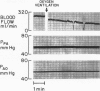
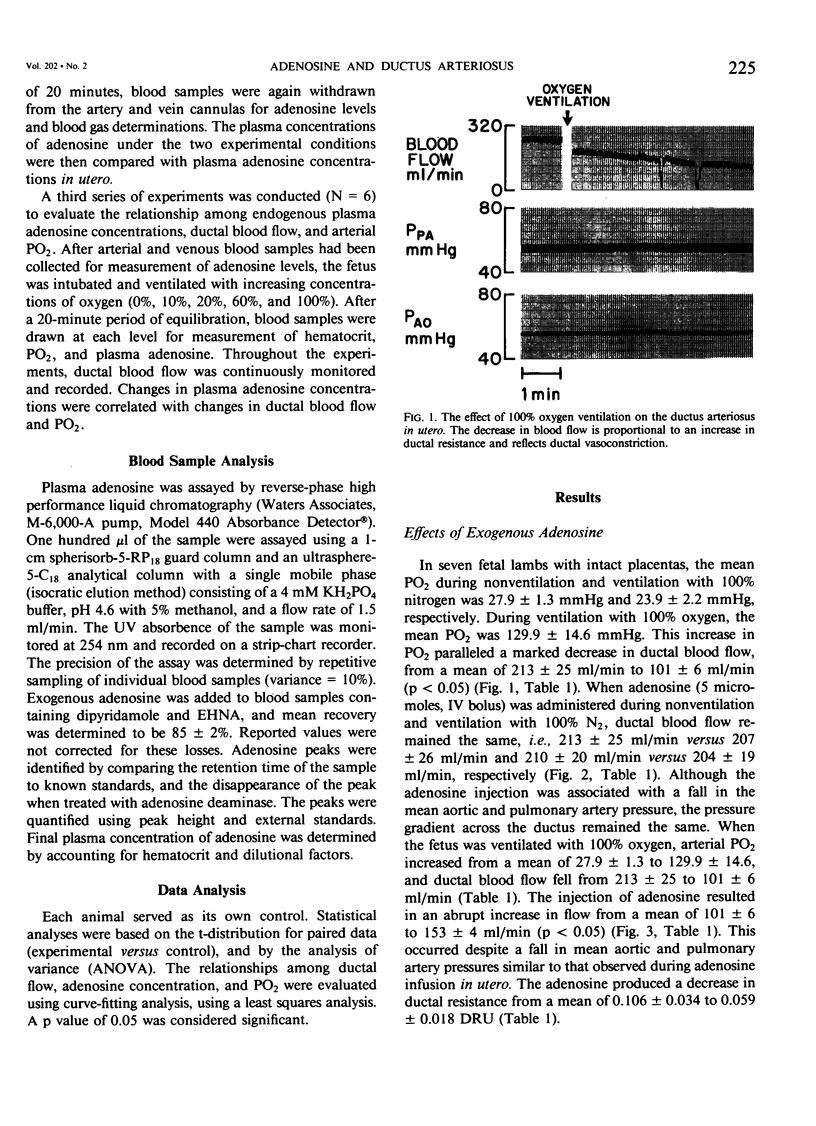
Abstract
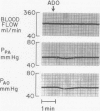
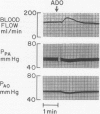
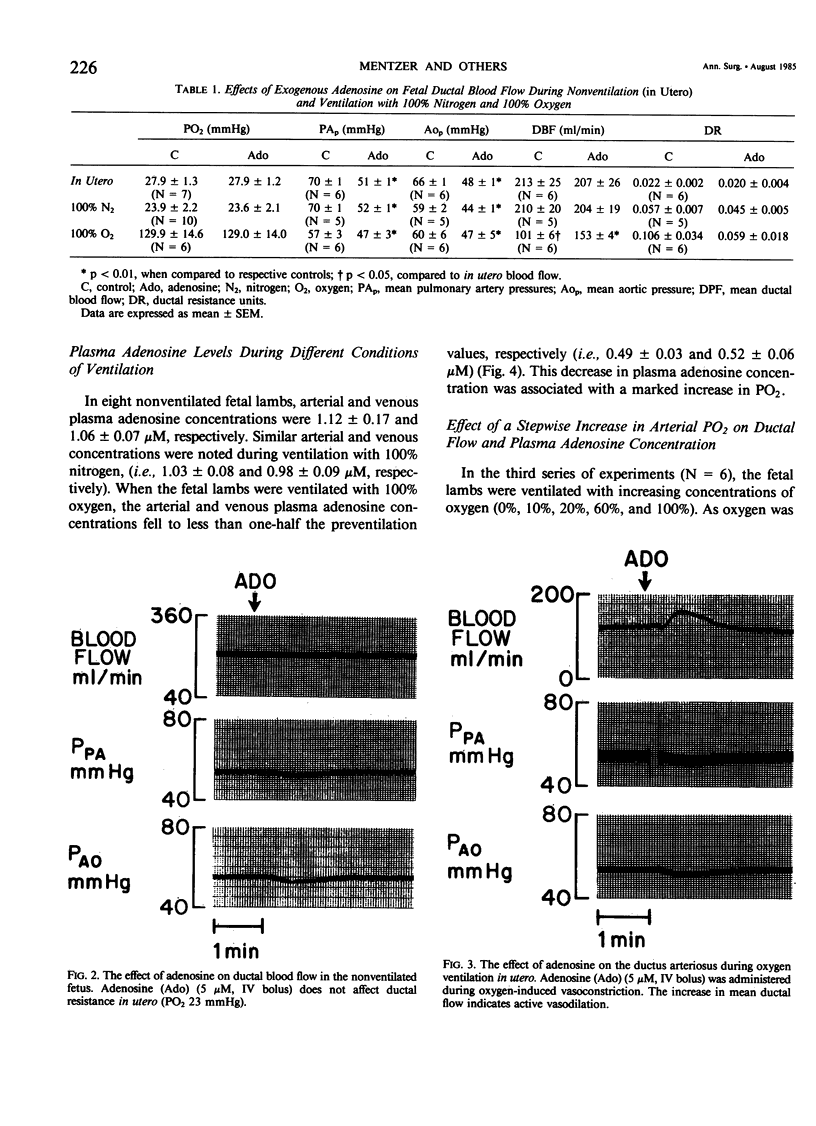
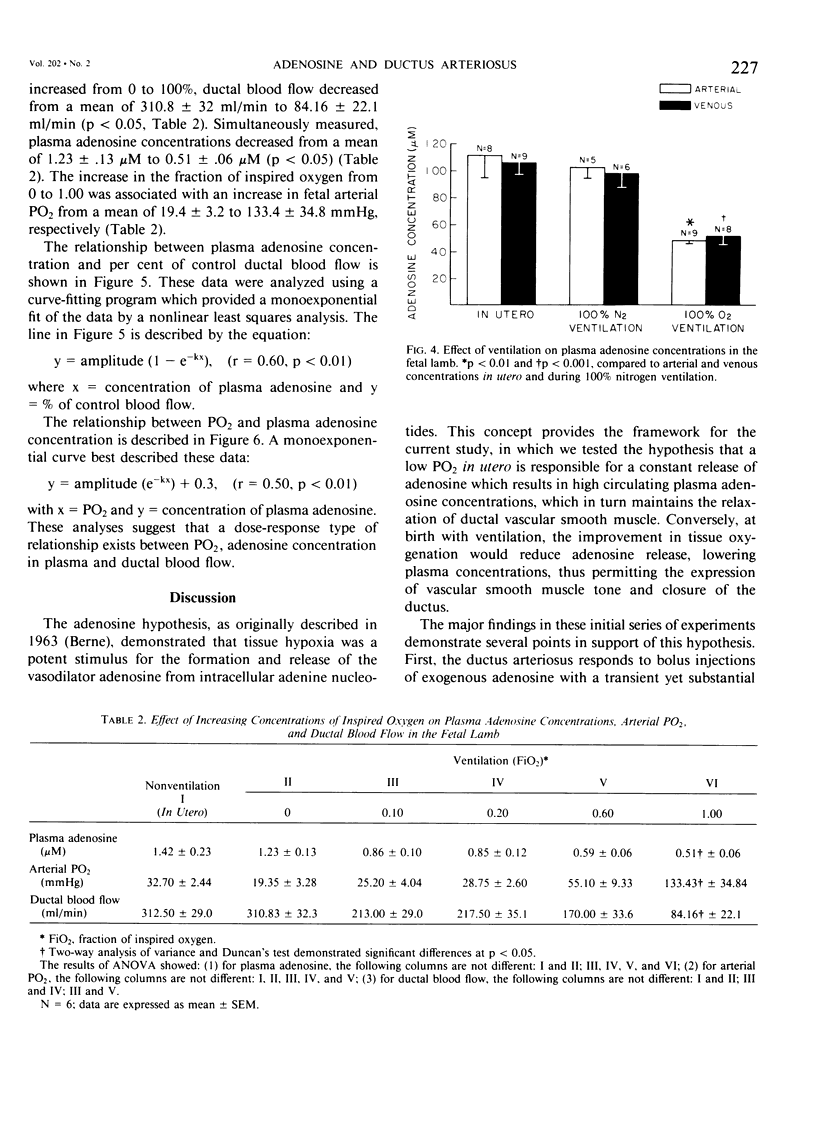
The hypothesis that endogenously released adenosine plays an important role in maintaining patency of the fetal lamb ductus arteriosus was tested. The design of the study was (1) to determine the effect, if any, of exogenous adenosine on blood flow through the ductus arteriosus and (2) to evaluate the relationship among the partial pressure of oxygen in arterial blood, circulating endogenous plasma adenosine concentration, and the rate of blood flow through the ductus. When exogenous adenosine (5 mumoles) was administered during oxygen-induced ductal constriction, ductal blood flow increased from 101 +/- 6 ml/min to 153 +/- 4 ml/min (p less than 0.01). When fetal blood adenosine concentrations were measured during nonventilation and ventilation with 100% oxygen, endogenous adenosine concentrations fell to less than one-half of the preventilation levels, i.e., from 1.12 +/- 0.17 to 0.49 +/- 0.03 microM (p less than 0.01). Finally, when fetal lambs were ventilated with increasing concentrations of oxygen (0%, 10%, 20%, 60%, and 100%) and measurements obtained simultaneously at each level, there was a significant monoexponential relationship among the rise in PO2, the fall in plasma adenosine concentration, and the decrease in ductal blood flow. These data suggest that: (1) adenosine is a potent vasodilator of the lamb ductus arteriosus during oxygen-induced vasoconstriction; (2) fetal endogenous plasma adenosine levels fall significantly when PO2 is increased; and (3) the fall in adenosine concentrations parallels a decrease in ductal blood flow. The findings suggest that the endogenous vasodilator adenosine plays an important role in maintaining ductal patency in utero.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNE R. M. Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am J Physiol. 1963 Feb;204:317–322. doi: 10.1152/ajplegacy.1963.204.2.317. [DOI] [PubMed] [Google Scholar]
- BORN G. V., DAWES G. S., MOTT J. C., RENNICK B. R. The constriction of the ductus arteriosus caused by oxygen and by asphyxia in newborn lambs. J Physiol. 1956 May 28;132(2):304–342. doi: 10.1113/jphysiol.1956.sp005526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchus A. N., Ely S. W., Knabb R. M., Rubio R., Berne R. M. Adenosine and coronary blood flow in conscious dogs during normal physiological stimuli. Am J Physiol. 1982 Oct;243(4):H628–H633. doi: 10.1152/ajpheart.1982.243.4.H628. [DOI] [PubMed] [Google Scholar]
- Berne R. M. The role of adenosine in the regulation of coronary blood flow. Circ Res. 1980 Dec;47(6):807–813. doi: 10.1161/01.res.47.6.807. [DOI] [PubMed] [Google Scholar]
- Clyman R. I. Ontogeny of the ductus arteriosus response to prostaglandins and inhibitors of their synthesis. Semin Perinatol. 1980 Apr;4(2):115–124. [PubMed] [Google Scholar]
- Coceani F., Hamilton N. C., Labuc J., Olley P. M. Cytochrome P 450-linked monooxygenase: involvement in the lamb ductus arteriosus. Am J Physiol. 1984 Apr;246(4 Pt 2):H640–H643. doi: 10.1152/ajpheart.1984.246.4.H640. [DOI] [PubMed] [Google Scholar]
- Coceani F., Olley P. M., Lock J. E. Prostaglandins, ductus arteriosus, pulmonary circulation: current concepts and clinical potential. Eur J Clin Pharmacol. 1980 Jul;18(1):75–81. doi: 10.1007/BF00561482. [DOI] [PubMed] [Google Scholar]
- Ely S. W., Knabb R. M., Bacchus A. N., Rubio R., Berne R. M. Measurements of coronary plasma and pericardial infusate adenosine concentrations during exercise in conscious dog: relationship to myocardial oxygen consumption and coronary blood flow. J Mol Cell Cardiol. 1983 Oct;15(10):673–683. doi: 10.1016/0022-2828(83)90257-2. [DOI] [PubMed] [Google Scholar]
- Fay F. S. Guinea pig ductus arteriosus. I. Cellular and metabolic basis for oxygen sensitivity. Am J Physiol. 1971 Aug;221(2):470–479. doi: 10.1152/ajplegacy.1971.221.2.470. [DOI] [PubMed] [Google Scholar]
- Herlihy J. T., Bockman E. L., Berne R. M., Rubio R. Adenosine relaxation of isolated vascular smooth muscle. Am J Physiol. 1976 May;230(5):1239–1243. doi: 10.1152/ajplegacy.1976.230.5.1239. [DOI] [PubMed] [Google Scholar]
- KOVALCIK V. THE RESPONSE OF THE ISOLATED DUCTUS ARTERIOSUS TO OXYGEN AND ANOXIA. J Physiol. 1963 Nov;169:185–197. doi: 10.1113/jphysiol.1963.sp007249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredi J. P., Sparks H. V., Jr Adenosine's role in coronary vasodilation induced by atrial pacing and norepinephrine. Am J Physiol. 1982 Oct;243(4):H536–H545. doi: 10.1152/ajpheart.1982.243.4.H536. [DOI] [PubMed] [Google Scholar]
- Mitchell M. D., Lucas A., Etches P. C., Brunt J. D., Turnbull A. C. Plasma prostaglandin levels during early neonatal life following term and pre-term delivery. Prostaglandins. 1978 Aug;16(2):319–326. doi: 10.1016/0090-6980(78)90033-3. [DOI] [PubMed] [Google Scholar]
- Rubio R., Berne R. M., Katori M. Release of adenosine in reactive hyperemia of the dog heart. Am J Physiol. 1969 Jan;216(1):56–62. doi: 10.1152/ajplegacy.1969.216.1.56. [DOI] [PubMed] [Google Scholar]
- Schrader J., Haddy F. J., Gerlach E. Release of adenosine, inosine and hypoxanthine from the isolated guinea pig heart during hypoxia, flow-autoregulation and reactive hyperemia. Pflugers Arch. 1977 May 6;369(1):1–6. doi: 10.1007/BF00580802. [DOI] [PubMed] [Google Scholar]