Abstract
Although cellulose biosynthesis among the cyanobacteria has been suggested previously, we present the first conclusive evidence, to our knowledge, of the presence of cellulose in these organisms. Based on the results of x-ray diffraction, electron microscopy of microfibrils, and cellobiohydrolase I-gold labeling, we report the occurrence of cellulose biosynthesis in nine species representing three of the five sections of cyanobacteria. Sequence analysis of the genomes of four cyanobacteria revealed the presence of multiple amino acid sequences bearing the DDD35QXXRW motif conserved in all cellulose synthases. Pairwise alignments demonstrated that CesAs from plants were more similar to putative cellulose synthases from Anabaena sp. Pasteur Culture Collection 7120 and Nostoc punctiforme American Type Culture Collection 29133 than any other cellulose synthases in the database. Multiple alignments of putative cellulose synthases from Anabaena sp. Pasteur Culture Collection 7120 and N. punctiforme American Type Culture Collection 29133 with the cellulose synthases of other prokaryotes, Arabidopsis, Gossypium hirsutum, Populus alba × Populus tremula, corn (Zea mays), and Dictyostelium discoideum showed that cyanobacteria share an insertion between conserved regions U1 and U2 found previously only in eukaryotic sequences. Furthermore, phylogenetic analysis indicates that the cyanobacterial cellulose synthases share a common branch with CesAs of vascular plants in a manner similar to the relationship observed with cyanobacterial and chloroplast 16s rRNAs, implying endosymbiotic transfer of CesA from cyanobacteria to plants and an ancient origin for cellulose synthase in eukaryotes.
Cellulose is the most abundant biopolymer on earth with some 1011 tons produced annually (Hess et al., 1928). To date, clear examples of this process have been found in prokaryotes (Acetobacter xylinus, Agrobacterium tumefaciens, Rhizobium spp. [Ross et al., 1991]; Escherichia coli, Klebsiella pneumoniae, Salmonella typhimurium, [Zogaj et al., 2001]; Sarcina ventriculi [Roberts, 1991]) and eukaryotes, including animals (tunicates), algae, fungi, vascular plants such as mosses and ferns, gymnosperms and angiosperms (Brown, 1985), and the cellular slime mold Dictyostelium discoideum (Blanton et al., 2000). Thus far, evidence is lacking for cellulose biosynthesis among the Euryarchaeota, although we have found putative cellulose synthases in the genome databases of Thermoplasma (http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/framik?db=Genome&gi=168) and Ferroplasma (http://spider.jgi-psf.org/JGI_microbial/html/).
One of the most ancient extant groups of living organisms is the cyanobacteria, having been in existence for more than 2.8 billion years (Knoll, 1992, 1999). Fossil records of cyanobacteria-like forms date as far back as 3.5 billion years (Schopf and Walter, 1982). Cyanobacteria produce a wide array of extracellular polysaccharides (EPS), which can take the form of released polysaccharides (Kawaguchi and Decho, 2000; Nicolaus et al., 1999), a tightly bound sheath that is often highly fibrillar and sometimes crystalline (Frey-Wyssling and Stecher, 1954; Singh, 1954; Tuffery, 1969; Hoiczyk, 1998), mucilaginous slime loosely associated with cells (often partially water soluble; Drews and Weckesser, 1982), or a transiently attached slime tube, present in motile filaments (Castenholz, 1982; Drews and Weckesser, 1982; Hoiczyk, 1998). Cyanobacterial EPS are involved in a wide range of functions including desiccation tolerance, protection from UV light, and adhesion to substrates, as well as motility (Ehling-Schulz et al., 1997; Phillipis and Vincenzini, 1998; Kodani et al., 1999; Nicolaus et al., 1999). Although several reports in the literature have suggested the presence of cellulose in cyanobacterial EPS (Frey-Wyssling and Stecher, 1954; Singh, 1954; Tuffery, 1969; Winder 1990), none has conclusively demonstrated cellulose biosynthesis among this group of organisms. Therefore, we sought to examine representatives from diverse genera of the cyanobacteria for the presence of cellulose, employing stringent methods for positive identification. Using cellobiohydrolase I (CBHI)-gold labeling and x-ray diffraction, we demonstrate the presence of cellulose in six strains of five genera. Four additional strains appear to have cellulose as evidenced by CBHI-gold labeling. Three of the five sections of cyanobacteria are represented among cellulose producing strains.
Recent genome sequencing projects allowed us to mine databases of cyanobacteria and other prokaryotes for protein sequences with similarity to cellulose synthases. In all, 17 prokaryotic (five of which were cyanobacterial) and eight eukaryotic cellulose synthase homologs were aligned and compared. The results show a close relationship between vascular plant and cyanobacterial cellulose synthases. This supports the hypothesis that plants acquired cellulose synthase from cyanobacteria through non-evolutionary means.
RESULTS
Electron Microscopy
Microfibrils of varied morphology were observed in the EPS isolates of eight cyanobacteria and in the slime tube isolates of Oscillatoria princeps (Table I). These microfibrils were strongly labeled with CBHI-gold, indicating that they are composed of β-1,4-glucans (Okuda et al., 1993; Tomme et al., 1995; Fig. 1). The narrow and wide axes of the microfibrils were measured from representative samples of all cyanobacterial cellulose microfibrils. The mean microfibril thickness is rather constant: 1.7 nm (±0.4 nm) based on 65 measurements, ranging from 1.1 nm to 2.8 nm. The mean microfibril width was more variable, with a mean 10.3 nm (±4.1 nm) based on 10 measurements, ranging from 5 nm to more than 17 nm.
Table I.
Summation of cyanobacteria investigated and results of experiments
| Organism | Motility | Acid Insolubility | Microfibrils (TEMa) | Location | CBHI-Gold | X-Ray Diffraction | Cellulose |
|---|---|---|---|---|---|---|---|
| Section I | |||||||
| Synechocystis sp. ATCC 27184 | Yes | Yes | No | NAb | No | 0.46 and 0.40 nm | No |
| Synechococcus sp. UTCC 100 | No | Yes | No | NA | No | Not tested | No |
| Gloeocapsa sp. UTEX L795 | No | Yes | Yes | Sheath | Yes | 0.60, 0.54, and 0.39 nm | Yes |
| Section III | |||||||
| O. princeps | Yes | Yes | Yes | Slime tube | Yes | Not tested | Tentative |
| Oscillatoria sp. UTEX L2435 | Yes | Yes | Yes | Slime tube | Yes | 0.59, 0.53, and 0.39 nm | Yes |
| Phormidium autumnale | Yes | Yes | Yes | Slime tube/sheath | Yes | 0.59, 0.53, and 0.39 nm | Yes |
| Crinalium epipsammum | No | Yes | Yes | ? | Yes | Inconclusive | Tentative |
| Section IV | |||||||
| Anabaena sp. | No | Yes | Yes | Sheath? | Yes | 0.60, 0.53, and 0.39 nm | Yes |
| Nostoc punctiforme | Yes | Yes | Yes | Sheath/slime? | Yes | Inconclusive | Tentative |
| Nostoc muscorum UTEX 2209 | No | Yes | Yes | Sheath? | Yes | 0.60, 0.53, and 0.39 nm | Yes |
| N. muscorum UTEX 1037 | Yes | Yes | Yes | Sheath? | Yes | Not tested | Tentative |
| Scytonema hofmanni | Yes | Yes | Yes | Sheath | Yes | 0.60, 0.53, and 0.40 nm | Yes |
TEM, Transmission electron microscopy.
NA, Not applicable.
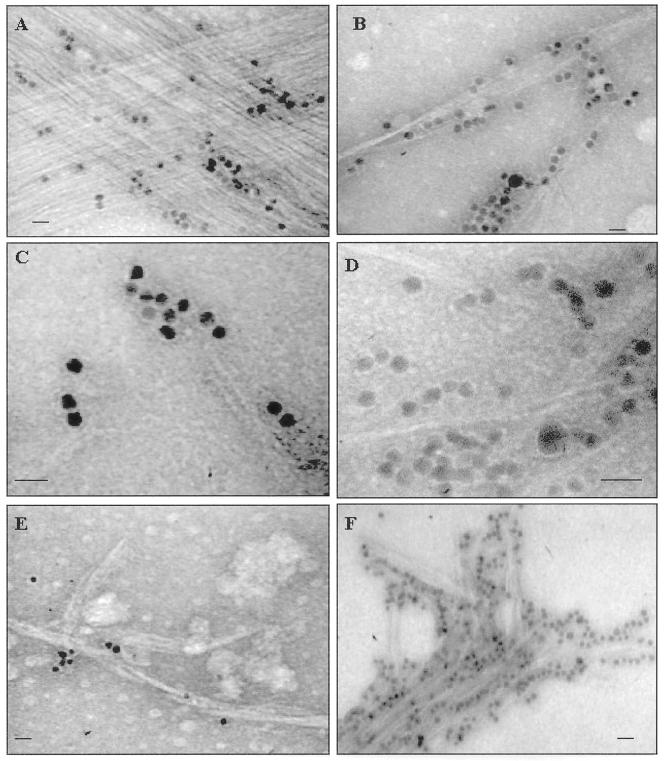
Figure 1.
A through F, Various cellulose microfibrils isolated from cyanobacteria (all negatively stained with 1% [v/v] aqueous uranyl acetate and labeled with CBHI-gold; the gold complex is 10 nm in diameter). A, Oriented bundles of microfibrils from Gloeocapsa sp. L795, many of which are stained with the CBHI-gold complex, which specifically binds to the surface of crystalline cellulose (Okuda et al., 1993; Tomme et al., 1995). B, Cellulose microfibrils from N. muscorum UTEX 2209. These microfibrils frequently aggregate into twisted ribbon-like structures reminiscent of the cellulose ribbons of A. xylinum. C, Cellulose microfibrils from C. epipsammum ATCC 49662. These microfibrils appear to be very thin in both dimensions in comparison with the other cyanobacteria tested. D, Microfibrils from P. autumnale, which appear more discontinuous and irregular. This could be caused by amorphous regions or regions of low crystallinity rendering the microfibrils more altered from acid treatments. E, Microfibrils from Nostoc punctiforme ATCC 29133. These microfibrils are shorter and many have tapered ends, suggesting the possibility of cellulose II. F, Large bundles of elongated microfibrils from Anabaena UTEX 2576. A through E, Bar = 20 nm; F, bar = 30 nm.
Cellulose microfibrils exist as only a fraction of the EPS investments of cyanobacteria, as demonstrated by thin sections of S. hofmanni UTEX 2349 (Fig. 2) and untreated slime tubes from O. princeps (Fig. 3A). In the case of S. hofmanni, a single layer of CBHI-gold labeled material is seen near the outer boundary of the thick sheath. The labeled region is approximately 300 nm wide, slightly less than one-third of the thickness of the sheath. Microfibrils, which seem to be enveloped in an extracellular matrix, are somewhat difficult to resolve in the untreated slime tubes of O. princeps. The chemical composition of the matrix is unknown since the EPS investments of a variety of cyanobacteria can be complex, containing proteins, glycoproteins, and lipids in addition to a variety of polysaccharides (Kawaguchi and Decho, 2000). Digestion of the slime tubes with TFA removes the majority of matrix material, and microfibrils are easily visualized (Fig. 3B).
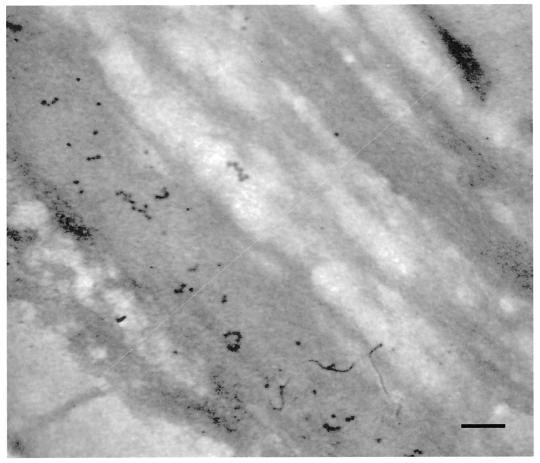
Figure 2.
Negatively stained thin section of S. hofmanni UTEX 2349 with Epon removed and labeled with CBHI-gold. Note the layering of the sheath and the position of the cellulose region near the outer boundary of the sheath. Sheath thickness is designated by the white line. Bar = 60 nm.
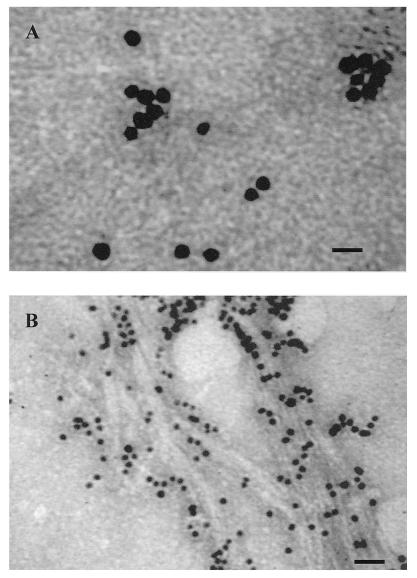
Figure 3.
A, Untreated slime tube material collected from the liquid culture O. princeps. The microfibrils are somewhat masked in the EPS matrix. Bar = 20 nm. B, Trifluoroacetic-digested slime tube material collected from liquid media. Microfibrils are more apparent here as is a decrease in matrix material. Bar = 40 nm.
X-Ray Diffraction
X-ray analysis of acetic nitric-treated EPS fractions revealed patterns typical for cellulose I in Oscillatoria sp. UTEX 2435 (data not shown), Nostoc sp. UTEX 2209 (data not shown), Gloeocapsa sp. UTEX L795, S. hofmanni UTEX 2349, Anabaena sp. UTEX 2576, and P. autumnale UTEX 1580. Figure 4 represents the diffraction patterns of four genera. Line broadening in these diffractograms suggests a low crystallinity, which is consistent with the small microfibril size observed. Electron diffraction patterns were also obtained (data not shown), but were very diffuse, supporting the size measurements and the x-ray line broadening. The presence of contaminating crystalline materials is evidenced by the existence of peaks not related to cellulose. Note that these peaks do not produce uniform overlaps with all four genera.
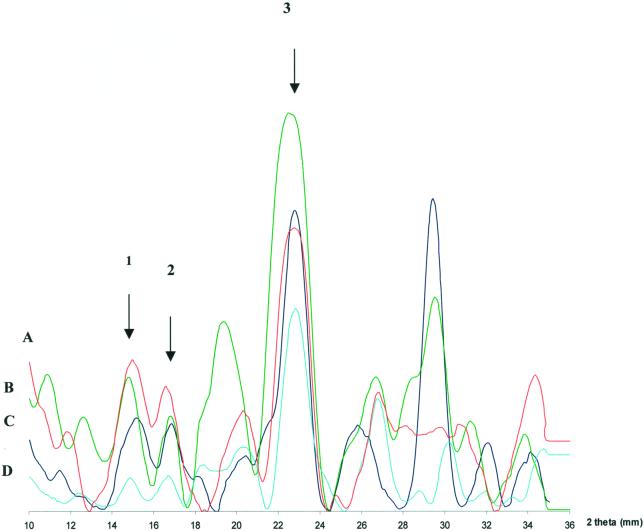
Figure 4.
X-ray analysis of cellulose from four different
cyanobacterial strains. A, Gloeocapsa; B,
Scytonema; C, Phormidium; D, Anabaena.
All genera exhibit a typical cellulose I pattern specified by the
overlapping peaks. Peak 1 is the 101 d-spacing; peak 2 is the −101
d-spacing; and peak 3 is the 002 d-spacing. For the 002 spacings, all
genera with the exception of S. hofmanni (4.0  ) have
reflections of 3.9
) have
reflections of 3.9  . For the −101 spacings, all genera with
the exception of Gloeocapsa (5.4
. For the −101 spacings, all genera with
the exception of Gloeocapsa (5.4 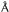 ) have 5.3-
) have 5.3-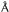 reflections. For the 101 spacings, all genera with the exception of
P. autumnale (5.9
reflections. For the 101 spacings, all genera with the exception of
P. autumnale (5.9 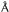 ) have 6.0-
) have 6.0-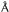 reflections.
The presence of contaminating crystalline materials is evidenced by the
existence of peaks not related to cellulose. Note that these peaks do
not always produce uniform overlaps with all four
genera.
reflections.
The presence of contaminating crystalline materials is evidenced by the
existence of peaks not related to cellulose. Note that these peaks do
not always produce uniform overlaps with all four
genera.
The acid-insoluble EPS fraction from Synechocystis sp. American Type Culture Collection (ATCC) 27184 yielded an x-ray diffraction pattern with d-spacings of 4.6 and 4.0 Å, indicating a crystalline product. These reflections, however, do not correspond with reflections of cellulose I or II. It is interesting to note that the contaminating peak preceding the 002 reflection in the S. hofmanni x-ray diffraction pattern also represents a d-spacing of 4.6 Å and therefore may indicate the presence of a similar crystalline product.
Phylogenetic Analysis
Having established the presence of cellulose among the cyanobacteria, we searched genome databases for open reading frames (ORFs) with sequencesimilarity to cellulose synthases. Searches identified sequences containing conserved DDD35QXXRW motif found in cellulose synthases and other processive glycosyltransferases (Saxena et al., 1995). BLAST (Altschul et al., 1990) searches of databases (http://www.kazusa.or.jp/cyano/cyano.html; http://spider.jgi-psf.org/JGI_microbial/html/) revealed cellulose-synthase-like (CSL) sequences in Synechocystis sp. Pasteur Culture Collection (PCC) 6803, Anabaena sp. PCC 7120, and N. punctiforme. Searches of the Anabaena sp. PCC 7120, N. punctiforme, and Synechococcus sp. WH8102 databases revealed the presence of ORFs with significant sequence similarity to known prokaryotic and eukaryotic cellulose synthases.
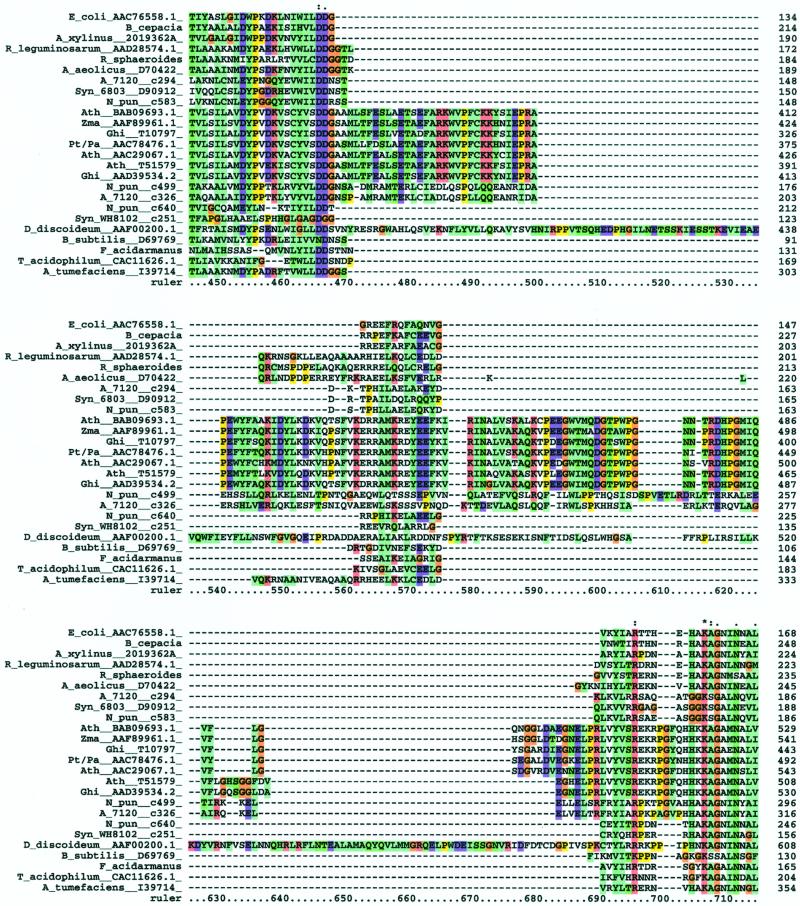
We were interested in determining the relationship of the putative cellulose synthases from cyanobacteria to known cellulose synthases. BLAST searches with the amino acid sequences from known cellulose synthases of prokaryotes and D. discoideum against the Arabidopsis genome database demonstrated that the CesA of D. discoideum had the lowest expectation value when compared with CesAs of Arabidopsis. Subsequent pairwise alignments showed that the putative cyanobacterial cellulose synthases represented by Anabaena sp. PCC 7120 (contig 326) and N. punctiforme ATCC 29133 (contig 499) had expectation values significantly lower (2 × 10−20 and 2 × 10−18 times, respectively) than that of the cellulose synthase of D. discoideum when compared with IRX3 of Arabidopsis. Although BLAST searches are sometimes used to infer phylogenetic relationships, pairwise alignments are inherently limited in this capacity. Thus, we chose to perform multiple alignments for the construction of phylograms. Toward this end, we sought to identify as many prokaryotic cellulose synthase sequences as possible from the above databases in order to minimize the possibility of systematic errors such as long branch attraction when constructing phylogenetic trees (Xiong et al., 2000). Criteria for inclusion of ORFs were simply the presence of the conserved motif and an arbitrary expectation value <10−9 when compared with any known cellulose synthase. The search yielded sequences from both gram-negative and gram-positive Eubacteria as well as members of Euryarchaeota. Multiple alignments using all prokaryotic amino acid sequences (17 sequences total) as well as sequences from Arabidopsis, Gossypium hirsutum, corn (Zea mays), Populus alba × Populus tremulus, and D. discoideum were constructed with ClustalX (Thompson et al., 1994). The presence of an insertion region corresponding to the plant-specific and conserved region (CR-P; Delmer, 1999) between regions U1 and U2 was observed in eukaryotic sequences as previously described by Blanton et al. (2000). The amino acids of this insertion block were highly conserved among plants but showed very little sequence similarity to the insert of D. discoideum. Two putative cellulose synthases from Anabaena sp. PCC 7120 and N. punctiforme showed the presence of the insertion region found in D. discoideum and vascular plants but lacking in other prokaryotes (Delmer, 1999; Blanton et al., 2000). Additionally, the cyanobacterial insertions showed greater similarity to those of plants than D. discoideum (Fig. 5). The sequences of cyanobacteria and D. discoideum lacked a second insertion region between U2 and U3 present only in plants (Delmer, 1999; Blanton et al., 2000).
Figure 5.
Multiple alignment of amino acid sequences from 17 prokaryotic cellulose synthase homologs with CesA sequences from Arabidopsis, Gossypium hirsutum, corn, Populus tremulus × Populus alba, and D. discoideum. The alignment demonstrates the presence of a CR-P region between the U1 and U2 domains present only in eukaryotic and cyanobacterial sequences.
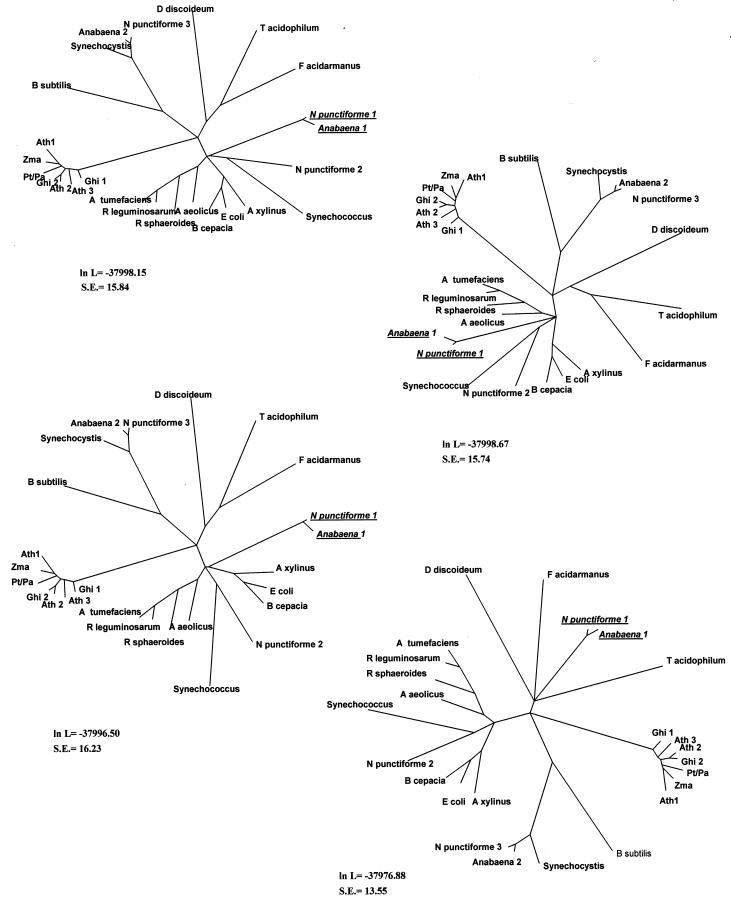
Trees generated using both neighbor joining (NJ) and maximum parsimony (MP) showed similar overall results (Fig. 6). As expected, the vascular plant sequences formed a monophyletic group. As described previously (Holland et al., 2000), CesA orthologs were seen to be more similar than paralogs. NJ and MP methods demonstrated a close phylogenetic relationship between the amino acid sequences of cyanobacteria and plants supported by high bootstrap values (Fig. 6). The branch connecting the cyanobacteria to plants is very deep, thus raising the possibility that the placement of the cyanobacterial branch was a long branch attraction artifact. To test the validity of the cyanobacterial/plant grouping, a quartet puzzling (QP) maximum likelihood tree was constructed from the multiple alignment. The maximum likelihood phylogeny reinforced the cyanobacteria/plant relationship demonstrated with NJ and MP methods (Fig. 7). Of 954 trees generated, only 1.3% failed to show this relationship. Additionally, alternative topologies were generated by manually grouping the cyanobacterial clade with other groups in the tree. The topologies were analyzed using the test (5% significance) of Kishino and Hasegawa (1989) and compared to the original topology. All other topologies showed log likelihood values smaller (even when ses were taken into account) and were rated by TreePuzzle as significantly worse trees than the maximum likelihood topology (Figs. 7 and 8).
Figure 6.
Comparison of NJ and MP trees. The tree shown is an NJ tree subjected to 5,000 bootstrap trials. Bootstrap values are shown as percentages with MP bootstrap values shown in parentheses. Differences in the MP tree are denoted by bold lines (multifurcations) and dashed arrow (variable position), and an asterisk indicates rooting at the base of the tree. Note the distribution of cyanobacterial sequences in the tree: two sequences from Anabaena sp. PCC 7120 and N. punctiforme branch with vascular plants; two sequences from N. punctiforme and Synechococcus branch distantly with Thermotogales and Proteobacteria; and three sequences from Synechocystis, N. punctiforme, and Anabaena, which are most likely CSL proteins, group with Bacillus subtilis. The high bootstrap values support the validity of the tree.
Figure 7.
The maximum likelihood phylogeny for the cellulose synthase sequences showing confidence values and the log likelihood. With the exception of D. discoideum (which groups with the Euryarchea in this tree), the relationships in this tree are nearly identical to those shown in Figure 6.
Figure 8.
Four distinct phylogenies alternative to the maximum likelihood phylogeny for N. punctiforme 1 and Anabaena 1 sequences (underlined). The log likelihood and the se are shown for each tree.
The trees generated place cyanobacterial sequences in three distinct branches (Figs. 6 and 7). Synechocystis sp. PCC 6803 (D90912), N. punctiforme ATCC 29133 (contig 583), Anabaena sp. PCC 7120 (contig 294), and B. subtilus (CAB12237) display a close grouping in all trees. Based on their small size and low similarity to known cellulose synthase sequences, these sequences most likely represent CSLs rather than cellulose synthases. Two cyanobacterial sequences (N. punctiforme ATCC 29133 [contig 640] and Synechococcus sp. WH8102 [contig 251]), present in a second main branch of the tree, group distantly with cellulose synthases of other gram-negative bacteria. Anabaena sp. PCC 7120 (contig 326) and N. punctiforme (contig 499) share a sister branch with vascular plants, indicating a distinct phylogenetic relationship between the cellulose synthase homologs of cyanobacteria and CesAs of vascular plants.
DISCUSSION
Cellulose Structure
Although several previous reports suggested the presence of cellulose among the cyanobacteria, it was actually a surprise to find that the product is so widespread among this group of organisms. Thus far, we have found three of the five sections of cyanobacteria to have genera exhibiting cellulose biosynthesis. It is intriguing to note cellulose production, but it is also necessary to understand where it is assembled. We have found cellulose in slime tubes, sheaths, and extracellular slime, the three major classes of extracellular polysaccharides in the cyanobacteria. The presence of cellulose in the sheath of S. hofmanni represents the first report of prokaryotic secretion of cellulose as an attached, capsular-type EPS. This is also the first report of cellulose in slime tubes of motile cyanobacterial trichomes.
What is known about the molecular machinery involved in cyanobacterial gliding motility? Gliding motility takes place with the concomitant secretion of polysaccharides. Circumstantial evidence implicates junctional pore complexes (JPCs) as points of polysaccharide secretion associated with gliding motility (Hoiczyk and Baumeister, 1998). Recently, outer membrane pores necessary for the secretion of a Type 1 capsular polysaccharide in E. coli were described (Drummelsmith and Whitfield, 2000). These pores are exposed on the outer surface and, based on transmission electron microscopy examinations, have structural similarity with cyanophycean JPCs. Cellulose present in the slime tubes of actively motile cyanobacterial filaments suggests a possible tie to gliding motility. The maximum rate of gliding in cyanobacteria is more than 100 times the rate of cellulose ribbon synthesis in A. xylinus, suggesting that the cyanobacterial movement is based on a more complex set of materials and processes than A. xylinus. In A. xylinus, the rate of movement is approximately 2 to 3 um min−1, whereas cyanobacterial filaments have demonstrated the ability to glide at up to 10 um s−1 (Castenholz, 1982). We can only speculate on the role of cellulose assembly in cyanobacterial gliding motility at present. Perhaps the slime tube is a frictional bearing for anchoring of other motility elements responsible for the gliding; however, relatively little is known about this process.
Interestingly, the microfibrils of cyanobacteria have smaller dimensions than those of other organisms, including A. xylinus (Brown et al., 1976), certain algae such as Valonia (Itoh and Brown, 1984), and Boergesenia (Itoh et al., 1984), and vascular plants (Brown, 1985). The microfibrils of the Rhodophycean alga Erythrocladia also have small dimensions of 1 to 1.5 × 10 to 33 nm (Okuda et al., 1994). Distinct linear terminal complexes assemble these thin yet wide microfibrils. Evidence implicating the JPCs as sites of polysaccharide secretion in the cyanobacteria is circumstantial; however, it is interesting to note that the JPCs are located in a ring around the filament, thus in a favorable position to secrete polysaccharides in general. It appears that the cellulose from cyanobacteria is only a small percentage of the total extracellular polysaccharide, as compared with A. xylinum and cell walls of vascular plants (Brown, 1985). It has been demonstrated that when A. xylinus synthesizes cellulose in the presence of carboxymethyl cellulose and other polysaccharides, the crystallinity of the cellulose is reduced (Hirai et al., 1998). Perhaps, in a similar fashion, the copious production of extracellular materials other than cellulose by cyanobacteria prevents lateral aggregation of microfibrils into larger units, thus accounting for the small observed crystallite size.
Phylogenetic Analysis
With the exciting progress in genome database mapping, researchers have an extremely valuable tool for determining phylogenetic relationships. Using these advances, we were able to gain insight into possible relationships of cellulose synthase among the cyanobacteria and other organisms. Pairwise alignments against the Arabidopsis genome database demonstrate that cyanobacterial cellulose synthase homologs have significantly lower expectation values than any other cellulose synthases not originating from plants. Further evidence for a close relationship of cyanobacterial and plant cellulose synthases was gained by performing a multiple alignment of the 17 prokaryotic sequences with cellulose synthases of D. discoideum and vascular plants. Unlike other prokaryotic sequences, two cyanobacterial sequences share a CR-P region with D. discoideum and vascular plants. In addition, plant CR-P regions show greater sequence similarity to the corresponding cyanobacterial regions than to the insert of D. discoideum.
NJ, MP, and QP trees based on the above multiple alignment showed that putative cyanobacterial cellulose synthases branch closely with plant cellulose synthases. Even though the bootstrap and confidence scores are high, the branch connecting the cyanobacterial sequences of vascular plant sequences is quite deep, leaving open the possibility of a long branch attraction artifact. However, maximum likelihood evaluation shows that tree topologies separating the cyanobacterial and plant clades are not viable. An alternative explanation for the long branch lengths may lie in the ancient origin of chloroplast endosymbiosis which, according to the fossil record, must have occurred more than 2.1 billion years ago (Han and Runnegar, 1992). Such a vast time for divergent evolution could easily account for the formation of deep branches.
There is substantial evidence that chloroplasts of vascular plants have a cyanobacterial origin and that genes can be transferred from the chloroplast to the nuclear genome (Weeden, 1981; Martin and Schnarrenberger, 1997; Martin and Herrmann, 1998; Martin et al., 1998; Krepinsky et al., 2001; Rujan and Martin, 2001). Chloroplasts, originating from the endosymbiotic capture of cyanobacteria, may have shed their cellulose synthases and donated them to the nuclear genome. It is known that a subgroup of cellulose synthases in Arabidopsis exists that bears minor sequence similarity to prokaryotic cellulose synthases (Taylor et al., 1999). Our findings offer a possible explanation for this similarity. The relationship between cyanobacterial cellulose synthase homologs and vascular plant cellulose synthases shown in our phylogram closely resembles the relationship of 16srRNAs from cyanobacteria and chloroplasts (Olsen et al., 1994). This evidence suggests that vascular plant cellulose synthases originated in the cyanobacteria. If invalid trees and convergent evolution are eliminated as possibilities, the relationship of cyanobacterial sequences to vascular plant sequences seems inexplicable except in terms of synology. Unfortunately, the current lack of CesA sequences from the algae and from more primitive cyanobacteria leaves a significant gap in the tree and makes it impossible to determine, with any certainty, the precise nature of the relationship between cyanophycean and plant cellulose synthases. Recently, however, the McCesA3 gene from Mesotaenium caldariorum UTEX 41 was sequenced by Roberts et al. (2000). Multiple alignments and phylograms (data not shown) demonstrate not only the expected close relationship of the algal sequence to vascular plants CesAs, but also a branch point closer to the cyanobacterial/vascular plant divergence point.
No sequences with similarity to cellulose synthase exist in genomes of chloroplasts or cyanelles sequenced to date. Given the lack of any such homologs in the cyanelle genome of the primitive alga Cyanophora paradoxa, it is reasonable to assume that if the cellulose synthases of plants were obtained via cyanobacterial endosymbioses, translocation of the gene to the nucleus must have occurred relatively early in the evolution of algae. The divergence of cyanobacterial and plant sequences from the branch point reinforces the idea of an early transfer of cellulose synthase from cyanobacteria to a eukaryotic cell.
Proteins associated with vascular plant cellulose synthases have been elusive, and cyanobacteria could offer a simplified model system for identification of these proteins. Homologs to the known cellulose synthase-associated proteins found in A. xylinus and A. tumefaciens are lacking in all cyanobacteria analyzed in this study. In fact, many of the ORFs in the vicinity of the putative cyanobacterial cellulose synthases have no sequence similarity to any proteins of known function. Therefore, it is likely that cyanobacteria possess a heretofore undescribed regulatory system for cellulose biosynthesis in prokaryotes. Curatti et al. (2000) recently isolated a prokaryotic Suc synthase gene from Anabaena sp. PCC 7119. This gene, which is very similar to the Suc synthase of vascular plants, also has substantial sequence similarity with ORFs found in databases of the cellulose producing N. punctiforme ATCC 29133 and Anabaena sp. PCC 7120. Since it is thought that SusY from vascular plants is tied to cellulose synthesis (Amor et al., 1995), it would not be surprising to find that the cyanobacterial homolog to SusY is associated with cellulose synthesis in the cyanobacteria. If this is the case, perhaps other cellulose synthase-associated proteins are shared between vascular plants and the cyanobacteria.
Two putative cellulose synthase proteins and one likely CSL protein exist in N. punctiforme ATCC 29133, a nitrogen fixing, facultatively heterotrophic, symbiotically competent cyanobacterium capable of differentiation into heterocysts, akinetes, and motile hormogonia, (Cohen et al., 1994). These different genes may be conditionally expressed in specific differentiated cell types serving different functions in each. For example, cellulose could serve as a means of attachment to the host plant in the formation of symbiotic relationships in a manner similar to A. tumefaciens' attachment to its host plant (Matthysse, 1983). In addition, cellulose could have roles in gliding motility of hormogonia, desiccation tolerance, nitrogen fixing efficiency of heterocysts, enhancing viability of akinetes, or protection from UV light.
In conclusion, the proof of cellulose biosynthesis in the strains of Anabaena UTEX 2576 and Nostoc punctiforme ATCC 29133 correlates directly with the discovery of the putative cellulose synthase homologs from these same organisms. This adds validity to the identity of these sequences as cellulose synthases. Given that the cyanobacteria were probably among the earliest forms of life on earth, contributing to the oxygen of the planet's atmosphere over eons, it is interesting to speculate why these organisms, of all potential photosynthetic life forms, gained prominence in the primitive earth environment. In the reducing atmosphere, severe UV radiation could have posed a serious hazard; however, a cellulose sheath, slime tube, or extracellular matrix could have shielded the cell from UV radiation. If the cellulose contributed to motility, this ability could have been important to allow these early cells to move into shade and avoid the intense radiation. It is ironic that the earliest producers of cellulose may be the last major group of organisms to have cellulose discovered among them!
MATERIALS AND METHODS
Strains and Growth Conditions
All cultures were grown photoautotrophically with a 12-h light/dark cycle under fluorescent light. Cultures of Oscillatoria sp. UTEX L2345, Oscillatoria princeps CE-95-OP Cl1 CC 5122, Phormidium autumnale UTEX 1580, Synechocyctis sp. ATCC 27184 (also known as PCC 6803), Crinalium epipsammum ATCC 49662, and Synechococcus sp. UTCC 100 (syn. PCC 7942) were maintained in BG11 medium. Anabaena sp. UTEX 2576 (syn. PCC 7120), N. muscorum UTEX 2209, Nostoc punctiforme ATCC 29133, N. muscorum UTEX 1037, Scytonema hofmanni UTEX 2349, Fischerella ambigua UTEX 1908, and Gloeocapsa sp. UTEX L795 were maintained in BG110 medium. Cultures were routinely maintained on agar plates prepared as previously described (Golden et al., 1987). When possible, contaminated cultures were purified by the method of Rippka (1988). With the exceptions of Scytonema hofmanni, Fischerella ambigua, N. muscorum UTEX 1037, and O. princeps, all cultures were axenic and were periodically monitored for contamination by microscopic examination and by growth, in the dark, on BG11 or BG110 plates supplemented with 0.5% (w/v) Glc and .05% (w/v) Vitamin-Free Casamino Acids (Difco Laboratories, Beckton Dickinson & Co., Franklin Lakes, NJ). Batch cultures were grown either as 500-mL cultures in 2-Lflasks on an orbital shaker or as 2.5-L cultures in growth chambers with agitation by magnetic stirrers and aeration. All cultures were maintained at 28°C with the exception of O. princeps, which was maintained at 35°C. Cultures were harvested by centrifugation and used directly for experiments, washed, and resuspended in 0.1 m K2HPO3 buffer, pH 7.0 (PB), and frozen at −80°C, or washed in deionized H2O and lyophilized.
Isolation of EPS
EPSs were isolated through a variation of the method previously described (Barclay and Lewin, 1985). Lyophilized cells resuspended in 30 mL of PB and frozen cells thawed at 37°C were broken by three to five passages through a pre-chilled French Press at 20,000 psi. A microscope was used to monitor cell breakage. The lysate was spun at 9,180g for 40 min. The supernatant was decanted and the pellet resuspended in 30 mL of PB, and the suspension was incubated with an excess of lysozyme (1:5 ratio dry protein weight to wet weight of pellet) at 37°C with gentle shaking for 24 to 48 h. The insoluble fraction was collected by centrifugation at 14,300g and the pellet washed with PB. The pellet was resuspended in 30 mL of 10 mm Tris, pH 7.5, 5 mm EDTA, 0.4% (w/v) SDS, and 66.67 μm proteinase K and incubated overnight at 37°C. The insoluble fraction was collected as above and the pellet washed twice with PB. The pellet was extracted twice each with 30 mL of chloroform/methanol 1:1 and acetone, washed twice in PB, and incubated with 165 units of α-amylase (A-6380, Sigma, St. Louis) and 360 units of amyloglucosidase (Sigma A-3042B) in 30 mL of PB, at 28°C, with gentle shaking, for 24 to 48 h. The insoluble fraction was collected as above, washed twice with deionized water, and lyophilized. Alternatively, sheaths were isolated essentially as described previously (Hoiczyk, 1998). In this case, cells were broken by passage through a French Press at 20,000 psi rather than by sonication.
Collection of Slime Tubes
Slime tube material was pipetted from liquid cultures of actively motile O. princeps 24 to 72 h postinoculation.
CBHI-Gold Labeling
Slime tube material harvested from liquid cultures of O. princeps and EPS isolates from all other cultures were either observed without further treatment or digested with 1 n TFA for 1 h at 100°C and/or acetic nitric reagent (Updegraff, 1969) for 30 min at 100°C. Slime tube material collected from motile filaments on Formvar (polyvinyl formyl; Monsanto, St. Louis)-coated grids was labeled without further treatment. CBHI-gold labeling was performed essentially as described previously (Okuda et al., 1993) with the following exceptions: (1) 10 nm gold was used for the CBHI-gold complex, (2) rather than floating grids, 8-μL drops of enzyme complex were added to Formvar grids with the EPS isolates, and (3) enzyme complex and product were incubated for 3 min at room temperature (RT). Grids were negatively stained with 2% (w/v) uranyl acetate.
Specimen Fixation
Cells were initially fixed in 0.1 m K2HPO3 buffer, pH 7.0 with 2% (v/v) glutaraldehyde for 4 h at RT. Fixed cells were given three 15-min washes and post-fixed with 2% (v/v) osmium tetroxide in 0.1 m buffer, in the dark, for 4 h at 4°C. Cells were washed three times (10 min each) with deionized water and stained with 1% (v/v) aqueous uranyl acetate for 1 h, in the dark, at 4°C. Cells were washed with deionized water for 10 min at 4°C and taken through a graded ethanol series: 30%, 50%, 70%, and 90% for 15 min each at RT. This was followed by two 15-min dehydrations each with 100% ethanol and 100% acetone. Cells were infiltrated with 33% and 66% EMbed 812 (Electron Microscopy Sciences, Fort Washington, PA) in absolute acetone for 12 h each at RT and polymerized for 24 h at 60°C.
CBHI-Gold Staining of Sectioned Material
Ultrathin sections of fixed specimens were placed on carbon-coated Formvar grids. Grids were incubated with Epoxy Resin Remover (Polysciences, Inc., Warrington, PA) for 10 min and washed with glass-distilled water. Grids were labeled and negatively stained in the same manner as EPS and slime tube material.
X-Ray Diffraction
X-ray diffraction analysis was performed with Ni-filtered CuKα (1.542 Å) at 35 kV and 25 mA on a PW 729 x-ray generator (Philips, Eindhoven, The Netherlands) and a Debye/Sherer camera system (Philips).
Acetic nitric-digested, lyophilized EPS isolates were packed into 0.3- or 0.7-mm capillary tubes and diffraction patterns collected under the above conditions for 1 h. Reflections were measured manually with measurements corrected for film shrinkage. Digital images of film negatives were generated using the Interactive Bild Analysung System image processing system. The images were converted to digital diffractograms using NIH Image, Image J, and Microsoft Excel.
Phylogenetic Analysis
Sequences with similarity to cellulose synthases were identified with BLAST (Altschul et al., 1990) searches against the following databases: (a) Department of Energy Joint Genome Institute, http://spider.jgi-psf.org/JGI_microbial/html/; (b) National Center for Biotechnology Information, http://www.ncbi.nlm.nih.gov/PMGifs/Genomes/micr.html; and (c) Kazuza, http://www.kazusa.or.jp/cyano/cyano.html.
Putative cellulose synthase amino acid sequences were then compared with sequences at National Center for Biotechnology Information Arabidopsis Genome database (http://www.ncbi.nlm.nih.gov:80/cgi-bin/Entrez/mapsearch?chr=arabid.inf) with BLAST (Altschul et al., 1990). The contig listings and amino acid accession numbers of the sequence legend in the phylognetic trees are as follows: E. coli, AAC76558.1; Burkholderia cepacia, contig 411; A. xylinus, 2019362A; Rhizobium leguminosarum, AAD28574.1; Rhodobacter sphaeroides, contig 168; Aquifex aeolicus, D70422; Anabaena1, contig 326; Anabaena2, contig 294; Synechocystis, D90912; N. punctiforme1, contig 499; N. punctiforme2, contig 640; N. punctiforme3, contig 583; Ath1, BAB09693.1; Zma, AAF89961.1; Ghi1, T10797; Pt/Pa, AAC78476.1; Ath2, AAC29067.1; Ath3, T51579; Ghi2, AAD39534.2; Dictyostelium discoideum, AAF00200.1; Bacillus subtilis, D69769; Thermoplasma acidophilum, CAC11626.1; Agrobacterium tumefaciens, I39714; and Ferroplasma acidarmanus, contig 137.
Multiple alignments for the creation of unrooted phylogenetic trees were constructed with ClustalX (default settings; Thompson, et al., 1994). Sequences in which the U1, U2, U3, or U4 conserved regions were misaligned were aligned manually. All trees were constructed with original, gapped alignments. For NJ trees, 5,000 bootstrap trials were performed and trees were constructed by the method of Saitou and Nei (1987). MP trees were created with PAUP 4.08 beta version (Sinaur Associates, Sunderland, MA) using a heuristic search algorithm with 100 replicates. Bootstrap analysis was performed with maximum parsimony as optimization criterion with resampling (100 replicates). QP maximum likelihood topologies were constructed with TreePuzzle (http://www.tree-puzzle.de) using the variable time model of substitution (Muller and Vingron, 2000) and assuming uniform rates of heterogeneity with 1,000 puzzling steps. Tree evaluations were made with the test of Kishino and Hasegawa (1989). Trees were drawn and edited with Treeview (http://taxonomy.zoology.gla.ac.uk/rod/treeview.html).
Footnotes
This work was supported in part by grants from the Division of Energy Biosciences, the Department of Energy (grant no. DE–FG03–94ER20145), and the Welch Foundation (grant no. F–1217).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010557.
LITERATURE CITED
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Amor Y, Haigler CH, Johnson S, Wainscott M, Delmer DP. A membrane-associated form of sucrose synthaseand its potential role in synthesis of cellulose and callose in plants. Proc Natl Acad Sci USA. 1995;92:9353–9357. doi: 10.1073/pnas.92.20.9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay W, Lewin R. Microalgal polysaccharide production for the conditioning of agricultural soils. Plant and Soil. 1985;88:159–169. [Google Scholar]
- Blanton RL, Fuller D, Iranfar N, Grimson MJ, Loomis WF. The cellulose synthase gene of Dictyostelium. Proc Natl Acad Sci USA. 2000;97:2391–2396. doi: 10.1073/pnas.040565697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM., Jr Cellulose microfibril assembly and orientation: recent developments. J Cell Sci Suppl. 1985;2:13–32. doi: 10.1242/jcs.1985.supplement_2.2. [DOI] [PubMed] [Google Scholar]
- Brown RM, Jr, Willison JH, Richardson CL. Cellulose biosynthesis in Acetobacter xylinum: visualization of the site of synthesis and direct measurement of the in vivo process. Proc Natl Acad Sci USA. 1976;73:4565–4569. doi: 10.1073/pnas.73.12.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castenholz RW. Motility and taxes. In: Carr NG, Whitton BA, editors. The Biology of Cyanobacteria. Boston: Blackwell Scientific Publications; 1982. pp. 413–440. [Google Scholar]
- Cohen MF, Wallis JG, Campbell EL, Meeks JC. Transposon mutagenesis of Nostoc sp. strain ATCC 29133, a filamentous cyanobacterium with multiple cellular differentiation alternatives. Microbiology. 1994;140:3233–3240. doi: 10.1099/13500872-140-12-3233. [DOI] [PubMed] [Google Scholar]
- Curatti L, Porchia AC, Herrera-Estrella L, Salerno GL. A prokaryotic sucrose synthase gene (susA) isolated from a filamentous nitrogen-fixing cyanobacterium encodes a protein similar to those of plants. Planta. 2000;211:729–735. doi: 10.1007/s004250000343. [DOI] [PubMed] [Google Scholar]
- Delmer D. Cellulose biosyntheis: exciting times for a difficult field of study. Annu Rev Plant Physiol Mol Biol. 1999;50:245–276. doi: 10.1146/annurev.arplant.50.1.245. [DOI] [PubMed] [Google Scholar]
- Drews G, Weckesser J. Function, structure and composition of cell walls and external layers. In: Carr NG, Whitton BA, editors. The Biology of Cyanobacteria. Boston: Blackwell Scientific Publications; 1982. pp. 333–357. [Google Scholar]
- Drummelsmith J, Whitfield C. Translocation of group 1 capsular polysaccharide to the surface of Escherichia colirequires a multimeric complex in the outer membrane. EMBO J. 2000;19:57–66. doi: 10.1093/emboj/19.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehling-Schulz M, Bilger W, Scherer S. UV-B-induced synthesis of photoprotective pigments and extracellular polysaccharides in the terrestrial cyanobacterium Nostoc commune. J Bacteriol. 1997;179:1940–1945. doi: 10.1128/jb.179.6.1940-1945.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey-Wyssling A, Stecher H. 471'55ben Fenbau Des Nostoc-Schleimes. Z Zellforsch Mikrosk Anat Abt Histochem. 1954;39:515. [PubMed] [Google Scholar]
- Golden SS, Brusslan J, Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- Han T, Runnegar B. Megascopic eukaryotic algae from the 2.1-billion-year-old Negaunee iron-formation, Michigan. Science. 1992;257:232–235. doi: 10.1126/science.1631544. [DOI] [PubMed] [Google Scholar]
- Hess K, Haller R, Katz JR. Die Chemie der Zellulose und ihrer Begleiter. Leipzig: Akademische Verlagsgesellschaft; 1928. [Google Scholar]
- Hirai A, Tsuji M, Yamamoto H, Horii F. In situ crystallization of bacterial cellulose: III. Influences of different polymeric additives on the formation of microfibrils as revealed by transmission electron microscopy. Cellulose. 1998;5:201–213. [Google Scholar]
- Hoiczyk E. Structural and biochemical analysis of the sheath of Phormidium uncinatum. J Bacteriol. 1998;180:3923–3932. doi: 10.1128/jb.180.15.3923-3932.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiczyk E, Baumeister W. The junctional pore complex, a prokaryotic secretion organelle, is the molecular motor underlying gliding motility in cyanobacteria. Curr Biol. 1998;8:1161–1168. doi: 10.1016/s0960-9822(07)00487-3. [DOI] [PubMed] [Google Scholar]
- Holland N, Holland D, Helentjaris T, Dhugga K, Xoconostle-Cazares B, Delmer D. A comparative analysis of the plant cellulose synthase (CesA) gene family. Plant Physiol. 2000;123:1313–1323. doi: 10.1104/pp.123.4.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Brown RM., Jr The assembly of cellulose microfibrils in Valonia macrophysa. Planta. 1984;160:372–381. doi: 10.1007/BF00393419. [DOI] [PubMed] [Google Scholar]
- Itoh T, O'Neil RM, Brown RM., Jr Interference of cell wall regeneration of Boergesenia forbesiiprotoplasts by Tinopal LPW, a fluorescent brightening agent. Protoplasma. 1984;123:174–183. [Google Scholar]
- Kawaguchi T, Decho A. Biochemical characterization of cyanobacterial extracellular polymers (EPS) from modern marine stromatolites (Bahamas). 2000. 30: 321–330. [DOI] [PubMed] [Google Scholar]
- Krepinsky K, Plaumann M, Martin W, Schnarrenberger C. Purification and cloning of chloroplast 6-phosphogluconate dehydrogenase from spinach. Eur J Biochem. 2001;268:2678–2686. doi: 10.1046/j.1432-1327.2001.02154.x. [DOI] [PubMed] [Google Scholar]
- Kishino H, Hasegawa M. Evaluation of the maximum likelihood estimate of the evolutionary tree topologies from DNA sequence data, and the branching order in Hoinoidea. J Mol Evol. 1989;29:170–179. doi: 10.1007/BF02100115. [DOI] [PubMed] [Google Scholar]
- Knoll A. The early evolution of eukaryotes: a geological perspective. Science. 1992;256:622–627. doi: 10.1126/science.1585174. [DOI] [PubMed] [Google Scholar]
- Knoll A. A new molecular window on early life. Science. 1999;285:1025–1030. doi: 10.1126/science.285.5430.1025. [DOI] [PubMed] [Google Scholar]
- Kodani S, Ishida K, Murakami M. Occurrence and identification of UDP-N-acetylmuramyl-pentapeptide from the cyanobacterium Anabaena cylindrica. FEMS Lett. 1999;176:321–325. doi: 10.1111/j.1574-6968.1999.tb13678.x. [DOI] [PubMed] [Google Scholar]
- Martin W, Herrmann R. Gene transfer from organelles to the nucleus: how much, what happens, and why? Plant Physiol. 1998;118:9–17. doi: 10.1104/pp.118.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W, Schnarrenberger C. The evolution of the Calvin cycle from prokaryotic to eukaryotic chromosomes: a case study of functional redundancy in ancient pathways through endosymbiosis. Curr Genet. 1997;32:1–18. doi: 10.1007/s002940050241. [DOI] [PubMed] [Google Scholar]
- Martin W, Stoebe B, Goremykin V, Gansmann S, Hasegawa M, Kowallik K. Gene transfer to the nucleus and the evolution of chloroplasts. Nature. 1998;393:162–165. doi: 10.1038/30234. [DOI] [PubMed] [Google Scholar]
- Matthysse A. Role of bacterial cellulose fibrils in Agrobacterium tumefaciensinfection. J Bacteriol. 1983;154:906–915. doi: 10.1128/jb.154.2.906-915.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller T, Vingron M. Modeling amino acid replacement. J Comput Biol. 2000;7:761–776. doi: 10.1089/10665270050514918. [DOI] [PubMed] [Google Scholar]
- Nicolaus B, Panico A, Lama L, Romano I, Cristina M, De Giulio A, Gambacorta A. Chemical composition and production of exopolysaccharides from representative members of heterocystous and non-heterocystous cyano-bacteria. Phytochemistry. 1999;52:639–647. [Google Scholar]
- Okuda K, Li L, Kudlicka K, Kuga S, Brown RM., Jr β-Glucan synthesis in the cotton fiber: I. Identification of β-1,4- and β-1,3-glucans synthesized in vitro. Plant Physiol. 1993;101:1131–1142. doi: 10.1104/pp.101.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda K, Tsekos I, Brown RM., Jr Cellulose microfibril assembly in Erythrocladia subintegraRosenv.: an ideal system for understanding the relationship between synthesizing complexes (TCs) and microfibril crystallization. Protoplasma. 1994;180:49–58. [Google Scholar]
- Olsen GJ, Woese CR, Overbeek R. The winds of (evolutionary) change: breathing new life into microbiology. J Bacteriol. 1994;176:1–6. doi: 10.1128/jb.176.1.1-6.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipis R, Vincenzini M. Exocellular Polysaccharides from cyanobacteria and their possible applications. FEMS Microbiol Rev. 1998;22:151–175. [Google Scholar]
- Rippka R. Isolation and purification of cyanobacteria. In: Packer L, Glazer A, editors. Methods in Enzymology. Vol. 167. San Diego: Academic Press; 1988. pp. 3–27. [DOI] [PubMed] [Google Scholar]
- Roberts A, Roberts E, Delmer D. The cellulose synthase (CesA) genes of ferns, mosses, and algae. Poster, Year 2000 Annual Meetings, American Association of Plant Biologists, San Diego. 2000. [Google Scholar]
- Roberts E. Biosynthesis of cellulose II and related carbohydrates. PhD thesis. Austin: Univeristy of Texas; 1991. [Google Scholar]
- Ross P, Mayer R, Benziman M. Cellulose biosynthesis and function in bacteria. Microbiol Rev. 1991;55:35–58. doi: 10.1128/mr.55.1.35-58.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rujan T, Martin W. How many genes in Arabidopsis come from cyanobacteria?: an estimate from 386 protein phylogenies. Trends Genet. 2001;17:113–20. doi: 10.1016/s0168-9525(00)02209-5. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:405–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Saxena IM, Brown RM, Jr, Fevre M, Geremia RA, Henrissat B. Multidomain architecture of β-glycosyl transferases: implications for mechanism of action. J Bacteriol. 1995;177:1419–1424. doi: 10.1128/jb.177.6.1419-1424.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopf JW, Walter MR. Origin and early evolution of cyanobacteria: the geological evidence. In: Carr N, Whitton B, editors. The Biology of Cyanobacteria. Vol. 19. Boston: Blackwell Scientific Publications; 1982. [Google Scholar]
- Singh R. Electron Micrographs of the Mucilage of the Blue-Green Algae I. Scytonema pseudogyanense. Ghent, Belgium: Rapport Europees Congres Toegeposte Electronen-microscopie; 1954. pp. 63–67. [Google Scholar]
- Taylor NG, Scheible WR, Cutler S, Somerville CR, Turner SR. The irregular xylem3 locus of Arabidopsis encodes a cellulose synthase required for secondary cell wall synthesis. Plant Cell. 1999;11:769–780. doi: 10.1105/tpc.11.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomme P, Driver DP, Amandoron EA, Miller RC, Jr, Antony R, Warren J, Kilburn DG. Comparison of fungal (family I) and bacterial (family II) cellulose-binding domain. J Bacteriol. 1995;177:4356–4363. doi: 10.1128/jb.177.15.4356-4363.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuffery AA. Light and electron microscopy of the sheath of a blue-green alga. J Gen Microbiol. 1969;57:41–50. [Google Scholar]
- Updegraff DM. Semimicro determination of cellulose in biological materials. Anal Biochem. 1969;32:420–424. doi: 10.1016/s0003-2697(69)80009-6. [DOI] [PubMed] [Google Scholar]
- Weeden NF. Genetic and biochemical implications of the endosymbiotic origin of the chloroplast. J Mol Evol. 1981;17:133–139. doi: 10.1007/BF01733906. [DOI] [PubMed] [Google Scholar]
- Winder B. Crinalium epipsammumsp. nov.: a filamentous cyanobacterium with trichomes composed of elliptical cells and containing poly-β-1,4-glucan cellulose. J Gen Microbiol. 1990;136:1645–1653. [Google Scholar]
- Xiong J, Fischer WM, Inoue K, Nakahara M, Bauer CE. Molecular evidence for the early evolution of photosynthesis. Science. 2000;289:1724–1730. doi: 10.1126/science.289.5485.1724. [DOI] [PubMed] [Google Scholar]
- Zogaj X, Nimtzv M, Rohde M, Bokranz W, Romling U. The multicellular morphotypes of Salmonella typhimurium and Escherichia coliproduce cellulose as the second component of the extracellular matrix. Mol Microbiol. 2001;39:1452–1463. doi: 10.1046/j.1365-2958.2001.02337.x. [DOI] [PubMed] [Google Scholar]