed | 118 (6.1) | <11 | 26 (7.2) | 87 (5.8) | |
| Poverty indicator a | <.001 b | ||||
| <5% | 571 (29.7) | <11 | 58 (16.0) | 512 (34.1) | |
| 5%-10% | 616 (32.0) | 19 (31.7) | 114 (31.4) | 483 (32.2) | |
| 10%-20% | 486 (25.2) | 37 (61.7) | 137 (37.7) | 312 (20.8) | |
| >20% | 168 (8.7) | <11 | 38 (10.5) | 129 (8.6) | |
| Distance to hospital, mi | <.001 | ||||
| First facility (SD) | 20.7 (38.5) | 95.9 ± 69.7 | 50.0 ± 66.5 | 10.7 ± 11.3 |
Abbreviations: SD, standard deviation; NOS, not otherwise specified.
Bold values are significant.
Percentages of total number of patients within each population classification unless otherwise specified. Categorical variables compared with chi-square test. Continuous variables compared with ANOVA test. Categories with less than 11 patients are not explicitly listed to protect from possible patient identification.
aPercentage of a patient’s community who live at or below the poverty line.
bCategorical variables compared with Fisher-Freeman-Halton exact test.
Within Frontier counties, 66% of patients lived in communities with >10% of neighbors who lived at or below the poverty line. In contrast, within Urban counties, 34.1% of patients lived in communities with <5% of neighbors who lived at or below the poverty line (P < .001). Patients from Frontier counties traveled significantly farther to receive care (95.9 ± 69.7 miles) compared with patients in Rural (50.0 ± 66.5 miles) and Urban (10.7 ± 11.3 miles) counties (P < .001).
HGG Characteristics and Treatment
HGGs were diagnosed as GBMs in 71.7% (43/60) of patients living in Frontier counties, 81.2% (297/363) of patients living in Rural counties, and 73.0% (1096/1502) of patients living in Urban counties (P = .004) (Table 2). Overall, most patients with HGG (72.0%) underwent resection, and 57.6% of patients with HGG underwent resection with adjuvant chemotherapy and/or radiation, whereas 11.6% had nonsurgical treatment only and 15.3% had no treatment; no significant difference was observed across population density groups with regard to treatment courses. No significant difference in the length of time between diagnosis and surgery was observed in any group (P = .592).
Table 2.
Cohort 1: effect of population density on high-grade glioma characteristics and treatment
| Variable | Total (%) N = 1925 |
Frontier (%) N = 60 |
Rural (%) N = 363 |
Urban (%) N = 1502 |
P-value |
|---|---|---|---|---|---|
| Diagnosis | .004 a | ||||
| Glioma malignant, NOS | 202 (10.5) | 11 (18.3) | 23 (6.3) | 168 (11.2) | |
| Astrocytoma, anaplastic | 203 (10.5) | <11 | 27 (7.4) | 172 (11.5) | |
| Glioblastoma | 1436 (74.6) | 43 (71.7) | 297 (81.2) | 1096 (73.0) | |
| Oligodendroglioma, anaplastic | 84 (4.4) | <11 | 16 (4.4) | 66 (4.4) | |
| Treatment | |||||
| Surgery only | 278 (14.4) | <11 | 61 (16.8) | 208 (13.8) | .334a |
| Surgery with adjuvant treatment | 1108 (57.6) | 33 (55.0) | 212 (58.4) | 863 (57.5) | .872 |
| Non-surgical treatment only | 244 (11.6) | <11 | 36 (10.0) | 200 (13.3) | .208a |
| No treatment | 295 (15.3) | 10 (16.7) | 54 (14.9) | 231 (15.4) | .931 |
| Treatment time, months | |||||
| Diagnosis to surgery | 0.3 (1.4) | 0.5 (0.7) | 0.2 (0.6) | 0.3 (1.6) | .592 |
Abbreviation: NOS, not otherwise specified.
Bold values are significant.
Percentages given out of total number of patients within each population classification unless otherwise specified. Categorical variables compared with Chi-square test. Continuous variables compared with ANOVA test. Categories with less than 11 patients are not explicitly listed to protect patients from possible identification.
aCategorical variables compared with Fisher-Freeman-Halton exact test.
HGG Survival by Socioeconomic Factors
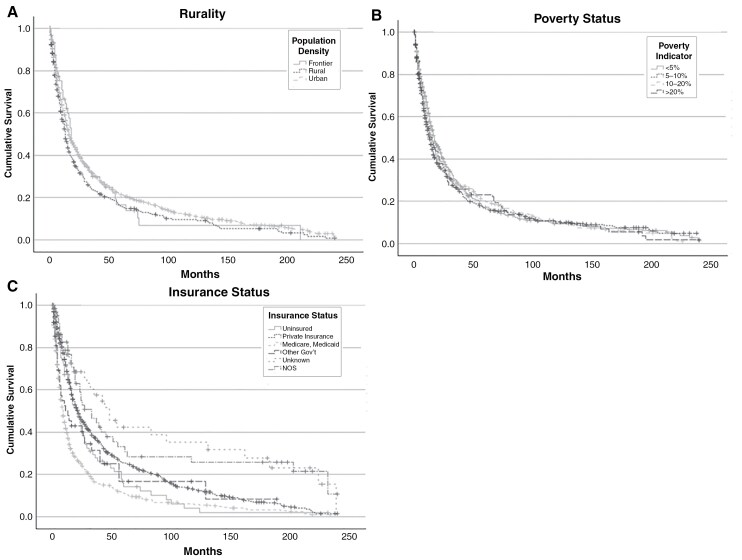
Rurality was significantly associated with OS (P = .049), with Frontier patients having a median OS of 18.0 months (95% CI 13.8-22.2), Rural patients having 13.0 months (95% CI 11.2-14.8), and Urban patients having 16.0 months (95% CI 14.6-17.4) (Figure 2A, Table 3). A higher poverty rate in the community was not significantly associated with OS (P = .485) (Figure 2B, Table 3). Insurance status was also not significantly associated with median OS (P = .267) (Figure 2C, Table 3). When all variables were included in a multivariable Cox regression analysis, rurality and poverty were not significantly associated with OS (Table 3).
Figure 2.
Cohort 1: overall survival. (A) Kaplan-Meier curve displaying overall survival by rurality for patients with HGG in Utah from 2000 to 2019 calculated from the date of diagnosis to the date of last known follow-up. (—) Frontier counties; (···) Rural counties; (- -) Urban counties. (B) Overall survival by poverty indicator for patients with HGG in Utah from 2000 to 2019, delineated by the percentage of a patient’s community living at or below the poverty line. (—) <5% poverty; (···) 5%-10% poverty; (light - -) 10%-20% poverty; (dark - -) >20% poverty. (C) Overall survival by insurance status for patients with HGG in Utah from 2000 to 2019. (—) Uninsured; (···) private insurance; (light - -) Medicare/Medicaid; (dark - -) other government insurance (including Tricare, Military, Veteran’s Affairs, and Indian Health Services); (· · ·) unknown; (-·-) not otherwise specified (NOS). (+) indicates censored patients.
Table 3.
Cohort 1: survival analysis of socioeconomic factors in HGG
| Variable | Median (SE)a | 95% CIa | P-valuea | HRb | 95% CIb | P-valueb |
|---|---|---|---|---|---|---|
| Rurality | .049 | |||||
| Frontier | 18.0 (2.1) | 13.8-22.2 | Ref | |||
| Rural | 13.0 (0.9) | 11.2-14.8 | 0.54 | 0.24-1.20 | .542 | |
| Urban | 16.0 (0.9) | 14.3-17.7 | 0.71 | 0.35-1.47 | .712 | |
| Poverty indicator c | .485 | |||||
| <5% | 17.0 (1.5) | 14.1-19.9 | Ref | |||
| 5%-10% | 13.0 (0.9) | 11.2-14.8 | 1.07 | 0.76-1.51 | .708 | |
| 10%-20% | 15.0 (1.0) | 13.0-17.0 | 1.38 | 0.96-1.99 | .084 | |
| >20% | 15.0 (2.6) | 9.9-20.1 | 1.31 | 0.81-2.11 | .269 | |
| Insurance | .267 | |||||
| Uninsured | 21.0 (3.1) | 14.8-27.2 | Ref | |||
| Insured, NOS | 33.0 (6.8) | 19.7-46.3 | 0.33 | 0.15-0.72 | .005 | |
| Private insurance | 22.0 (1.5) | 19.0-25.0 | 0.07 | 0.36-1.04 | .068 | |
| Medicare, Medicaid | 9.0 (0.6) | 7.9-10.1 | 0.05 | 0.31-1.01 | .054 | |
| Other gov’t | 13.0 (4.8) | 3.6-22.4 | 0.43 | 0.24-1.84 | .425 | |
| Unknown | 48.0 (9.6) | 29.1-66.9 | 0.01 | 0.17-0.78 | .009 |
Abbreviations: NOS, not otherwise specified; Other gov’t, other government program.
Bold values are significant.
aKaplan-Meier analysis and log-rank test.
bMultivariable Cox regression analysis.
cPercentage of a patient’s community who live at or below the poverty line.
HGG Adjuvant Treatment
A further analysis of additional patients (cohort 2) was performed using an available poverty indicator. Of the 2264 patients diagnosed with HGG who underwent resection, 1163 (51%) underwent both chemotherapy and radiation, 93 (4%) underwent chemotherapy alone, 270 (12%) underwent radiation alone, and 738 (33%) did not receive adjuvant therapy (Table 4). Similar findings in sex, ethnicity, treatment undergone, insurance status, and diagnosis were seen compared with cohort 1.
Table 4.
Cohort 2: demographics of patients who received HGG adjuvant therapy
| Variables | Total (%) N = 2264 |
Chemo and radiation (%) N = 1163 |
Chemo (%) N = 93 |
Radiation (%) N = 270 |
No adjuvant treatment (%) N = 738 |
P-value |
|---|---|---|---|---|---|---|
| Sex, male | 1300 (57.4) | 699 (60.1) | 58 (62.4) | 144 (53.3) | 399 (54.1) | .099 |
| Age (SD) | 54.1 (20.4) | 52.4 (17.3) | 47.3 (23.5) | 57.8 (20.8) | 56.6 (23.6) | <.001 |
| Race | .686a | |||||
| American Indian/Alaska Native | <11 | <11 | <11 | 0 | <11 | |
| Asian | 21 (0.9) | 13 (1.1) | 0 | <11 | <11 | |
| Pacific Islander | 15 (0.7) | <11 | 0 | <11 | <11 | |
| Black | 10 (0.4) | <11 | 0 | <11 | <11 | |
| White | 2208 (97.5) | 1133 (97.4) | 92 (98.9) | 263 (97.4) | 720 (97.6) | |
| More than one race | <11 | 0 | 0 | 0 | <11 | |
| Not listed | <11 | 0 | 0 | 0 | <11 | |
| Ethnicity | .349 | |||||
| Non-Hispanic | 2106 (93.0) | 1071 (92.1) | 88 (94.6) | 253 (93.7) | 694 (94.0) | |
| Marital status | <.001 a | |||||
| Single, never married | 390 (17.2) | 169 (14.5) | 26 (28.0) | 40 (14.8) | 155 (21.0) | |
| Married | 1496 (66.1) | 851 (73.2) | 56 (60.2) | 182 (67.4) | 407 (55.1) | |
| Single, previously married | 346 (15.3) | 133 (11.4) | 10 (10.8) | 44 (16.3) | 159 (21.5) | |
| Not listed | 32 (1.4) | 10 (0.9) | <11 | <11 | 17 (2.3) | |
| Insurance status | <.001 a | |||||
| Uninsured | 94 (4.2) | 48 (4.1) | <11 | 16 (5.9) | 27 (3.7) | |
| Insurance, NOS | 72 (3.2) | 39 (3.4) | <11 | 10 (3.7) | 18 (2.4) | |
| Private insurance | 1003 (44.3) | 623 (53.6) | 44 (47.3) | 102 (37.8) | 234 (31.7) | |
| Medicare and Medicaid | 898 (39.7) | 396 (34.0) | 22 (23.7) | 125 (46.3) | 355 (48.1) | |
| Other government program | 61 (2.7) | 30 (2.6) | <11 | <11 | 22 (3.0) | |
| Not listed | 136 (6.0) | 27 (2.3) | 17 (18.3) | 10 (3.7) | 82 (11.1) | |
| Diagnosis | <.001 a | |||||
| Malignant glioma, NOS | 256 (11.3) | 32 (2.8) | 11 (11.8) | 35 (13.0) | 178 (24.1) | |
| Astrocytoma, anaplastic | 217 (9.6) | 156 (13.4) | <11 | 26 (9.6) | 30 (4.10) | |
| Astrocytoma, diffuse | 66 (2.9) | 18 (1.5) | <11 | 15 (5.6) | 30 (4.1) | |
| Glioblastoma | 1639 (72.4) | 918 (78.9) | 62 (66.7) | 177 (65.6) | 482 (65.3) | |
| Oligodendroglioma, anaplastic | 86 (3.8) | 39 (3.4) | 12 (12.9) | 17 (6.3) | 18 (2.4) | |
| Distance traveled, mi | .801 | |||||
| First facility (SD) | 37.6 (58.3) | 37.4 (57.1) | 31.1 (46.4) | 39.8 (67.8) | N/A | |
| Income quartiles (%) | .007 | |||||
| Q1 (lowest income) | 413 (18.2) | 188 (16.2) | 16 (17.2) | 46 (17.0) | 163 (22.1) | |
| Q2 | 573 (25.3) | 274 (23.6) | 23 (24.7) | 74 (27.4) | 202 (27.4) | |
| Q3 | 641 (28.3) | 338 (29.0) | 27 (29.0) | 83 (30.7) | 193 (26.2) | |
| Q4 (highest income) | 637 (28.1) | 363 (31.2) | 27 (29.0) | 67 (24.8) | 180 (24.4) | |
| Yost Index quartile (%) | .005 | |||||
| Q1 (lowest SES) | 399 (17.6) | 181 (15.6) | 20 (21.5) | 44 (16.3) | 154 (20.9) | |
| Q2 | 570 (25.2) | 270 (23.2) | 21 (22.6) | 72 (26.7) | 207 (28.0) | |
| Q3 | 638 (28.2) | 340 (29.2) | 27 (29.0) | 84 (31.1) | 187 (25.3) | |
| Q4 (highest SES) | 657 (29.0) | 372 (32.0) | 25 (26.9) | 70 (25.9) | 190 (25.7) | |
| RUCA code (%) | .836 | |||||
| Metropolitan | 1938 (85.6) | 988 (85.0) | 85 (91.4) | 237 (87.8) | 628 (85.1) | |
| Micropolitan | 164 (7.2) | 91 (7.8) | <11 | 15 (5.6) | 54 (7.3) | |
| Small town | 96 (4.2) | 52 (4.5) | <11 | 11 (4.1) | 30 (4.1) | |
| Rural | 66 (2.9) | 32 (2.8) | <11 | <11 | 26 (3.5) |
Abbreviations: HGG, high-grade glioma; SD, standard deviation; NOS, not otherwise specified; SES, socioeconomic status; RUCA, Rural-Urban Commuting Area code; N/A, not applicable.
Bold values are significant.
Percentages given out of total number of patients within adjuvant treatment group. Income quartiles, Yost Index Quartiles, and RUCA codes are given as percentages of total within each quartile. Categorical variables compared with Chi-square test. Continuous variables compared with ANOVA test. Categories with less than 11 patients are not explicitly listed to protect patients from possible identification. Distance is not provided for patients not receiving adjuvant therapy because they did not travel to a treatment facility for adjuvant therapy.
aCategorical variables compared with Fisher-Freeman-Halton exact test.
Patients who received both chemotherapy and radiation were more likely to be part of the highest Yost Index quartile, whereas patients who received either chemotherapy or radiation were more likely to be part of the third quartile, and patients who received no adjuvant therapy were most likely to be part of the second quartile (P = .005) (Table 4). Patients in higher income quartiles were also more likely to receive both therapies, and patients in lower quartiles were more likely to receive a single or no therapy (P = .007). No significant differences in pursued adjuvant therapies were seen across RUCA codes (P = .742).
Discussion
Our examination of SDOHs in relation to HGG treatment and outcomes in Utah reveals insights into the factors influencing patient care across the socioeconomic and geographic contexts in Utah. Notably, although patients from Frontier counties in Utah traveled significantly farther to receive care and were more likely to come from regions with higher poverty rates, they did not experience significant delays in undergoing resection and had longer median survival compared with patients from Urban counties. In contrast, patients from Rural counties, while still traveling farther and residing in regions with similar SDOHs as Frontier patients, had significantly shorter median survival.
The correlation of longer travel distances and lower socioeconomic status highlights the logistical challenges faced by individuals in remote areas when accessing specialized healthcare services. This impact is most notable in our study as patients of lower socioeconomic status experienced significantly lower rates of treatment with adjuvant therapies. These data suggest patients initially receive timely care but may face challenges in returning to receive additional care.
Access to Care in HGG
Our median OS findings by population density are multifaceted. Our Frontier cohort was much smaller than the Rural and Urban cohorts, and a larger cohort of Frontier patients would enhance the findings among this population. The shorter OS among Rural patients is supported by the lower socioeconomic status that was concurrently observed in this population. The literature has reported the impact of these known SDOHs on HGG outcomes, suggesting lower socioeconomic status notably impacts access to treatment and, subsequently, survival.
In 2020, Bower et al4 found patients from low-income communities had significantly shorter median OS and subsequent survival at 1-, 3-, and 5-year intervals when compared with their high-income counterparts. Additionally, a multivariable analysis suggested patients from high-income communities had a lower risk of death.4 Although our multivariate analysis results suggest that a rural location alone was not associated with OS, the differences in OS among the various groups highlight the nuanced and multifaceted interaction of rurality with other SDOHs affecting these communities. Similarly, in their 2023 review of the literature, Gorenflo et al14 found an underlying strong association between socioeconomic status and GBM prognosis, and proposed that this observation was a multifactorial consequence of insurance status, available adjuvant therapy, and clinical trial participation.
In 2020, Wang et al15 found that GBM patients of lower socioeconomic status were less likely to receive radiation, and those who did receive radiation had significantly longer delays in starting and completing their radiation course. Additionally, they found that temozolomide use was lower among patients with lower socioeconomic status. Overall, however, survival was not significantly different across patient populations.15 Although our data do not include the granularity of specific adjuvant therapy agents and course, they do support the disparity among patients receiving adjuvant therapies, because patients receiving the standard of care with chemotherapy and radiation were more likely to have private insurance, whereas those not receiving the standard of care were more likely to have Medicare or Medicaid.
Impact of Insurance Status on Outcomes in HGG
The literature consistently shows that insurance status significantly affects treatment access and outcomes in patients with GBM. Patients who are uninsured or who are on Medicaid have been found to be less likely to receive surgery, radiotherapy, and chemotherapy compared with patients with private insurance.16 Patients with Medicaid also face higher hospital costs and longer lengths of stay compared with patients with Medicare or private insurance.17 By contrast, in our study, patients using Medicare or Medicaid did not have significantly shorter OS compared with patients with private insurance. We did note, however, significant evidence of patients living in Frontier or Rural counties predominantly utilizing Medicare and Medicaid as insurance, whereas those living in Urban areas predominantly maintained private insurance, highlighting the contrast across population densities with regard to access to higher-level coverages in areas closer to higher-level care.
Impact of an NCI-Designated Center
Although socioeconomic factors play a role, access to multidisciplinary care, like that available at our academic cancer center, may help patients overcome some barriers to treatment quality.15 Zhu et al18 found that academic facilities offer superior outcomes because there is greater access to clinical trials, multidisciplinary resources, and specialized healthcare providers. Similarly, Ranjan et al19 found that multidisciplinary approaches (eg, targeted therapies based on genomic profiling) may also prolong survival. Specialized neuro-oncology programs with coordinated care from various specialists can also produce outcomes comparable to clinical trials.20 Our dataset does not explicitly state where patients received their treatment. Utah has only one NCI-designated cancer center: the Huntsman Cancer Institute at the University of Utah. Although all of our patients were diagnosed in Utah and this is the only center in Utah that offers patients access to specialized brain tumor care, including neuro-oncology, we are unable to definitively determine whether patients were treated at our facility or at one of the private hospitals in the state. Our data may show self-control for treatment in our only academic center, which could explain the notable differences in OS as patients traveled farther to receive care. Additionally, the intersection of independent socioeconomic factors (eg, income and insurance status), in addition to rurality, warrants continued study to develop predictive models and outreach programs to help lower-income and uninsured patients receive the standard of care.
Limitations
Our study has inherent limitations relating to the granularity of our database. Our sample size was limited to the state of Utah and to those patients for whom the information was available in the UCR. We were unable to control for duplicate entries made in the UCR, and accuracy of the information obtained from the UCR may be subjective based upon the accuracy of those manually entering the data. Our survival data suggesting that some patients with HGG were still surviving nearly 10 years after their diagnosis likely reflects the diagnostic changes over the last 20 years, namely as the criteria for GBM diagnoses have changed rather significantly.
Conclusion
Our data suggest that patients from Frontier and Rural counties in Utah encounter more barriers related to SDOH but do not necessarily experience delays in surgical care. Patients in the different regions demonstrated different median OS, and the complex relationship of intersecting SDOHs is likely a driving factor in these patients receiving the full standard of care. We conclude from this comprehensive analysis of data pertaining to both preoperative and postoperative characterization of SDOHs that patients in Utah who experience SDOH barriers appear to experience little difference in care related to the resection of HGG, although they do experience a significant difference in receiving adjuvant therapies after surgery, which could affect OS. Patients with more SDOH barriers may need greater follow-up management to ensure adequate accessibility of adjuvant therapies such as chemotherapy and radiation. Further investigation is warranted to understand the nuanced epidemiological characteristics that have contributed to these findings and to widen the generalizability of this study in order to develop actionable predictive models.
Acknowledgments
This research was completed through participation in the Rural and Underserved Utah Training Experience (RUUTE) at the University of Utah School of Medicine. We thank Kristin Kraus and Cortlynd Olsen for their editorial assistance.
Contributor Information
Emma R Earl, Spencer Fox Eccles School of Medicine, University of Utah, Salt Lake City, Utah, USA.
Cody J Orton, Spencer Fox Eccles School of Medicine, University of Utah, Salt Lake City, Utah, USA.
Samuel A Tenhoeve, Spencer Fox Eccles School of Medicine, University of Utah, Salt Lake City, Utah, USA.
Clayton Rawson, Noorda College of Osteopathic Medicine, Provo, Utah, USA.
Michael Karsy, Department of Neurosurgery, University of Michigan, Ann Arbor, Michigan, USA.
Randy L Jensen, Huntsman Cancer Institute, University of Utah, Salt Lake City, Utah, USA; Departments of Neurosurgery, Radiation Oncology, and Medical Oncology, University of Utah, Salt Lake City, Utah, USA.
Funding
None declared.
Conflict of interest statement
M.K. received royalties from Thieme Medical Publishing. The other authors have no conflicts of interest to declare.
Authorship statement
Design: E.R.E. and C.J.O. Analysis: E.R.E., C.J.O., and S.A.T. Writing: E.R.E., C.J.O., S.A.T., C.R., M.K., and R.L.J. Revising: E.R.E., C.J.O., S.A.T., C.R., M.K., and R.L.J. Supervision: M.K. and R.L.J.
Data availability
Data will be made available by the corresponding author upon reasonable request.
References
- 1. Marmot M. Social determinants of health: from observation to policy. Med J Aust. 2000;172(8):379–382. [DOI] [PubMed] [Google Scholar]
- 2. Asfaw ZK, Hernandez-Marquez GC, Naik A, et al. 100 The impact of race and social determinants of health on clinical outcome of glioblastoma multiforme patients over a decade. J Clin Transl Sci. 2024;8(s1):28. [Google Scholar]
- 3. Aguirre AO, Lim J, Kuo C-CJ, et al. Social determinants of health and associations with outcomes in pediatric patients with brain tumors. Neurosurgery. 2023;94(1):108–116. [DOI] [PubMed] [Google Scholar]
- 4. Bower A, Hsu FC, Weaver KE, et al. Community economic factors influence outcomes for patients with primary malignant glioma. Neurooncol Pract. 2020;7(4):453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sibih Y, Perera S, DiGiorgio AM, Hervey-Jumper SL.. DISP-06. Examining the influence of neurosurgical oncology provider access on diffuse glioma survival outcomes: a nationwide county-level approach. Neuro Oncol. 2023;25(Supplement_5):v137–v138. [Google Scholar]
- 6. Bergqvist J, Iderberg H, Mesterton J, Henriksson R.. The effects of clinical and sociodemographic factors on survival, resource use and lead times in patients with high-grade gliomas: a population-based register study. J Neurooncol. 2018;139(3):599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stockdill ML, Vo JB, Celiku O, et al. Abstract 2155: the impact of urban residence on symptom duration, treatment initiation, and survival among primary brain tumor patients: a large cohort analysis. Cancer Res. 2024;84(6_Supplement):2155.38635900 [Google Scholar]
- 8. Best B, Nguyen HS, Doan N, et al. Gliomas: survival differences between metropolitan and non-metropolitan counties. J Neurosurg Sci. 2019;63(2):114–120. [DOI] [PubMed] [Google Scholar]
- 9. Earl ER, Colman H, Mendez J, Jensen RL, Karsy M.. An evaluation of biobanking and therapeutic clinical trial representation among adult glioma patients from rural and urban Utah. Neurooncol Pract. 2023;10(5):472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. National Cancer Institute. Overview of the SEER program. US Department of Health and Human Services. https://seer.cancer.gov/about/overview.html. Accessed May 1, 2024. [Google Scholar]
- 11. United States Census Bureau. QuickFacts. U.S. Department of Commerce. https://www.census.gov/quickfacts/fact/table/UT,US/PST045223. Published 2021. Accessed June 1, 2022. [Google Scholar]
- 12. Utah Department of Health and Human Services. County classifications map. Utah Office of Primary Care and Rural Health. https://ruralhealth.health.utah.gov/, https-ruralhealth-health-utah-gov-utah-state-profile/county-classifications-map/. Published 2018. Accessed June 1, 2022. [Google Scholar]
- 13. Yost K, Perkins C, Cohen R, Morris C, Wright W.. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12(8):703–711. [DOI] [PubMed] [Google Scholar]
- 14. Gorenflo MP, Shen A, Murphy ES, Cullen J, Yu JS.. Area-level socioeconomic status is positively correlated with glioblastoma incidence and prognosis in the United States. Front Oncol. 2023;13:1110473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang T, Pham A, Yoo S, et al. Identifying disparities in care in treating glioblastoma: a retrospective cohort study of patients treated at a safety-net versus private hospital setting. World Neurosurg. 2020;137:e213–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brown DA, Himes BT, Kerezoudis P, et al. Insurance correlates with improved access to care and outcome among glioblastoma patients. Neuro Oncol. 2018;20(10):1374–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chandra A, Young JS, Dalle OC, et al. Insurance type impacts the economic burden and survival of patients with newly diagnosed glioblastoma. J Neurosurg. 2020;133(1):89–99. [DOI] [PubMed] [Google Scholar]
- 18. Zhu P, Du XL, Esquenazi Y, Zhu J-J.. Access to clinical trials, multidisciplinary environment, and health care providers by facility type and outcomes of patients with newly diagnosed glioblastoma using SEER-Medicare dataset. J Clin Oncol. 2019;37(15_suppl):e13576. [Google Scholar]
- 19. Ranjan T, Yu A, Wegner RE, et al. INNV-39. The impact of multidisciplinary management on the overall survival of glioblastoma and anaplastic glioma patients in the era of precision medicine—a community physician’s experience and dilemma. Neuro Oncol. 2019;21(Supplement_6):vi138. [Google Scholar]
- 20. Banerji N, Hultman M, Tipps ME, et al. INNV-12. Outcomes in a real-world practice for patients with primary glioblastoma: impact of a specialized neuro-oncology cancer care program. Neuro Oncol. 2019;21(Supplement_6):vi133. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available by the corresponding author upon reasonable request.