Abstract
Why the leaves of many woody species accumulate anthocyanins prior to being shed has long puzzled biologists because it is unclear what effects anthocyanins may have on leaf function. Here, we provide evidence for red-osier dogwood (Cornus stolonifera) that anthocyanins form a pigment layer in the palisade mesophyll layer that decreases light capture by chloroplasts. Measurements of leaf absorbance demonstrated that red-senescing leaves absorbed more light of blue-green to orange wavelengths (495–644 nm) compared with yellow-senescing leaves. Using chlorophyll a fluorescence measurements, we observed that maximum photosystem II (PSII) photon yield of red-senescing leaves recovered from a high-light stress treatment, whereas yellow-senescing leaves failed to recover after 6 h of dark adaptation, which suggests photo-oxidative damage. Because no differences were observed in light response curves of effective PSII photon yield for red- and yellow-senescing leaves, differences between red- and yellow-senescing cannot be explained by differences in the capacities for photochemical and non-photochemical light energy dissipation. A role of anthocyanins as screening pigments was explored further by measuring the responses PSII photon yield to blue light, which is preferentially absorbed by anthocyanins, versus red light, which is poorly absorbed. We found that dark-adapted PSII photon yield of red-senescing leaves recovered rapidly following illumination with blue light. However, red light induced a similar, prolonged decrease in PSII photon yield in both red- and yellow-senescing leaves. We suggest that optical masking of chlorophyll by anthocyanins reduces risk of photo-oxidative damage to leaf cells as they senesce, which otherwise may lower the efficiency of nutrient retrieval from senescing autumn leaves.
Senescing leaves of many temperate deciduous plants turn brilliant red in autumn (Wheldale, 1916; Sanger, 1971; Chang et al., 1989; King, 1997; Kozlowski and Pallardy, 1997). Unlike yellow and orange autumn leaves where chlorophyll breakdown unmasks the already present carotenoid pigments, most red leaves result from de novo synthesis of anthocyanins (Matile et al., 1992; King, 1997; Kozlowski and Pallardy, 1997; Matile, 2000). An exception to this is the winter accumulation of red carotenoids in the shoots of some conifers and leaves of a few evergreen flowering plants (Ida et al., 1995). Anthocyanins are a group of water-soluble flavonoids (glycosides of phenolic aglycons with a flavan C6-C3-C6 skeleton) produced in the cytoplasm and then transported into the vacuole (Harborne, 1988; Marrs et al., 1995; Shirley, 1996). It has been unclear why anthocyanins are synthesized in autumn leaves just before they are shed (Mohr and Schopfer, 1994; Archetti, 2000; Matile, 2000). A recent study proposed that autumn anthocyanins have no direct physiological significance to plants but instead reflect co-evolutionary interactions with aphids, where anthocyanins act as “warning coloration” to deter herbivores (Archetti, 2000). Ford (1986) hypothesized that accumulation of anthocyanins may represent an excretion process to load toxins into the soon-to-be-discarded leaves. The prevailing view among plant physiologists is that anthocyanins are a nonfunctional by-product of leaf senescence (Mohr and Schopfer, 1994; Archetti, 2000; Matile, 2000). Anthocyanins are end products of the flavonoid pathway and the induction of anthocyanin synthesis has been suggested to result from carbohydrate “overflow” during the active recycling of photosynthetic proteins (Matile, 2000). However, the induction of anthocyanin synthesis by high light in tissues that are unlikely to have an excess of carbon reserves, such as germinating seedlings, is inconsistent with the carbon overflow hypothesis (Christie et al., 1994; Yanovsky et al., 1998). Anthocyanin synthesis in autumn leaves often precedes chlorophyll breakdown and the color intensity of red-senescing leaves is increased by high light, cool (but not freezing) temperatures, and mild drought (Wheldale, 1916; Kozlowski and Pallardy, 1997; Dodd et al., 1998; Chalker-Scott, 1999). These conditions affect the capacity for photosynthesis in ways that increase the requirement for protective dissipation of excess light energy (Demmig-Adams and Adams, 1992; Long et al., 1994; Horton et al., 1996; Huner et al., 1996, 1998; Thomas, 1997; Matile et al., 1999). We suggest that the buildup of anthocyanins in autumn leaves contributes to the shielding of leaf chloroplasts from excess sunlight during senescence.
A protective role for anthocyanins as “sunscreens” (for review, see Wheldale, 1916; Chalker-Scott, 1999) and as scavengers of reactive oxygen has been suggested previously for young, expanding leaves (Baker and Hardwick, 1973; Lee et al., 1987; Dodd et al., 1998), developing fruits (Hetherington, 1997; Smillie and Hetherington, 1999; Merzlyak and Chivkunova, 2000), the leaves of cold-stressed plants (Krol et al., 1995; Grace et al., 1998; Close et al., 2000; Grace and Logan, 2000), and the leaves of plants of deeply shaded tropical rainforest understories (Gould et al., 1995, 2000). Because anthocyanins strongly absorb blue-green light (Harborne, 1988; Neill and Gould, 1999; Smillie and Hetherington, 1999; Barnes et al., 2000; Merzlyak and Chivkunova, 2000), the accumulation of anthocyanins in red autumn leaves may attenuate the quality and quantity of light captured by chlorophylls and carotenoids as leaves senesce. The major activity during leaf senescence is nutrient resorption for leaf production during the next growing season (Killingbeck, 1996; Buchanan-Wollaston, 1997; Thomas, 1997; Matile et al., 1999; Matile, 2000; Quirino et al., 2000). Thus, protection from excess irradiance may play a role in limiting oxidative damage that may interfere with the retrieval of inorganic nutrients from senescing autumn leaves. The effects of anthocyanins on the ability of senescing autumn leaves to cope with excess sunlight have not been previously investigated. Here, we measure leaf optical properties and use modulated chlorophyll a fluorescence measurements to probe PS II function in senescing autumn leaves of red-osier dogwood (Cornus stolonifera) with and without anthocyanins.
RESULTS
Senescing autumn leaves of red-osier dogwood exposed to direct sunlight turn reddish-purple due to anthocyanin accumulation in the vacuoles of the palisade mesophyll cells (data not shown). We refer to these leaves as “red-senescing.” In contrast, leaves senescing in sub-canopy environments do not produce anthocyanins (Table I) and become pale yellow-green during senescence. We refer to these leaves as “yellow-senescing.” At the time of these measurements (late August to mid-September), leaves from both microsites contained approximately 70% of the total chlorophyll content of summer (July) leaves from the same environment. Colorless anthocyanins or leucoanthocyanins were not present in yellow-senescing leaves as indicated by the low absorption of leaf extracts at 540 nm in HCl (Table I; Gould et al., 2000). Thus, red coloration was a reliable indicator of anthocyanin presence. For up to 3 weeks after the beginning of autumn senescence, the lower surfaces of red- and yellow-senescing leaves of similar age appeared equally green in color. Consistent with this observation, red- and yellow-senescing leaves had similar photosynthetic pigment contents (chlorophyll a plus b and total carotenoids; Table I). In addition, there were no differences in leaf structural features that could alter the internal optical environment between red-and yellow-senescing leaves (Table I; Vogelmann, 1993). Finally, maximum photosystem II (PSII) photon yields (Fv/Fm) were not significantly different between red- and yellow-senescing leaves (Table I). These observations demonstrate that red- and yellow-senescing leaves form an appropriate system to investigate the effects of autumn anthocyanins on light utilization by chloroplasts (Gould et al., 2000).
Table I.
Physiological and pigment characteristics of senescing leaves of red-osier dogwood with and without anthocyanins
| Variable | Yellow-Senescing Leaves | Red-Senescing Leaves |
|---|---|---|
| Fv/Fm (n = 15, relative units) | 0.80 ± 0.05 | 0.79 ± 0.03, NS |
| Anthocyanins (n = 10; μg cm−2) | 0.0 | 0.45 ± 0.0249*** |
| Chlorophylls (n = 10) | – | |
| a+b (μg cm−2) | 15.7 ± 1.7 | 13.2 ± 0.9, NS |
| b (μg cm−2) | 3.8 ± 1.6 | 2.1 ± 0.1, NS |
| Total carotenoids (n = 10, μg cm−2) | 3.1 ± 0.2 | 3.3 ± 0.2, NS |
| Total leaf thickness (n = 6, μm) | 74.6 ± 2.7 | 76.3 ± 5.6, NS |
| Palisade mesophyll (n = 6, μm) | 24.5 ± 1.8 | 27.3 ± 2.4, NS |
| Spongy mesophyll (n = 6, μm) | 44.3 ± 1.8 | 43.5 ± 5.5, NS |
| Lower epidermis (n = 6, μm) | 5.8 ± 0.8 | 5.5 ± 0.8, NS |
Chlorophyll fluorescence parameters and pigment contents (expressed on a fresh, leaf area basis) were determined as in Wellburn (1994). Fv/Fm, Maximal dark-adapted photosystem II photon yield (means ± sd, n = sample size; *, **, and *** denote the degree of statistical significance, <0.05, 0.01, and 0.001, respectively); NS, not significantly different (Student's t test). Red- and yellow-senescing leaves were the same age; low chlorophyll content reflects chlorophyll loss during senescence.
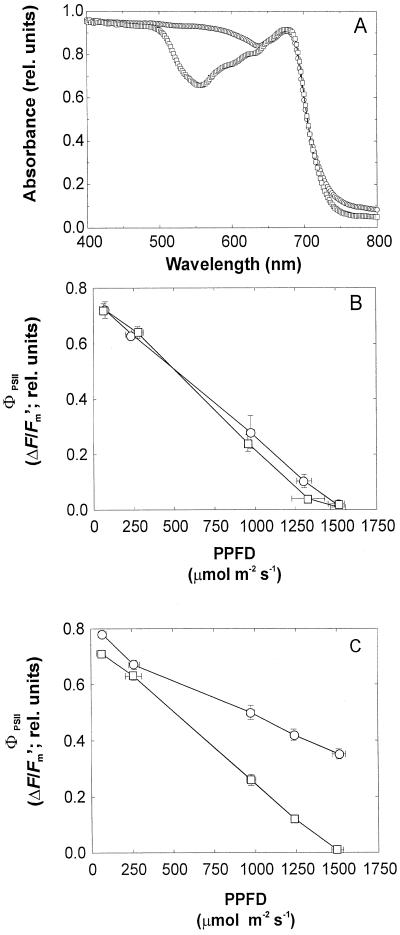
Red-senescing leaves absorbed more light of blue-green to orange wavelengths (495–644 nm) compared with yellow-senescing leaves (Fig. 1A). In response to increasing photosynthetic photon flux density (PPFD) applied to the leaf lower surface, red- and yellow-senescing leaves had similar responses of effective PSII photon efficiency (ΦPSII, Fig. 1B). However, if light was applied to the top leaf surface, the response of ΦPSII in red- and yellow-senescing leaves differed. Lower surface chloroplasts in red-senescing leaves maintained a 50% higher ΦPSII (at 1,500 μmol m−2 s−1), compared with yellow-senescing leaves (Fig. 1C).
Figure 1.
Leaf absorbance spectra of red- (circles) and yellow- (squares) senescing leaves of red-osier dogwood (A). Light response of effective PSII photon efficiency (ΦPSII; calculated from ΔF/Fm′) in red- (circles) and yellow- (squares) senescing leaves illuminated from the leaf undersurface (B) as compared with those illuminated on the leaf upper surface (C). Measurements were made on detached leaves in a humidified chamber at constant gas concentration (380 μL L−1 carbon dioxide, 21% [v/v] oxygen balanced with nitrogen gas) and temperature (20°C ± 2°C). Results for A through C are means for three leaves and error bars denote the sd.
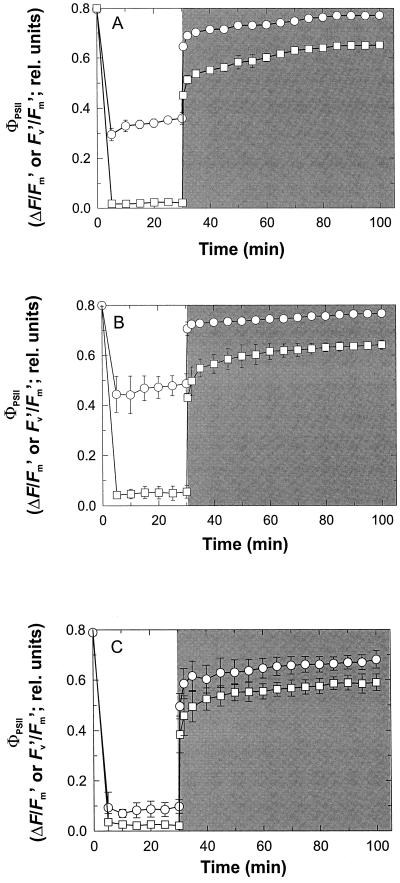
Exposure to high white light (1,500 ± 50 μmol m−2 s−1) for 30 min led to a large reduction in the ΦPSII of yellow-senescing leaves compared with red-senescing leaves (Fig. 2A). When the light was turned off, ΦPSII (measured as Fv′/Fm′ in the dark) in red-senescing leaves returned quickly (approximately 80 min) to the dark-adapted state (Fig. 2A). In contrast, yellow-senescing leaves exhibited a sustained depression of ΦPSII that did not recover to the pretreatment state despite prolonged dark adaptation (Fig. 2A). Measurements of ΦPSII after 6 h of dark adaptation were 23% lower than the pretreatment state (mean = 22.7; sd = 3.5; n = 5).
Figure 2.
Changes in effective PSII photon efficiency (ΦPSII under irradiation and Fv′/Fm′ following darkening) to excess PPFD (1,500 ± 50 μmol m−2 s−1) treatments of varying wavelength distribution. A, Illuminated with white (400–800 nm) light; B, illuminated with blue (400–550 nm) light; C, illuminated with red (640–710 nm light) for red- (circles) and yellow- (squares) senescing red-osier dogwood leaves. The light was turned off after 30 min (as indicated by the shaded box) and ΦPSII recovery measured as described in “Materials and Methods.” Measurements were made under the conditions described in Figure 1. Each curve for A through C is an average of five leaves per treatment and error bars denote the sd.
The influence of anthocyanins on the relaxation kinetics of ΦPSII were explored further by illuminating the upper surfaces of red- and yellow-senescing leaves with blue-enriched light, which is preferentially absorbed by anthocyanins, versus red-enriched light, which is poorly absorbed (Harborne, 1988; Smillie and Hetherington, 1999; Neill and Gould, 1999). Lower leaf surface chloroplasts in red-senescing leaves maintained a higher ΦPSII when illuminated with blue light and recovered to the pretreatment maximum ΦPSII, whereas those in yellow-senescing leaves did not recover fully in darkness (Fig. 2B). In contrast, red-enriched light induced a similar light-dependent decrease in ΦPSII in red and yellow-senescing leaves, and neither recovered fully following dark adaptation (Fig. 2C).
There were no differences in leaf nitrogen content (grams nitrogen per grams dry leaf tissue) for the leaves from the two microsites sampled at mid-summer (July 18) or immediately following leaf abscission (November 5; Table II). The percentage leaf nitrogen retranslocated was approximately 57% and did not differ between red- and yellow-senescing leaves (Table II).
Table II.
Leaf nitrogen content (percentage of leaf dry mass) of red-osier dogwood leaves collected at mid-summer and at leaf abscission
| Time of Year | Nitrogen Content | Nitrogen Translocated |
|---|---|---|
| % of leaf dry mass | % | |
| Green, exposed (July 18, n = 5) | 1.70 ± 0.18 | – |
| Red senescing (November 5, n = 5) | 0.719 ± 0.13 | 56.6 |
| Green, shaded (July 18, n = 5) | 1.55 ± 0.08 | – |
| Yellow senescing (November 5, n = 5) | 0.66 ± 0.06 | 57.6 |
DISCUSSION
This study provides evidence that anthocyanins in senescing leaves of red-osier dogwood form a pigment screen that shields the photosynthetic apparatus from excess light energy. Our experiments show that the major component of PSII down-regulation in lower surface chloroplasts of red-senescing leaves under high light is most likely attributable to non-photochemical processes associated with the pH gradient across the thylakoid membrane (Fig. 2A; Krause and Weis, 1991; Demmig-Adams and Adams, 1992; Long et al.,. 1994; Horton et al., 1996). In contrast, the failure of effective PSII photon yield (ΦPSII) to recover to dark-adapted values in yellow-senescing leaves treated with excess light likely may result from photo-oxidative damage to PSII (Fig. 2A; Powles, 1984; Genty et al., 1989; Krause and Weis, 1991; Demmig-Adams and Adams, 1992; Foyer et al., 1994; Long et al., 1994; Horton et al., 1996). However, the sustained level of non-photochemical quenching observed in yellow-senescing leaves may originate from other prolonged processes that are not necessarily indicative of damage to PSII (Demmig-Adams and Adams, 1992; Horton et al., 1996). Differences between red- and yellow-senescing leaves in the dynamics of ΦPSII following excess light treatment cannot be explained by differences in photochemical utilization, or the capacity for non-photochemical photon energy dissipation. We found that light response curves of ΦPSII for both red- and yellow-senescing leaves illuminated on their lower surface (thus eliminating any light-shielding effect of the anthocyanin layer) were similar (Fig. 1B). In addition, red light, which is poorly captured by anthocyanins, induces a prolonged depression of ΦPSII in both red- and yellow-senescing leaves (Fig. 2C).
The evidence presented here that anthocyanins protect senescing red-osier dogwood leaves from excess light is based on laboratory studies in which we were able to impose on red- and yellow-senescing leaves identical treatments of high light intensity. We based our light treatments on the maximum PPFDs that a red-senescing leaf might experience under natural conditions. Yellow-senescing leaves are those that occur in more shaded microsites, and thus they would not normally experience these PPFDs (see “Materials and Methods”). The absence of any difference in maximum PSII photon efficiency of dark-adapted between red- and yellow-senescing leaves is consistent with the idea that yellow-senescing leaves do not experience high PPFDs at sufficient duration (i.e. sun flecks are short lived) to cause photodamage under natural conditions (Table I). If PPFDs are sufficiently high and prolonged, then anthocyanin accumulation is induced. Red-osier dogwood appears to show a facultative anthocyanin production. Manipulations of red-osier dogwood canopies (i.e. removal of shading branches) in early autumn to expose leaves normally senescing yellow resulted in anthocyanin accumulation. Furthermore, leaves flipped in their orientation accumulate anthocyanins in the spongy mesophyll cells, whereas the palisade mesophyll remains anthocyanin-less during senescence. This indicates that anthocyanin accumulation is not developmentally programmed at the leaf or leaf tissue level. Anthocyanin production and expression of key regulatory enzymes are known to be up-regulated by high light intensity or treatments that limit photochemical utilization of excitation energy (Christie et al., 1994; Nooden et al., 1996; Chalker-Scott, 1999), suggesting that anthocyanins play a physiological role in coping with excess light.
A need for protecting chloroplasts from excess light absorption during autumn senescence at first seems counterintuitive. Given that light interception declines during autumn and thylakoid membranes already contain xanthophyll pigments to dissipate excess light energy (Demmig-Adams and Adams, 1992; Horton et al., 1996), why should an additional mechanism for reducing light captured by chloroplasts be deployed? One hypothesis is that the metabolic changes that occur during leaf senescence increase the susceptibility of light-induced oxidative damage to leaf cells (Nooden et al., 1996; Merzlyack and Hendry, 1994).
Leaf senescence is a programmed transformation of leaf metabolism and ultrastructure whose functional significance is best understood from the perspective of nutrient salvage (Smart 1994; Killingbeck, 1996; Buchanan-Wollaston, 1997; Quirino et al., 2000). This is paramount in plastids where as much as 90% of the nitrogen recycled from senescing leaves comes from the degradation of stroma proteins and thylakoid membranes (Evans, 1983; Killingbeck, 1996; Thomas, 1997; Matile et al., 1999). However, before nitrogen can be mobilized, chlorophyll molecules must be unbound from their associated proteins and enzymatically degraded (Hinder et al., 1996; Thomas, 1997; Matile et al., 1999; Matile, 2000). Chlorophyll breakdown apparently does not result in the release of nutrients that are resorbed by the leaf; instead, chlorophyll is catabolized and the degradation products stored in the vacuole using a detoxification pathway shared with xenobiotic compounds (Peisker et al., 1990; Hinder et al., 1996; Thomas, 1997; Matile et al., 1999; Matile, 2000). This special handling reflects the high phototoxicity of unbound chlorophyll and its derivatives, which readily produce highly reactive singlet oxygen in the presence of light and oxygen (Merzlyack and Hendry, 1994; Thomas, 1997; Marder et al., 1998; Matile et al., 1999). If free chlorophyll is not catabolized or protected from light, the uncontrolled generation of singlet oxygen could jeopardize the viability of senescing leaf cells through photo-oxidative damage (such as per-oxidation of membrane lipids; Merzlyack and Hendry, 1994; Asada, 1999). Because autumn senescence involves the rapid liberation of the entire pool of chlorophyll (Sanger, 1971; Matile, 2000), it presents a substantial opportunity for oxidative damage that may reduce the efficiency of nutrient recovery from senescing leaves. By acting as an optical screen that reduces the light capture of senescing chloroplasts, anthocyanins provide an additional degree of photoprotection during the dismantling of the photosynthetic apparatus.
Although we hypothesize that the functional significance of anthocyanins in autumn leaves relates to nutrient retrieval, we did not observe differences in nitrogen retranslocation between red- and yellow-senescing leaves (Table II). This observation is consistent with our hypothesis because red- and yellow-senescing leaves occupy different microsites in the field and experience different exposures to light. Yellow-senescing leaves, which occur exclusively in the shade, do not naturally experience light intensities sufficient to cause photo-inhibition and thus the likelihood of photo-oxidative damage is low (Table I). Red-senescing leaves, which receive high light intensities, are internally shaded due to the presence of the anthocyanin layer. An experimental approach that compares plants with and without the ability to synthesize anthocyanins, which thus can be forced to senesce in the same light environment, is needed to rigorously test this hypothesis.
Given the variety of pigments and enzymatic pathways that can mitigate light-dependent oxidative stress in plant cells (Demmig-Adams and Adams, 1992; Horton et al., 1996; Asada, 1999), why are anthocyanins used? Anthocyanins appear to be particularly appropriate during autumn senescence for intercepting light that would otherwise be captured by chloroplasts undergoing massive chlorophyll turnover. The high absorbance of anthocyanins in blue wavelengths of light that could be captured by free chlorophyll and some of its breakdown products may represent a cost-effective solution that resides outside the chloroplast at a time when the normal mechanisms for curtailing photodamage are diminished (Merzlyack and Hendry, 1994). Because anthocyanins are localized in the cell vacuole, they may be poised to scavenge oxygenated radicals leaking from chloroplasts as well as mitochondria and peroxisomes (Yamasaki et al., 1996; Yamasaki, 1997; Grace and Logan, 2000). In addition, anthocyanins strongly attenuate green wavelengths of light (Fig. 1A), which although absorbed less efficiently by chlorophyll (Nishio, 2000) nevertheless would penetrate more deeply into the leaf to excite the more shade-adapted chloroplasts (Vogelmann, 1993). This mechanism is, of course, incompatible with photosynthetic carbon gain during the growing season but well suited for autumn senescence when photosynthetic rates decline (Kozlowski and Pallardy, 1997).
Given a functional role for anthocyanins in autumn leaves, how do we account for the fact that a large a number of temperate deciduous species do not produce anthocyanins? Plants possess a number of mechanisms for coping with excess light levels and it is possible that species that do not produce anthocyanins during autumn senescence depend more heavily on alternative mechanisms (for example carotenoid accumulation; Goodwin, 1958; Asada, 1999; Grace and Logan, 2000). In addition, species are known to differ in the efficiency with which they resorb nutrients from senescing leaves (Killingbeck, 1996). In particular, early successional species are frequently much less efficient in nutrient resorption than late successional species (Killingbeck, 1996). The importance of photoprotection during autumn senescence is likely to be related to both the ecology and nitrogen economy of different species. The diversity of coloration patterns during autumn senescence therefore may have important ecological significance and the availability of anthocyanin-deficient mutants provides further opportunities for understanding the physiological role of anthocyanins in leaf senescence.
MATERIALS AND METHODS
Plant Materials
Five shrubs of red-osier dogwood (Cornus stolonifera, Cornaceae) were studied at Fresh Pond Reservoir Reserve (Cambridge, MA) from July to early November of 1998 and 1999. Red-osier dogwood is a common shrub in the understory to sub-canopy of low-lying areas of eastern deciduous forests in North America. In late-summer to early autumn (August to September), leaves exposed to direct sunlight turn reddish-purple due to the accumulation of anthocyanins in their top surface, whereas the lower surface remains green for as long as 3 weeks. Shaded leaves do not accumulate anthocyanins and turn yellow as they senesce. We used these populations of red- and yellow-senescing autumn leaves as an experimental system for investigating the effects of anthocyanins on light utilization by chloroplasts. Paired red- and yellow-senescing leaves were collected from each shrub. Leaves for physiological studies and pigment contents were sampled in the morning from 7 am to 9 am and placed in moist plastic bags until analysis later that day. PPFDs (400–700 nm) were measured directly above red- and yellow-senescing leaves using a hand-held light meter (LI-COR 190, LI-COR, Lincoln NE). Red-senescing leaves occurred in environments that were exposed to full sunlight for several hours of the day. Maximal PPFDs on clear days in these environments ranged from 1,500 μmol m−2 s−1during the 1st week of September to 1,350 μmol m−2 s−1 during the 2nd week of October. Yellow-senescing leaves were not exposed to direct sunlight; however, leaves were exposed to sun fleck PPFDs ranging from 950 to 1,130 μmol m−2 s−1.
Leaf Anatomy and Optical Properties
Fresh red- and yellow-senescing leaves of red-osier dogwood were hand sectioned with a razor blade. Leaf sections, approximately 15 μm thick, were photographed with slide film (Ektachrome 100, Kodak-Eastman, Rochester, NY) at 1,000×. Using a slide projector, anatomical features (thicknesses of the total leaf, the adaxial and abaxial epidermal layer, the palisade, and the spongy mesophyll layer) were measured with a ruler and converted to micrometers relative to a scale standard that was also photographed at 1,000×.
Absorption spectra leaves were calculated from measurements with a Li-1800 spectroradiometer (LI-COR) attached to integrating sphere by fiber optics, using a barium sulfate block as a reference. Prior to making these measurements, leaves were washed with distilled water and blotted gently with a paper towel. Leaf reflectance and transmittance are respectively defined as the proportion of incident, diffuse light that is reflected from, and transmitted through the leaf. Absorbance was calculated as: absorbance = 1 − reflectance − transmittance. Absorption spectra were determined from 350 to 800 nm at a scanning interval of 2 nm.
Pigment Quantification
Chlorophylls (chlorophyll a and b) and total carotenoids (xanthophylls, lutein, and β-carotene) were extracted from a 0.685-cm2 leaf disc using 100% (v/v) N,N-dimethylforamide without tissue disruption for 48 h at 3°C in darkness. Concentrations were determined spectrophotometrically using the equations provided by Wellburn (1994) for a 0.2-nm wavelength bandwidth and a Cary model 219 spectrophotometer (Varian Inc., Palo Alto, CA). Anthocyanins were extracted with N,N-dimethylforamide, acidified with 0.1 n HCl. We estimated total anthocyanins as mg cm−2 in leaves, by subtracting the interference by phaeophytin (Murray and Hackett, 1991; but using 0.55 × A554, appropriate for this solvent). We modified the specific extinction coefficient for cyanidin-3-glucoside determined by Fuleki and Francis (1968) for this solvent at 525 nm: 3.8 × 104 L g−1 cm−1. We also checked for additional interference of soluble tannins by bleaching extracts with 30% (v/v) hydrogen peroxide (Lee et al., 1987).
Chlorophyll a Fluorescence Measurements
Chlorophyll a fluorescence parameters were determined using a pulse amplitude modulated fluorometer (PAM-2000, Heniz Walz, Effeltrich, Germany). For all experiments, the fiber-optic light guide was positioned at 90° relative to the lower leaf surface. Because most of the fluorescence escaping from a leaf surface illuminated with a red measuring beam (centered at 655 nm) originates from the chloroplasts contained in the first few cell layers, our measurements should sample a population of chloroplasts located near the lower leaf surface (Bornmann et al., 1991; Vogelmann, 1993; Vogelmann and Han, 2000). Minimal fluorescence emission (Fo) was determined using a nonactinic measuring beam, following exposure to 10 s of far-red illumination (at 710 nm) to ensure maximal PS II re-oxidation (Feild et al., 1998). An 800-ms saturation pulse was then used to determine the maximal fluorescence yield (Fm). Dark-adapted values for Fm and Fo were measured on leaves placed in darkness for a minimum of 4 h to calculate maximum PSII photon yield (Fv/Fm; Fv = Fm − Fo, Krause and Weis, 1991). Previous studies have shown that a dark period of 1 to 2 h at 20°C is generally sufficient for full relaxation of the fluorescence quenching that is related to the trans-thylakoid proton gradient (ΔpH), non-photochemical quenching caused by the state 1 to state 2 transition, and slower components possibly related to sustained zeaxanthin presence (Foyer et al., 1990; Long et al., 1994; Horton et al., 1996).
The effective photon yield of PSII (ΦPSII, or ΔF/Fm′) was calculated as (Fm′ − F)/Fm′, where F is the fluorescence yield of the light-adapted sample at steady state and Fm′ is the maximum light-adapted fluorescence yield when a saturating light pulse of 800-ms duration (PPFD approximately 3,000 μmol m−2 s−1) is superimposed on the prevailing environmental light intensity (Genty et al., 1989). Light response functions relating ΦPSII to increasing incident light intensity were measured on detached leaves in a humidified chamber at constant gas concentration (380 μL l−1 carbon dioxide, 21% [v/v] oxygen balanced with nitrogen gas) and temperature (20 ± 2°C). ΦPSII was measured as a function of increasing PPFD for leaves illuminated either on their upper or lower leaf surface. Measurements were made at approximately 50, 270, 950, 1,300, and 1,500 μmol m−2 s−1, with a minimum wait time of 20 min per change in light intensity. Light was produced by a halogen bulb filtered with 0.25 mm copper sulfate solution to smooth spectral out from 530 to 700 nm (i.e. intensity was even across these wavelengths because copper sulfate attenuates the red tail emission by a halogen bulb). Emission intensity across this waveband was checked with a spectroradiometer. F values were steady for at least 2 min before application of a saturation pulse. Irradiating the lower leaf surface allowed us to probe ΦPSII without anthocyanins (in the case of red-senescing leaves) affecting the PPFD reaching the chloroplasts sampled with the measuring beam. This provided baseline data on the comparability of the light utilization capacity of lower surface chloroplasts in red- and yellow-senescing leaves. Irradiating the top leaf surface allowed us to examine the effect of an anthocyanin layer on ΦPSII light response of lower surface chloroplasts in red-senescing leaves compared with yellow-senescing leaves.
Relaxation kinetics of PSII down-regulation were determined by measuring the recovery of effective PSII photon yield in the dark (Fv′/Fm′, relative to the dark-adapted value for each leaf measured before treatment) following 30 min of exposure to high light intensity similar to peak midday intensities for unshaded red-osier dogwood leaves in the field. In these experiments, the top surfaces of red- and yellow-senescing leaves were illuminated with actinic light and chlorophyll fluorescence measured from the leaf lower surface. We also investigated the effect of light quality on the relaxation kinetics of non-photochemical quenching. Appropriate wavelengths of light captured versus those that largely bypass anthocyanins were determined from analysis of leaf absorption spectra. Blue-enriched light (400–550 nm) was produced using a 550-nm low-pass filter (peak 537 nm, Andover Filters, Salem, NH) and red-enriched light (640–710 nm) produced with a 640-nm high-pass filter (peak 650 nm, Andover Filters). Actinic PPFD was 1,500 ± 50 μmol m−2 s−1. Tramittance was 80% to 90% across the respective wavelengths produced by blue- and red-light treatments.
Leaf Nitrogen Analysis
Leaf nitrogen concentration (percentage of leaf dry mass) was determined using a carbon-nitrogen analyzer (ANCA-sl, Europa Scientific, Cheshire, UK). Leaves were sampled at mid-summer (July 18, 1999) and at abscission (November 5, 1999). Abscised leaves were sampled by gently shaking branches and collecting the dislodged leaves. Leaf samples were cleaned with a dry paper towel prior to oven drying at 60°C for 24 h.
ACKNOWLEDGMENTS
We gratefully acknowledge Marilyn Ball, Fakhri Bazzaz, Alex Cobb, Chris Field, Peter Melcher, John Nevins, John O'Keefe, Lawren Sack, Mathew Thompson, Graham Timmins, and Maciej Zwieniecki for helpful discussions during the course of this research and comments on the manuscript. We also thank Eithne O'Brien and Baxter O'Brien for help in chlorophyll pigment extractions.
Footnotes
This research was supported by the Harvard Forest at Harvard University, by the Bullard Fellowship (to D.W.L.), and by the Andrew Mellon Foundation.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010063.
LITERATURE CITED
- Archetti M. The origin of autumn colors by coevolution. J Theor Biol. 2000;205:625–630. doi: 10.1006/jtbi.2000.2089. [DOI] [PubMed] [Google Scholar]
- Asada K. The water-water cycle in chloroplasts: scavenging of active oxygen and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:601–639. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- Baker NR, Hardwick Biochemical and physiological aspects of leaf development in cocoa (Theobroma cacao): I. Development of chlorophyll and photosynthetic activity. New Phytol. 1973;72:1315–1324. [Google Scholar]
- Barnes PW, Searles PS, Ballare CL, Ryel RJ, Caldwell MM. Non-invasive measurements of leaf epidermal transmittance of UV radiation using chlorophyll fluorescence: field and laboratory studies. Physiol Plant. 2000;109:274–283. [Google Scholar]
- Bornmann JF, Vogelmann TC, Martin G. Measurement of chlorophyll fluorescence within leaves with fiber-optic microprobes. Plant Cell Environ. 1991;14:719–725. [Google Scholar]
- Buchanan-Wollaston V. The molecular biology of leaf senescence. J Exp Bot. 1997;48:181–199. [Google Scholar]
- Chalker-Scott L. Environmental significance of anthocyanins in plant stress responses. Photochem Photobiol. 1999;70:1–9. [Google Scholar]
- Chang KG, Fechner GH, Schroeder HA. Anthocyanins in autumn leaves of quaking aspen in Colorado. Forest Sci. 1989;35:229–236. [Google Scholar]
- Christie PJ, Alfenito, Walbot V. Impact of low-temperature stress on general phenylpropanoid and anthocyanin pathways: enhancement of transcript abundance and anthocyanin pigmentation in maize seedlings. Planta. 1994;194:541–549. [Google Scholar]
- Close DC, Beadle CL, Brown PH. Cold-induced photoinhibition affects establishment of Eucalyptus nitens (Deane and Maiden) Maiden and Eucalyptus globulus Labill. Trees. 2000;15:32–41. [Google Scholar]
- Demmig-Adams B, Adams WW., III Photoprotection and other response of plants to high light stress. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:599–626. [Google Scholar]
- Dodd IC, Critchley C, Woodall GS, Stewart GR. Photoinhibition in differently colored juvenile leaves of Syzygium species. J Exp Bot. 1998;49:1437–1445. [Google Scholar]
- Evans JR. Nitrogen and photosynthesis in the flag leaf of wheat (Triticum aestivum) Plant Physiol. 1983;72:297–302. doi: 10.1104/pp.72.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feild TS, Nedbal L, Ort DR. Nonphotochemical reduction of the plastoquinone pool in sunflower leaves originated from chlororespiration. Plant Physiol. 1998;116:1209–1218. doi: 10.1104/pp.116.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford BJ. Even plants excrete. Nature. 1986;323:763. [Google Scholar]
- Foyer C, Furbank R, Harbinson J, Horton P. The mechanisms contributing to photosynthetic control of electron transport by carbon assimilation in leaves. Photosynth Res. 1990;25:83–100. doi: 10.1007/BF00035457. [DOI] [PubMed] [Google Scholar]
- Foyer C, Lelandais M, Kunert KJ. Photooxidative stress in plants. Physiol Plant. 1994;92:696–717. [Google Scholar]
- Fuleki T, Francis FJ. Quantitative methods for anthocyanins: I. Extraction and determination f total anthocyanin in cranberries. J Food Sci. 1968;3:72–77. [Google Scholar]
- Genty B, Briantais J-M, Baker NR. The relationship between quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta. 1989;990:87–92. [Google Scholar]
- Goodwin TW. Studies in carotenogensis: XXIIII. Changes in carotenoid and chlorophyll pigments in the leaves of deciduous trees during autumn necrosis. Biochem J. 1958;68:503–511. doi: 10.1042/bj0680503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould KS, Kuhn DN, Lee DW, Oberbauer SF. Why leaves are sometimes red. Nature. 1995;378:241–242. [Google Scholar]
- Gould KS, Markham KR, Smith RH, Goris JJ. Functional role of anthocyanins in the leaves of Quintinia serrata A. Cunn. J Exp Bot. 2000;51:1107–1115. doi: 10.1093/jexbot/51.347.1107. [DOI] [PubMed] [Google Scholar]
- Grace SC, Logan BA. Energy dissipation and radical scavenging by the plant phenylpropanoid pathway. Philos T R Soc B. 2000;355:1499–1510. doi: 10.1098/rstb.2000.0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace SC, Logan BA, Adams WW., III Seasonal differences in foliar content of chlorogenic acid, a phenylpropanoid antioxidant, in Mahonia repens. Plant Cell Environ. 1998;21:513–521. [Google Scholar]
- Harborne JR. The flavonoids: recent advances. In: Goodwin TW, editor. Plant Pigments. London: Academic Press; 1988. pp. 299–343. [Google Scholar]
- Hetherington SE. Profiling photosynthetic competence in mango fruit. J Hortic Sci. 1997;72:755–763. [Google Scholar]
- Hinder B, Schellenberg M, Rodon S, Ginsburg S, Vogt E, Martinoia E, Matile P, Hörtensteiner S. How plants dispose of chlorophyll catabolites: directly energized uptake of tetrapyrrolic breakdown products in isolated vacuoles. J Biol Chem. 1996;271:27233–27236. doi: 10.1074/jbc.271.44.27233. [DOI] [PubMed] [Google Scholar]
- Horton P, Ruban AV, Walters RG. Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- Huner NPA, Maxwell DP, Gray GR, Savitch LV, Krol M, Ivanov AG, Falk S. Sensing environmental temperature change through imbalances between energy supply and energy consumption: redox state of photosystem II. Physiol Plant. 1996;98:358–364. [Google Scholar]
- Huner NPA, Oquist G, Sarhan F. Energy balance and acclimation to light and cold. Trends Plant Sci. 1998;3:224–230. [Google Scholar]
- Ida A, Masamoto K, Maoka T, Fujiwara Y, Takeda S, Hasegawa E. The leaves of the common box, Buxus sempervirens, become red as the level of a red carotenoid, anhydroescholtzxanthin, increases. J Plant Res. 1995;108:369–376. [Google Scholar]
- Killingbeck KT. Nutrients in senesced leaves: keys to the search for potential resorption and resorption proficiency. Ecology. 1996;77:1716–1727. [Google Scholar]
- King J. Reaching for the Sun: How Plants Work. Cambridge: Cambridge University Press; 1997. [Google Scholar]
- Kozlowski TT, Pallardy SD. Physiology of Woody Plants. New York: Academic Press; 1997. [Google Scholar]
- Krause GH, Weis E. Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:313–349. [Google Scholar]
- Krol M, Gray GR, Hurry VM. Low temperature stress and photoperiod affect an increase tolerance to photoinhibition in Pinus banksiana seedlings. Can J Bot. 1995;73:1119–1127. [Google Scholar]
- Lee DW, Brmmeier S, Smith AP. The selective advantage of anthocyanins in developing leaves of mango and cacao. Biotropica. 1987;19:40–49. [Google Scholar]
- Long SP, Humpheries S, Falkowski PG. Photoinhibition of photosynthesis in nature. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:633–662. [Google Scholar]
- Marder JB, Droppa M, Caspi V, Raskin VI, Horvath G. Light-independent thermoluminescence from thylakoids of greening barley leaves: evidence for involvement of oxygen radicals and free chlorophyll. Physiol Plant. 1998;104:713–719. [Google Scholar]
- Marrs KA, Alfenito MR, Lloyd AM, Walbot V. A glutathione S-transferase involved in vaculolar transfer encoded by the maize gene bronze-2. Nature. 1995;375:397–400. doi: 10.1038/375397a0. [DOI] [PubMed] [Google Scholar]
- Matile P. Biochemistry of Indian summer: physiology of autumn leaf coloration. Exp Gerontology. 2000;35:145–158. doi: 10.1016/s0531-5565(00)00081-4. [DOI] [PubMed] [Google Scholar]
- Matile P, Flach BMP, Eller BM. Autumn leaves of Ginkgo biloba L.: optical properties, pigments and optical brighteners. Bot Acta. 1992;105:13–17. [Google Scholar]
- Matile R, Hörtensteiner S, Thomas H. Chlorophyll degradation. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:67–95. doi: 10.1146/annurev.arplant.50.1.67. [DOI] [PubMed] [Google Scholar]
- Merzlyak MN, Chivkunova OB. Light-stress-induced pigment changes and evidence for anthocyanin photoprotection in apples. J Photochem Photobiol B. 2000;55:155–163. doi: 10.1016/s1011-1344(00)00042-7. [DOI] [PubMed] [Google Scholar]
- Merzlyak MN, Hendry GAF. Free radical metabolism, pigment degradation and lipid peroxidation in leaves during senescence. P R Soc Edinb B. 1994;102:459–471. [Google Scholar]
- Mohr H, Schopfer P. Plant Physiology. New York: Springer-Verlag; 1994. [Google Scholar]
- Murray JR, Hackett WP. Dihydroflavonol reductase activity in relation to differential anthocyanin accumulation in juvenile and mature phase Hedra helix L. Plant Physiol. 1991;97:343–351. doi: 10.1104/pp.97.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill S, Gould KS. Optical properties of leaves in relation to anthocyanin concentration and distribution. Can J Bot. 1999;77:1777–1782. [Google Scholar]
- Nishio JN. Why are higher plants green? Evolution of the higher plant photosynthetic pigment complement. Plant Cell Environ. 2000;23:539–548. [Google Scholar]
- Nooden LD, Hillsberg JW, Schneider MJ. Induction of leaf senescence in Arabidopsis thaliana by long days through a light-dosage effect. Physiol Plant. 1996;96:491–495. [Google Scholar]
- Peisker C, Thomas H, Keller F, Matile P. Radiolabeling of chlorophyll for studies on catabolism. J Plant Physiol. 1990;136:544–549. [Google Scholar]
- Powles SB. Photoinhibition of photosynthesis induced by visible light. Ann Rev Plant Physiol. 1984;35:15–44. [Google Scholar]
- Quirino BF, Noh Y-S, Himelblau E, Amasino RM. Molecular aspects of leaf senescence. Trends Plant Sci. 2000;5:278–282. doi: 10.1016/s1360-1385(00)01655-1. [DOI] [PubMed] [Google Scholar]
- Sanger JE. Quantitative investigations of leaf pigments from their inception in buds through autumn coloration to decomposition in falling leaves. Ecology. 1971;52:1075–1081. [Google Scholar]
- Shirley BW. Flavonoid biosynthesis: “new” functions for an “old” pathway. Trends Plant Sci. 1996;1:377–382. [Google Scholar]
- Smart CM. Gene expression during leaf senescence. New Phytol. 1994;126:419–448. doi: 10.1111/j.1469-8137.1994.tb04243.x. [DOI] [PubMed] [Google Scholar]
- Smillie RM, Hetherington SE. Photoabatement by anthocyanin shields photosynthetic systems from light stress. Photosynthetica. 1999;36:451–463. [Google Scholar]
- Thomas H. Tansley review no. 92: chlorophyll: a symptom and a regulator of plastid development. New Phytol. 1997;136:163–181. [Google Scholar]
- Vogelmann TC. Plant tissue optics. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:489–499. [Google Scholar]
- Vogelmann TC, Han T. Measurement of gradients of absorbed light in spinach leaves from chlorophyll fluorescence. Plant Cell Environ. 2000;23:1303–1311. [Google Scholar]
- Wellburn AR. Determination of chlorophyll-a and chlorophyll-b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol. 1994;144:307–313. [Google Scholar]
- Wheldale M. The anthocyanin pigments of plants. Cambridge, UK: Cambridge University Press; 1916. [Google Scholar]
- Yamasaki H. A function of color. Trends Plant Sci. 1997;2:7–8. [Google Scholar]
- Yamasaki H, Uefuji H, Sakihama Y. Bleaching of the red anthocyanin induced by superoxide radical. Arch Biochem Biophys. 1996;332:183–186. doi: 10.1006/abbi.1996.0331. [DOI] [PubMed] [Google Scholar]
- Yanovsky MJ, Alconada-Magliano TM, Mazzella MA, Gatz C, Thomas B, Casal JJ. Phytochrome A affects stem growth, anthocyanin synthesis, sucrose-phosphate-synthase activity and neighbor detection in sunlight-grown potato. Planta. 1998;205:235–241. [Google Scholar]