Abstract
In most insect species, juvenile hormones regulate critical physiological processes such as metamorphosis and reproduction. In insects, these sesquiterpenoids are synthesized by retrocerebral endocrine organs, the corpora allata, via the classical mevalonate (MVA) pathway. One of these compounds, juvenile hormone III (JH III), has also been identified in the sedge Cyperus iria. In higher plants, biosynthesis of the sesquiterpenoid backbone may proceed through two distinct pathways: the MVA pathway or the 2C-methyl erythritol 4-phosphate pathway or through a combination of both pathways. Cell suspension cultures of C. iria were used to elucidate the biosynthetic pathway of JH III in the plant. Enzyme inhibition and labeling studies conclusively demonstrated that the biosynthesis of the sesquiterpenoid backbone of JH III proceeds via the MVA pathway. Inhibitor and precursor feeding studies also suggest that later steps of JH III biosynthesis in C. iria are similar to the insect pathway and that the final enzymatic reaction in JH III biosynthesis is catalyzed by a cytochrome P450 monooxygenase.
Juvenile hormones are a group of structurally related sesquiterpenoids that regulate critical physiological processes including metamorphosis and reproduction in most insect species (Gilbert et al., 1996). Juvenile hormone III (JH III), methyl-10R,11-epoxy-3,7,11-trimethyl-2E,6E-dodecadienoate, has also been identified in the sedge Cyperus iria (Toong et al., 1988).
The presence of an insect hormone and its high levels in C. iria throughout development suggest that this compound may play a role in protection of the plant against insect herbivory (Toong et al., 1988; Bede et al., 1999a). However, there is no evidence that JH III functions as a feeding deterrent. Therefore, any antiherbivory activity would arise from its ability to interfere with insect development. In two laboratory studies, contact of insects with C. iria effectively disrupted development (Toong et al., 1988; Schwartz et al., 1998). These effects were attributed to the JH III content of the plant. However, conclusive evidence that this hormone protects C. iria against insect herbivory has not yet been demonstrated. We have proposed alternatively that JH III may function as an allelopathic agent in C. iria (Bede and Tobe, 2000). This plant species is an extremely invasive weed, responsible for economic losses in many important crops such as rice (Oryza sativa; Holm et al., 1977; Catling, 1992). Treatment of seeds with JH III delays lettuce (Lactuca sativa) seed germination and inhibits shoot growth of rice seedlings (Bede and Tobe, 2000). Therefore, it is possible that this compound confers an ecological advantage to C. iria by leaching from the roots, in which the highest levels are localized, into the immediate environment and affecting the germination and growth of neighboring competing plant species.
Temporal and spatial changes in JH III levels are observed in C. iria throughout development (Bede et al., 1999a). JH III levels increased in the immature seedlings until flowering, at which time a transitory decrease in JH III content of all tissues was observed. In mature plants, JH III levels again increased. As the aerial tissues became senescent, the JH III levels in these tissues declined. This decrease was not observed in the root tissue, which contains the highest levels of JH III in C. iria. These dynamic fluctuations of JH III levels suggest that there is active biosynthesis, catabolism, and perhaps transport of this compound in the plant.
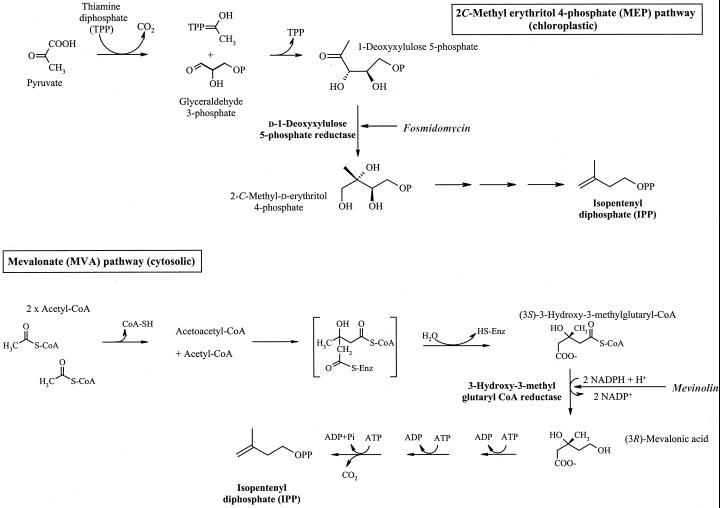
Elucidation of the JH III biosynthetic pathway in C. iria would permit investigation of its localization and metabolic regulation in the plant. Changes in gene expression and enzyme levels or activity then could be determined over development and in response to biotic stimuli such as wounding or insect herbivory. In insects, JH III is biosynthesized in retrocerebral endocrine organs, the corpora allata (CA; Tobe and Stay, 1985). The sesquiterpenoid skeleton of this compound is formed in the cockroach (Diploptera punctata) through the mevalonate (MVA) pathway (Fig. 1; Feyereisen et al., 1981). The regulatory enzyme, HMG-CoA reductase, which catalyzes the irreversible conversion of HMG-CoA to MVA, is thought to be rate limiting in this pathway (Monger, 1985), although its regulatory importance in JH III biosynthesis in the cockroach recently has been questioned (Sutherland and Feyereisen, 1996). MVA undergoes three phosphorylations (net two) and a decarboxylation to generate the isoprene intermediate, isopentenyl diphosphate (IPP; 5C). Covalent linkages of IPP and its isomer, dimethyl allyl diphosphate, form the prenyl diphosphate intermediates that give rise to monoterpenes (10C), sesquiterpenes (15C), diterpenes (20C), and sterols. Insects lack the enzymes to synthesize higher terpenoids and JH III is synthesized from the 15-carbon farnesyl diphosphate (FPP; Svoboda et al., 1975).
Figure 1.
Terpenoid biosynthesis: the MVA and MEP pathways. Mevinolin is an inhibitor of 3-hydroxy-3-methylglutaryl (HMG)-coenzyme A (CoA) reductase, a key regulatory enzyme in the MVA pathway. Fosmidomycin inhibits 1-deoxy-d-xylulose 5-phosphate reductoisomerase in the MEP pathway.
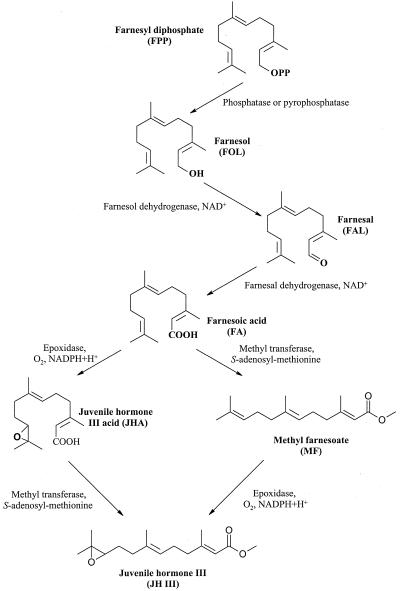
The alcohol farnesol, generated by the removal of pyrophosphate from FPP, undergoes oxidation to the aldehyde (farnesal) and then to the acid (farnesoic acid; Fig. 2). These steps are catalyzed by one or two NAD+-dependent dehydrogenase(s) (Baker et al., 1983). In the cockroach, methylation of farnesoic acid by an S-adenosyl-Met-dependent methyl transferase is followed by epoxidation between C10 and C11 to produce JH III (Schooley and Baker, 1985; Cusson et al., 1991; Cusson and Palli, 2000).
Figure 2.
JH III biosynthesis in insects. This schematic illustrates the putative biosynthetic pathway for JH III in insects. After farnesoic acid (FA), biosynthesis can proceed via an epoxidation at the C10, C11 followed by a methylation of the JH acid (Lepidopteran) or, alternatively, by methylation then the epoxidation (other insect orders).
This pathway, or variations of it, occurs in other insect species. The ethyl branches of the higher JH homologs found in the Lepidoptera are derived from iso-Leu and Val, which are first metabolized to propionate and incorporated, instead of acetate, in the early steps of biosynthesis (Brindle et al., 1987, 1992). The sequence of the final steps of JH biosynthesis may also be different. In the CA of some Lepidopteran species, epoxidation of farnesoic acid to JH acid occurs before final methylation (Fig. 2; Law, 1981; Schooley and Baker, 1985; Cusson et al., 1991). Prepupal stages of the tobacco hornworm (Manduca sexta) and adult males of the silkworm (Hyalophora cecropia) also synthesize JH acids in the CA but release these compounds into the hemolymph, where they then undergo methylation in the imaginal discs or accessory sex glands, respectively (Peter et al., 1981; Sparagana et al., 1985).
In plants, terpenoids may be biosynthesized either through the classical MVA pathway or the 2C-methyl erythritol 4-phosphate (MEP) pathway (Liechtenthaler, 1999). This second pathway has been characterized in bacteria, algae, and recently in plastids of higher plants (Rohmer et al., 1993; Bach, 1995; Eisenreich et al., 1996). In this alternate pathway, a pyruvate-derived, thiamine-activated acetaldehyde undergoes a transketolase-type reaction with glyceraldehyde 3-phosphate to generate 1-deoxy-d-xylulose-5-phosphate (DOXP; Fig. 1; Lichtenthaler et al., 1997). This compound undergoes intramolecular isomerization and reduction to form MEP that after a series of biosynthetic steps generates the 5C isoprene unit, IPP. It is assumed that there is separate compartmentalization of these two pathways and sesquiterpenes, triterpenes, and sterols are biosynthesized through the cytosolic MVA pathway whereas monoterpenes, diterpenes, and tetraterpenes are synthesized through the plastidic MEP pathway.
Characterization of terpenoid biosynthesis is further complicated by recent evidence of limited interchange of isoprene intermediates between these two pathways (Lichtenthaler, 1999; Rohmer, 1999). In chamomile (Matricaria recutita), labeling studies indicate that the first two C5 subunits of the sesquiterpene bisaboloxide A are biosynthesized through the MEP pathway whereas the last C5 unit is derived from either the MEP or MVA pathway (Adam and Zapp, 1998). Therefore, either isoprene subunits are able to traverse the thylakoid membrane or both pathways are localized in the same compartment. In the lima bean (Phaseolus lunatus), the volatile terpenoid, 4,8-dimethylnona-1,3,7-triene (DMNT), is synthesized de novo following treatment of freshly cut plantlets with the elicitor jasmonic acid (Piel et al., 1998). Labeling studies with deuterated intermediates demonstrated that jasmonic acid-induced DMNT is synthesized through the MVA pathway. However, if the MVA pathway is inhibited by mevastatin or cervastatin, labeled precursors can be incorporated into DMNT through the MEP pathway. The authors suggest that this flexibility allows the plant to increase the quantity of IPP precursors necessary for the biosynthesis of DMNT or defensive molecules, such as phytoalexins, during times of stress, such as herbivore or pathogen attack. It is unknown which of these pathways (or both) contribute to the sesquiterpenoid skeleton of JH III in C. iria.
Intermediates from the later steps of the insect biosynthetic pathway of JH III have been isolated from C. iria and related sedges. For example, methyl farnesoate, the immediate biosynthetic precursor to JH III in insects (Fig. 2), has been identified in C. iria, Cyperus microiria Steud, Cyperus monophyllus Vahl., Cyperus pilosus Vahl., and Cyperus serotinus Rottb. (Iwamura et al., 1978a, 1978b, 1978c; Iwamura et al., 1979; Komai et al., 1981; Toong et al., 1988). Another biosynthetic intermediate, farnesol (Fig. 2), has also been isolated from the last four plant species (Iwamura et al., 1978a, 1978c; Iwamura, 1979; Komai et al., 1981). The presence of these intermediates in both plants and insects suggests that the late steps of JH III biosynthesis are similar.
C. iria suspension cultures provide a relatively homogeneous biomass that can be experimentally manipulated under defined, controlled conditions, allowing the investigation of JH III biosynthesis (Bede et al., 1999b). Preliminary investigations of the biosynthesis of the sesquiterpenoid backbone of JH III were conducted through enzyme inhibition studies using the fungal metabolite mevinolin, a potent inhibitor of the MVA pathway, and fosmidomycin, an inhibitor of DOXP reductoisomerase in the MEP pathway, and labeling studies with [1-13C]Glc (Fig. 1). Elucidation of the later steps of the biosynthetic pathway were investigated through precursor feeding studies using intermediates of the insect JH III biosynthetic pathway.
RESULTS
JH III Accumulation in C. iria Cell Suspension Cultures
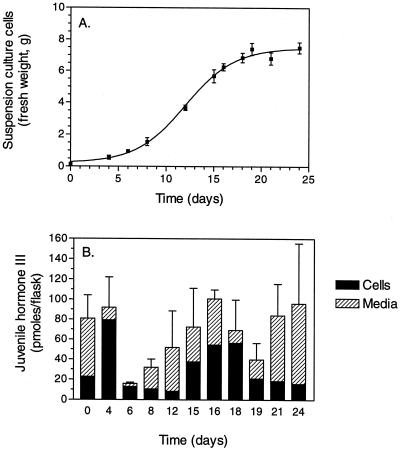
Typical cell growth was observed in the suspension cultures (Fig. 3A). At subculturing, cells are introduced into new medium (d 0). After a brief lag phase (d 0–4), the exponential phase (d 4–7), and the linear growth phase were observed (d 7–16). The progressive deceleration of suspension culture growth occurred at approximately d 16 to 19, followed by the stationary phase. Suspension cultures were subcultured on d 18.
Figure 3.
C. iria suspension cultures. A, Time course of cellular growth in C. iria suspension cultures. On d 18, suspension cells were subcultured into new medium. Each point represents the mean fresh weight of at least three determinations ± se. B, Time course of JH III accumulation in C. iria suspension cultures. JH III was measured by radioimmunoassay (RIA) using antiserum 31867 (1:1,750) and [3H-methyl]-10R, 11-JH III as the radiotracer. Bars represent the means ± se of three determinations.
In general, biosynthesis and accumulation of secondary metabolites in cell suspension cultures occur during the stationary phase (Charlwood and Charlwood, 1991; Banthorpe, 1994). During the exponential and linear phases, the carbon flux is directed toward primary metabolites necessary for growth. As cells enter the stationary phase, biomass production declines and production of secondary metabolites is often observed. As predicted, the level of JH III sharply declined as C. iria suspension cultures entered the growth stage (Fig. 3B). Total JH concentration decreased as cells entered the exponential growth phase and then steadily increased during the linear phase to its highest level in d-17 cultures. As the cells entered the progressive deceleration phase, a decrease in JH III was observed but the concentration of this compound again increased during the stationary phase. The time course of JH III accumulation illustrates temporal fluctuations of this compound in the suspension cultures. To account for these changes, there must be cellular synthesis, transport, release into the medium, and degradation, either intra- or extracellularly or both.
The high concentration of JH III detected in d-0 suspension cultures was surprising. On d 18, suspension cells are subcultured into new medium at approximately a 1:30 dilution and JH III extracted 2 h later. The JH III detected in these suspension cultures was over 20 times the amount expected, considering dilution. Therefore, this is not residual JH III produced in the d-18 cultures and introduced into the new medium, but rather must represent predominantly newly biosynthesized compound. This stimulation of synthesis may be attributable to the introduction of cells to new nutrients or to the stress of subculturing on the suspension culture. This time point was selected for further biochemical studies as it represents a period of high rates of JH III biosynthesis and low rates of degradation.
Biosynthesis of the Sesquiterpenoid Backbone
HMG-CoA reductase (E.C. 1.1.1.34), an important regulatory enzyme in the classical MVA pathway, catalyzes the formation of MVA from HMG-CoA (Fig. 1; Stermer et al., 1994; Chappell et al., 1995). The fungal metabolite mevinolin is a potent competitive inhibitor of this enzyme (Fig. 1; Bach et al., 1990); treatment of tobacco (Nicotiana tabacum L. cv BY-2) suspension cultures with low concentrations of mevinolin (10–30 μm) inhibited the MVA pathway as observed through a reduction in the incorporation of [14C]acetate into free sterols (Vögeli and Chappell, 1991). In vitro, microsomal HMG-CoA reductase activity was inhibited by over 90% in the presence of 3 μm mevinolin. However, in these tobacco cell cultures, higher mevinolin concentrations (100 μm) also inhibited the activity of sesquiterpene cyclase, which catalyzes the first step in the formation of cyclic sesquiterpenoids (Vögeli and Chappell, 1991).
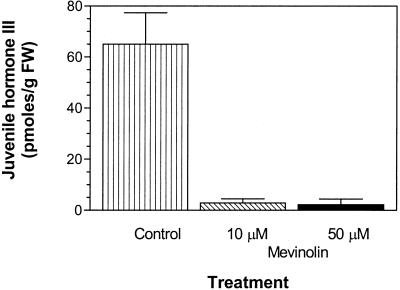
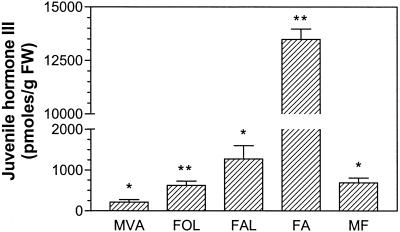
Treatment of C. iria suspension cultures with mevinolin (50 μm) did not affect cell growth at the concentrations tested (P = 0.911). However, >85% of JH III production was inhibited in the presence of 10 or 50 μm mevinolin (Fig. 4). To demonstrate that this effect was attributable to the specific inhibition of HMG-CoA reductase, the product of this enzymatic reaction, MVA, was added to mevinolin-treated cell suspension cultures. JH III production was restored (Fig. 5), confirming that biosynthesis of the terpenoid skeleton of JH III proceeds, at least partially, through the MVA pathway in C. iria.
Figure 4.
Effect of mevinolin on JH III production. JH III production was significantly inhibited in C. iria suspension cultures treated with mevinolin, a potent inhibitor of the key regulatory enzyme in the MVA pathway, HMG-CoA reductase (10 μm, P = 0.022, 96.2% inhibition; 50 μm, P = 0.021, 97.9% inhibition). Treatment with mevinolin did not affect cell growth (10 μm, P = 0.649; 50 μm, P = 0.911; data not shown). Bars represent the mean of three experiments ± se.
Figure 5.
Effect of insect biosynthetic intermediates on JH production in mevinolin-inhibited C. iria cell suspension cultures. JH III production in C. iria suspension cultures was inhibited by mevinolin (50 μm). Putative biosynthetic precursors, MVA lactone (MVA, 5 mm), farnesol (FOL, 0.77 mm), farnesal (FAL, 0.67 mm), farnesoic acid (FA, 0.52 mm), and methyl farnesoate (MF, 0.70 mm) were added to cultures and JH III production monitored by RIA. Addition of JH acid (0.06 mm) did not significantly increase JH III levels (P = 0.707; data not shown). Bars represent the mean of three experiments ± se. Asterisks indicate significant differences between treatments as determined by Student's t tests: *, 0.01 < P < 0.04; **, P < 0.01.
The MEP pathway is responsible for the biosynthesis of plastid isoprenoids, such as carotenoids and the prenyl side chains of chlorophyll (Lichtenthaler, 1999). The rate-limiting step of this pathway, the conversion of DOXP to MEP, is catalyzed by DOXP reductoisomerase (Lange and Croteau, 1999; Fig. 1). Treatment of C. iria suspension cultures with fosmidomycin (0.5 mm), a potent inhibitor of this enzyme and thereby plastid isoprenoid biosynthesis (Fig. 1; Kuzuyama et al., 1998; Zeidler et al., 1998), did not affect cell growth (P = 0.407) or JH III production (P = 0.807; data not shown). However, carotenoid levels were significantly depressed (22.3%, Student's t test, P = 0.017) in fosmidomycin-treated cultures (data not shown).
In an elegant series of experiments, Lichtenthaler et al. (1997) differentiated between terpenoids synthesized by the two isoprene pathways through the addition of [1-13C]-Glc to plants or plant suspension cultures. Incorporation of [1-13C]-Glc via the classical MVA pathway generates an IPP unit that is labeled at three sites, whereas two carbons are labeled through the alternate MEP pathway. Incorporation of labeled [1-13C]Glc into JH III resulted in addition of 9 mass units to the molecular ion (m/e = 266 synthetic JH III; m/e natural product = 275), indicating that three 13C molecules were incorporated per isoprene unit. This conclusively proves that the biosynthesis of the terpenoid skeleton of JH III in C. iria occurs through the MVA pathway. Fragmentation patterns of 13C-labeled JH III were consistent with expected results {ion 1 [M + 1 = 276]; ion 2 [M = 275]; ion 3 [ion 1 − CH3OH = 244]; ion 4 [ion 3 − HOH = 226]; ion 5 [ion 4 − CO = 198]; ion 6 [ion 5 − (13CH3)2 − C, from epoxide terminus = 154]; and ion 7 [13C3C4H11O (scission between C6 and 13C7 in skeleton after loss of CH3OH) = 114]; see Teal et al., 2000}.
Biosynthesis of JH III
Treatment of mevinolin-inhibited cell suspension cultures with farnesol, farnesal, farnesoic acid, or methyl farnesoate, all precursors of JH III biosynthesis in insects, significantly increased JH III production (Fig. 5). Although approximately equimolar amounts of the biosynthetic precursors were added to the suspension cultures, treatment with farnesoic acid resulted in more than a 6-fold increase in JH III production compared with the other compounds. This probably reflects the increased permeability of farnesoic acid to the cells. Controls, such as extraction and RIA analysis of intermediates added to medium alone or to suspension cell cultures in which JH III biosynthesis was inhibited by miconazole, confirmed that only a limited amount (<26% and <15%, respectively) of measured JH III production may have resulted from cross reactivity of the antiserum with the added biosynthetic precursors. Addition of JH acid to mevinolin-treated cultures did not significantly increase JH III levels over controls (P = 0.707), suggesting that this biosynthetic intermediate in some Lepidopteran species is not a precursor in the C. iria pathway. An alternative explanation is that this compound was degraded or was unable to enter the cell or compartment in which JH III biosynthesis occurs.
Hemmerlin and Bach (2000) observed that 0.1 mm farnesol was cytotoxic to a particularly sensitive tobacco cell culture. In the present study, addition of MVA lactone, farnesol, farnesal, JH acid, or methyl farnesoate to suspension cultures treated either with mevinolin or miconazole did not significantly affect C. iria cell biomass (MVA lactone, P = 0.17 [mevinolin]; farnesol, P = 0.51 [mevinolin], P = 0.25 [miconazole]; farnesal, P = 0.75 [mevinolin], P = 0.16 [miconazole]; JH acid, P = 0.63 [mevinolin]; and methyl farnesoate, P = 0.71 [mevinolin], P = 0.67 [miconazole]). However, treatment of mevinolin-inhibited C. iria suspension cultures with farnesoic acid resulted in brown, dead cells, possibly representing the autotoxicity of JH III to these cultures (Bede and Tobe, 2000). To determine whether this observation was attributable to the exposure of the cells to farnesoic acid or the resultant JH III, JH III biosynthesis was inhibited by miconazole and then cultures were treated with farnesoic acid. These cultures were viable and there was no significant difference in cell fresh weight between these cells and miconazole-treated control cultures (P = 0.10). Therefore, high levels of JH III appear to be autotoxic to C. iria cells. Similar autotoxicity of JH III was observed in allelopathic studies with C. iria seedlings (Bede and Tobe, 2000).
The final step in JH III biosynthesis (Orthoptera/Dictyoptera), the epoxidation of methyl farnesoate, is catalyzed by an NADPH-dependent cytochrome P450 monooxygenase, methyl farnesoate-reduced flavoprotein: oxygen oxidoreductase (EC 1.14.14.-; Fig. 2; Hammock, 1975; Hammock and Mumby, 1978; Feyereisen et al., 1981; Wang et al., 1994). In C. iria suspension cultures, precursor feeding studies suggest that the JH III biosynthetic pathways in plants and insects are similar. Therefore, it was predicted that the last step of this pathway in C. iria was also catalyzed by a cytochrome P450 monooxygenase. Three general cytochrome P450 inhibitors, ancymidol, a pyrimidine derivative, and two N-substituted imidazoles, clotrimazole and miconazole, were added to cell suspension cultures of C. iria. Their proposed mechanism of action is through binding to the heterocyclic nitrogen of the ferric protoheme of the cytochrome P450 enzyme through a lone pair of electrons, thereby excluding oxygen from the reaction site (Durst, 1991). Ancymidol, clotrimazole, and miconazole completely inhibited JH III production at the concentrations tested (ancymidol, P = 0.004; clotrimzole, P = 0.004; and miconazole, P = 0.004; data not shown).
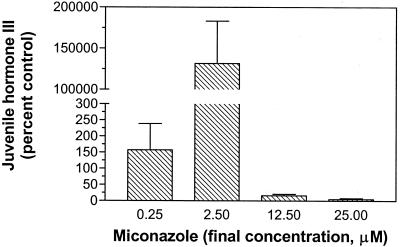
These compounds are broad-spectrum cytochrome P450 inhibitors, exhibiting wide-ranging activities (Durst, 1991). To demonstrate that these compounds specifically inhibited the putative enzyme that catalyzes the last step in JH III biosynthesis, methyl farnesoate-reduced flavoprotein: oxygen oxidoreductase (methyl farnesoate epoxidase), the immediate biosynthetic precursor, methyl farnesoate, was added to miconazole-inhibited C. iria suspension cultures (Fig. 6). If the observed reduction in JH III results from general inhibition of cytochrome P450 enzymes, then addition of methyl farnesoate should “rescue” JH III production. If miconazole specifically inhibits the methyl farnesoate epoxidase, JH III levels will be suppressed.
Figure 6.
Effect of the general cytochrome P450 inhibitor miconazole on JH III levels in C. iria suspension cell cultures. The biosynthetic intermediate methyl farnesoate (0.18 mm) was added to cell suspension cultures containing the general cytochrome P450 inhibitor, miconazole. Inhibition of JH III production by miconazole suggests that the enzyme that catalyzes the final step of biosynthesis is a methyl farnesoate-reduced flavoprotein: oxygen oxidoreductase (methyl farnesoate epoxidase). JH levels were monitored by RIA and bars represent percent controls (suspension cultures + methyl farnesoate) ± se.
In the presence of methyl farnesoate (0.18 mm) and low miconazole concentrations (0.25 μm), JH III production was stimulated over control levels (Fig. 6). This may have resulted from the inhibition of other cytochrome P450 enzymes by miconazole, such as lanosterol 14α-demethylase, possibly shunting FPP into JH III biosynthesis and/or alleviating phytosterol feedback inhibition of HMG-CoA reductase (Gupta et al., 1990), or an enzyme involved in JH III degradation. At higher concentrations of miconazole (12.5 μm, 0.18 mm methyl farnesoate), JH III production was inhibited by 83.7%, suggesting that in C. iria, the final step in the biosynthesis of JH III is catalyzed by a cytochrome P450 monooxygenase.
DISCUSSION
In plants, terpenoids are a diverse class of compounds ranging from photosynthetic pigments (chlorophylls and carotenoids) to defensive molecules (phytoalexins) and growth regulators (gibberellins and abscisic acid [ABA]). However, despite the identification of over 30,000 isoprenoid compounds, our current knowledge of the regulation of terpenoid biosynthetic pathways in plants is nascent (Bach, 1995; Wink, 1999). In fact, only recently has the occurrence of two distinct isoprene pathways been described in higher plants (Lichtenthaler, 1999; Rohmer, 1999): the classical MVA pathway and the MEP pathway. It is assumed that these pathways operate in separate compartments; the MEP pathway responsible for mono-, di-, and tetraterpenoid biosynthesis in the chloroplast and the MVA pathway for sesquiterpenoids, triterpenoids, and sterols in the cytoplasm (Lichtenthaler, 1999).
Little is known about the biosynthesis of JH III in the sedge, C. iria. In the cockroach, the sesquiterpenoid backbone of JH III is biosynthesized through the classical MVA pathway (Fig. 1; Cusson et al., 1991). From the C15 intermediate FPP, removal of the pyrophosphate group generates farnesol (Fig. 2). This alcohol undergoes sequential oxidation reactions to the aldehyde and then the acid. Methylation of the carboxylic acid produces methyl farnesoate. In the final step, epoxidation of methyl farnesoate results in the formation of JH III.
To elucidate the biosynthetic pathway of JH III in C. iria, enzyme inhibitor and labeling studies were conducted on cell suspension cultures. Two inhibitors, mevinolin, an inhibitor of HMG-CoA reductase in the MVA pathway, and fosmidomycin, an inhibitor of the DOXP reductoisomerase in the MEP pathway, were used to elucidate the biosynthetic origin of JH III (Fig. 1). Mevinolin significantly inhibited JH III production, whereas fosmidomycin did not affect JH III levels, suggesting that JH III is solely biosynthesized through the MVA pathway. This conclusion was supported by [1-13C]Glc labeling studies in which the molecular ion of JH III increased by 9 mass units (m/e = 274). Furthermore, the mass fragmentogram revealed the presence of ions indicative of JH III biosynthesis through the MVA pathway.
In other plant species, there is increasing evidence of terpenoid biosynthesis through both MVA and MEP pathways. In chamomile, the third isoprene subunit of the sesquiterpenoid bisabololoxide A may be produced through either pathway (Adam and Zapp, 1998). In the biosynthesis of DMNT in lima bean, inhibition of the MVA pathway by HMG-CoA reductase inhibitors results in biosynthetic compensation through the MEP pathway (Piel et al., 1998). In accordance, either these two pathways coexist in the same compartment or there is limited transfer of isopentenyl subunits between the chloroplast and the cytoplasm (Lichtenthaler, 1999; Rohmer, 1999). The full extent of this interchange has yet to be determined. Therefore, although enzyme inhibition and labeling studies strongly suggest that JH III is biosynthesized principally, if not exclusively, through the MVA pathway in C. iria, the possibility of intercompartmental exchange of isoprene units cannot be excluded. Only through [1-13C]Glc feeding studies and metabolite analysis by 13C-NMR spectroscopy can the unambiguous determination of the biosynthesis of the sesquiterpenoid skeleton be made. In the present work, such studies were not performed because C. iria cell suspension cultures did not provide sufficient quantities of JH III for analysis by NMR. Nonetheless, our results do conclusively demonstrate that the JH III biosynthesis in C. iria proceeds primarily through the cytoplasmic MVA pathway.
Characterization of the final steps of JH III biosynthesis in cell suspension cultures of C. iria was conducted through the use of enzyme inhibitors and precursor feeding studies. Putative biosynthetic precursors known to occur in the insect pathway were added to mevinolin-inhibited suspension cultures. MVA, farnesol, farnesal, farnesoic acid, and methyl farnesoate all rescued JH III production, suggesting that these compounds are intermediates in the biosynthesis of JH III in C. iria (Figs. 2 and 5). Some of these putative biosynthetic precursors have been isolated from several Cyperus spp. as well as unrelated plants. (Iwamura et al., 1978a, 1978b, 1978c, 1979; Iwamura, 1979; Toong et al., 1988; Kijjoa et al., 1990; Versini et al., 1994). However, JH acid, an intermediate in some Lepidopteran insect species, was not incorporated into JH III. This may be attributable to the degradation of this highly unstable compound or its impermeability (Goodman and Adams, 1984).
Although the phytohormone ABA previously was hypothesized to be biosynthetically derived through the MVA pathway, it has now been conclusively demonstrated in plants that ABA biosynthesis proceeds through the MEP pathway to form a C40 carotenoid precursor that undergoes subsequent metabolism to generate ABA (Cutler and Krochko, 1999). The substantial increase in JH III production after addition of farnesol, farnesal, farnesoic acid, and methyl farnesoate to mevinolin-inhibited cell suspension cultures strongly suggests that these compounds are authentic biosynthetic precursors to JH III and that it is unlikely that JH III biosynthesis proceeds through the cleavage of a larger terpenoid intermediate as in the case of ABA.
In Locusta migratoria, the final step in JH III biosynthesis, the epoxidation of the C10, C11 of methyl farnesoate, is catalyzed by an NADPH-dependent cytochrome P450 monooxygenase, methyl farnesoate-reduced flavoprotein: oxygen oxidoreductase (EC 1.14.14.-; Feyereisen et al., 1981). Inhibition of JH III production by the general cytochrome P450 inhibitor, miconazole, implies that a similar epoxidase may also be involved in the final steps of JH III biosynthesis in the plant. Addition of farnesol, farnesal, farnesoic acid (data not shown), and methyl farnesoate (Fig. 6) to miconazole-inhibited cell cultures did not rescue JH III production. Therefore, the reduction in JH III production by miconazole was the result of the specific inhibition of a monooxygenase in the later stages of JH III biosynthesis. Based on the results of these studies, we propose that the final steps of JH III biosynthesis are similar in plants and insects.
MATERIALS AND METHODS
Suspension Cultures
Suspension cultures were maintained on Schenk and Hildebrandt (SH) medium (Schenk and Hildebrandt, 1972) supplemented with 3% (w/v) Suc, 3.0 μm thiamine-HCl, 2.4 μm pyridoxine-HCl, 4.0 μm nicotinic acid, 0.5 g L−1 casein hydrolysate, 0.6 mm myo-inositol, and 2.5 mg L−1 2,4-d (Bede et al., 1999b). All chemicals used for tissue culture medium were obtained from Sigma (St. Louis), except cupric sulfate (CuSO4-5H2O; Fisher, Nepean, ON) and cobaltous chloride (CoCl2-6H2O; BDH Chemicals, Poole, Dorset, UK). The pH of the media was adjusted to 5.8 ± 0.1 with 1 m KOH (BDH Chemicals) prior to autoclaving for 28 min at 121°C. Cell suspension cultures were subcultured at 18-d intervals (approximately 1:30 dilution) and maintained in a shaking incubator at 26 ± 2°C in the dark with rotary gyration at 125 rpm.
Extraction and RIA
SH suspension cells were collected by filtration and homogenized in pentane (HPLC grade, Burdick and Jackson, Muskegon, MI). The medium (100 mL) was extracted overnight with 50 mL of pentane. The organic phase was evaporated under vacuum in a rotary evaporator (Buchler Instruments, Lenexa, KS) to near dryness. The remainder of the solvent was evaporated under a gentle stream of nitrogen and resuspended in toluene for analysis by RIA (Bede et al., 2000). The sensitivity limit of the RIA was approximately 65 pg (Goodman et al., 1990); samples below this detection limit were assumed not to contain JH III. As a control, SH medium alone was extracted and, as expected, JH III was not detected.
Enzyme Inhibition and Feeding Precursor Experiments
Inhibitors of the terpenoid pathways, either the HMG-CoA reductase inhibitor, mevinolin (final concentration 50 μm; Sigma; Bach et al., 1990), or the DOXP reductoisomerase inhibitor, fosmidomycin (final concentration 0.5 mm; Toronto Research Chemicals, North York, ON; Kuzuyama et al., 1998; Zeidler et al., 1998;), were added to d-0 suspension cultures of C. iria. After 48 h, cells and media were filtered and extracted in pentane as described above and the JH III content determined by RIA.
In complementary experiments, MVA lactone (final concentration 5 mm; Sigma) or putative biosynthetic precursors (96% [w/v] pure farnesol, final concentration 0.77 mm, Aldrich, Milwaukee), farnesal (synthesized by a modification of Corey et al. [1968]; Xiao and Prestwich, 1990; Bede et al., 2000; purity 83% [w/v], final concentration 0.67 mm), farnesoic acid (gift of Dr. Mark Feldlaufer, U.S. Department of Agriculture, Beltsville, MD; purity 70% [w/v], final concentration 0.52 mm), methyl farnesoate (synthesized according to Latli and Prestwich [1991]; Bede et al., 2000; purity 99% [w/v], final concentration 0.70 mm), and JH acid (synthesized according to Goodman and Adams [1984], final concentration 0.06 mm) were added to cultures 24 h after the addition of the MVA pathway inhibitor, mevinolin (final concentration 50 μm). MVA lactone was pre-incubated with 10 mm NaOH for 2 h on ice prior to addition to the cell suspension cultures to generate the free acid. After an incubation period of 24 h, cultures were filtered, extracted, and analyzed for JH III production as described above. As controls, biosynthetic precursors were added to medium alone and to cell suspension cultures containing the cytochrome P450 inhibitor miconazole (final concentration 12.5 μm; Sigma). Twenty-four hours after treatment, samples were subjected to extraction and RIA analysis as described above.
Analysis of Carotenoids
To monitor the inhibition of the MEP pathway by fosmidomycin, carotenoid levels were measured spectrophotometrically (Lichtenthaler, 1987). Suspension culture cells were homogenized in cold acetone (ACP Chemical Inc., St. Leonard, QB) in diffuse light. Centrifugation (450g × 5 min, VWR Scientific, West Chester, PA) separated the organic phase from the cell debris. Chlorophyll (Ca and Cb) and carotenoid (Cx + c) levels were measured using an Ultraspectrophotometer 450 (LKB Biochrom, Cambridge, UK) and levels were determined using the following equations:
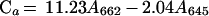 |
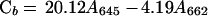 |
 |
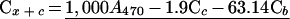 |
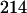 |
Glc Labeling Study
C. iria suspension cultures were subcultured into standard SH medium containing [1-13C]Glc (0.3% [w/v], 99% isotopic abundance; Sigma) substituted for Suc. After 48 h, media and cells were extracted as outlined above and analyzed by gas chromatography-mass spectroscopy (Bede et al., 1999b; Teal et al., 2000).
Inhibition of the Methyl Farnesoate-Reduced Flavoprotein: Oxygen Oxidoreductase
At the time of subculturing, general cytochrome P450 inhibitors (ancymidol, final concentration 0.03 mm, Sigma; clotrimazole, final concentration 0.30 mm, Sigma; and miconazole, final concentration 1.0 mm) were added to C. iria cell suspension cultures. Two days after treatment, cells and media were extracted and analyzed for JH III. To further demonstrate the specificity of the cytochrome P450 inhibitor miconazole for the putative plant methyl farnesoate reduced-flavoprotein: oxygen oxidoreductase, the experiment was repeated in the presence of the biosynthetic intermediate methyl farnesoate (final concentration 0.18 mm).
Statistics
Statistical analyses of the data were performed using SPSS 7.5. Statistical differences were determined by Student's t test or one-way analysis of variance followed by a Tukey's honestly significant difference mean-separation test.
ACKNOWLEDGMENTS
We thank Dr. Mark Feldlaufer for the generous gift of farnesoic acid and Jin Rui Zhang for performing dissections of cockroach CA. We also thank Chris Garside, Dr. Ken Korth, and two anonymous reviewers for critical reading of this manuscript and insightful comments.
Footnotes
This research was supported by the Natural Sciences and Engineering Research Council of Canada (operating grant to S.S.T.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010264.
LITERATURE CITED
- Adam KP, Zapp J. Biosynthesis of the isoprene units of chamomile sesquiterpenes. Phytochemistry. 1998;48:953–959. [Google Scholar]
- Bach TJ. Some new aspects of isoprenoid biosynthesis in plants: a review. Lipids. 1995;30:191–202. doi: 10.1007/BF02537822. [DOI] [PubMed] [Google Scholar]
- Bach TJ, Weber T, Motel A. Some properties of enzymes involved in the biosynthesis and metabolism of 3-hydroxy-3-methylglutaryl CoA in plants. In: Towers GHN, Stafford HA, editors. Biochemistry of Mevalonic Acid Pathway to Terpenoids. New York: Plenum Press; 1990. pp. 1–82. [Google Scholar]
- Baker FC, Mauchamp B, Tsai LW, Schooley DA. Farnesol and farnesal dehydrogenase(s) incorporate allata of the tobacco hornworm moth, Manduca sexta. J Lipid Res. 1983;24:1586–1594. [PubMed] [Google Scholar]
- Banthorpe DV. Secondary metabolism in plant tissue culture: scopes and limitations. Nat Prod Rep. 1994;11:303–328. doi: 10.1039/np9941100303. [DOI] [PubMed] [Google Scholar]
- Bede JC, Goodman WG, Tobe SS. Developmental distribution of insect juvenile hormone III in the sedge, Cyperus iria L. Phytochemistry. 1999a;52:1269–1274. [Google Scholar]
- Bede JC, Goodman WG, Tobe SS. Quantification of juvenile hormone III in the sedge, Cyperus iria L: comparison of high performance liquid chromatography and radioimmunoassay. Anal Phytochem. 2000;11:21–28. [Google Scholar]
- Bede JC, Teal P, Tobe SS. Production of insect juvenile hormone III and its precursors in cell suspension cultures of the sedge, Cyperus iria L. Plant Cell Rep. 1999b;19:20–25. doi: 10.1007/s002990050704. [DOI] [PubMed] [Google Scholar]
- Bede JC, Tobe SS. Activity of insect juvenile hormone III: seed germination and seedling growth studies. Chemoecology. 2000;10:89–97. [Google Scholar]
- Brindle PA, Baker FC, Tsai LW, Reuter CC, Schooley DA. Sources of propionate for the biogenesis of ethyl-branched insect juvenile hormones: role of isoleucine and valine. Proc Natl Acad Sci USA. 1987;84:7906–7910. doi: 10.1073/pnas.84.22.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindle PA, Baker FC, Tsai LW, Schooley DA. Comparative metabolism of isoleucine by corpora allata of nonlepidopteran insects versus lepidopteran insects, in relation to juvenile hormone biosynthesis. Arch Insect Biochem Physiol. 1992;19:1–15. [Google Scholar]
- Catling K. Rice in Deep Water. London: International Rice Research Institute; 1992. [Google Scholar]
- Chappell J, Wolf F, Proulx J, Cuellar R, Saunders C. Is the reaction catalyzed 3-hydroxy-3-methylglutaryl coenzyme A reductase a rate-limiting step for isoprenoid biosynthesis in plants. Plant Physiol. 1995;109:1337–1343. doi: 10.1104/pp.109.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlwood BV, Charlwood KA. Terpenoid production in plant cell cultures. In: Harbourne JB, Tomas-Barberan FA, editors. Ecological Chemistry and Biochemistry of Plant Terpenoids. Oxford: Clarendon Press; 1991. pp. 95–132. [Google Scholar]
- Corey EJ, Gilman NW, Ganem BE. New methods of the oxidation of aldehydes to carboxylic acids and esters. J Am Chem Soc. 1968;90:5616–5617. [Google Scholar]
- Cusson M, Palli SB. Can juvenile hormone research help rejuvenate integrated pest management. Can Entomol. 2000;132:263–280. [Google Scholar]
- Cusson M, Yagi KJ, Ding Q, Duve H, Thorpe A, McNeil JN, Tobe SS. Biosynthesis and release of juvenile hormone and its precursors in insects and crustaceans: the search for a unifying arthropod endocrinology. Insect Biochem. 1991;21:1–6. [Google Scholar]
- Cutler AJ, Krochko JE. Formation and breakdown of ABA. Trends Plant Sci. 1999;4:472–478. doi: 10.1016/s1360-1385(99)01497-1. [DOI] [PubMed] [Google Scholar]
- Durst F. Biochemistry and physiology of plant cytochrome P-450. In: Ruckpaul K, Rein H, editors. Frontiers in Biotransformation. 4. Microbial and Plant Cytochromes P-450: Biochemical Characteristics, Genetic Engineering and Practical Implications. London: Taylor and Francis; 1991. pp. 191–232. [Google Scholar]
- Eisenreich W, Menhard B, Hylands PJ, Zenk M, Bacher A. Studies on the biosynthesis of taxol: the taxane carbon skeleton is not of mevalonoid origin. Proc Natl Acad Sci USA. 1996;93:6431–6436. doi: 10.1073/pnas.93.13.6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyereisen R, Pratt GE, Hamnett AF. Enzymatic synthesis of juvenile hormone in locust corpora allata: evidence for a microsomal cytochrome P-450 linked methyl farnesoate epoxidase. Eur J Biochem. 1981;118:231–238. doi: 10.1111/j.1432-1033.1981.tb06391.x. [DOI] [PubMed] [Google Scholar]
- Gilbert LI, Rybczynski R, Tobe SS. Endocrine cascade in insect metamorphosis. In: Gilbert LI, Tata JR, Atkinson BG, editors. Metamorphosis. New York: Academic Press; 1996. pp. 59–107. [Google Scholar]
- Goodman WG, Adams B. Semipreparative synthesis and purification of juvenile hormone acids by high-performance liquid chromatography. J Chromatogr. 1984;294:447–451. [Google Scholar]
- Goodman WG, Coy DC, Baker FC, Xu L, Toong YC. Development and radioimmunoassay for the juvenile hormones. Insect Biochem. 1990;20:357–364. [Google Scholar]
- Gupta AK, Sexton RC, Rudney H. Differential regulation of low density lipoprotein suppression of HMG-CoA reductase activity in cultured cells by inhibitors of cholesterol biosynthesis. J Lipid Res. 1990;31:203–215. [PubMed] [Google Scholar]
- Hammock BD. NADPH dependent epoxidation of methyl farnesoate to juvenile hormone in the cockroach Blaberus giganteus L. Life Sci. 1975;17:323–328. doi: 10.1016/0024-3205(75)90479-8. [DOI] [PubMed] [Google Scholar]
- Hammock BD, Mumby SM. Inhibition of epoxidation of methyl farnesoate to juvenile hormone III by cockroach corpus allatum homogenates. Pest Biochem Physiol. 1978;9:39–47. [Google Scholar]
- Hemmerlin A, Bach TJ. Farnesol-induced cell death and stimulation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase activity in tobacco cv Bright Yellow-2 cells. Plant Physiol. 2000;123:1257–1268. doi: 10.1104/pp.123.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm LG, Pluchnett DL, Pancho JV, Herberger JP. The World's Worst Weeds. University Press of Hawaii, Honolulu. 1977. Cyperus iria L; pp. 240–242. [Google Scholar]
- Iwamura J. The constituents of essential oils from Cyperus polystachyos Rottb., Cyperus globosus Allioni and Cyperus difformis L. Nippon Nogai Kagaku Kaishi. 1979;53:343–347. [Google Scholar]
- Iwamura J, Kameda M, Komai K, Hirao N. Studies on constituents in the Cyperaceae: II. The constituents of the essential oil from Cyperus microiria Steud. Nippon Kagaku Kaishi. 1978a;XX:1552–1555. (Chemical Abstracts [1979] 90: 69086h) [Google Scholar]
- Iwamura J, Kameda M, Komai K, Hirao N. The constituents of the essential oil from Cyperus iria L. Nippon Nogei Kagaku Kaishi. 1978b;52:379–383. [Google Scholar]
- Iwamura J, Komaki K, Komai K, Hirao N. Studies on constituents in the Cyperaceae: V. The constituents of the essential oil from Cyperus monophyllus Vahl. Nippon Nogei Kagaku Kaishi. 1978c;52:561–563. (Chemical Abstracts [1979] 90: 135072d) [Google Scholar]
- Iwamura J, Komaki K, Komai K, Hirao N. Studies on constituents in Cyperaceae: IV. The constituents of essential oil from Cyperus pilosus Vahl. Nippon Kagaku Kaishi. 1979;52:255–2588. (Chemical Abstracts [1979] 90: 164723x) [Google Scholar]
- Kijjoa A, Pinto MMM, Pinho PMM, Tantisewie B, Herz W. Clerodone derivatives from Polyalthia viridis. Phytochemistry. 1990;29:653–655. [Google Scholar]
- Komai K, Sugiwaka Y, Sato S. Plant-growth retardant of extracts obtained from waternutgrass (Cyperus serotinus Rottb.) Mem Faculty Agric Kinki Univ. 1981;14:57–65. [Google Scholar]
- Kuzuyama T, Shimizu T, Takahashi S, Seto H. Fosmidomycin, a specific inhibitor of 1-deoxy-D-xylulose 5-phosphate reductoisomerase in the nonmevalonate pathway for terpenoid biosynthesis. Tetrahedron Lett. 1998;39:7913–7916. [Google Scholar]
- Lange BM, Croteau R. Isoprenoid biosynthesis via a mevalonate-independent pathway in plants: cloning and heterologous expression of 1-deoxy-D-xylulose-5-phosphate reductoisomerase from peppermint. Arch Biochem Biophys. 1999;265:170–174. doi: 10.1006/abbi.1999.1168. [DOI] [PubMed] [Google Scholar]
- Latli B, Prestwich GD. Synthesis of labeled (10R)-juvenile hormone III bisepoxide, and its photoaffinity analogue, [12-3H]-(10R)-6,7,10,11-bisepoxyfarnesyl diazoacetate (BEFDA) J Labeled Comp Radiopharmacol. 1991;29:1168–1173. [Google Scholar]
- Law JH. Biosynthesis of juvenile hormones in insects. In: Porter JW, Spurgeon SL, editors. Biosynthesis of Isoprenoid Compounds. Vol. 2. New York: John Wiley and Sons; 1981. pp. 507–534. [Google Scholar]
- Lichtenthaler H. Chlorophyll and carotenoids: pigments of photosynthetic biomembranes. In: Packer L, Douce R, editors. Methods in Enzymology. Vol. 148. New York: Academic Press; 1987. pp. 350–382. [Google Scholar]
- Lichtenthaler H, Schwender J, Disch A, Rohmer M. Biosynthesis of isoprenoids in higher plant chloroplasts proceeds via a mevalonate-independent pathway. FEBS Lett. 1997;400:271–274. doi: 10.1016/s0014-5793(96)01404-4. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK. The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:47–65. doi: 10.1146/annurev.arplant.50.1.47. [DOI] [PubMed] [Google Scholar]
- Monger D. Insect 3-hydroxy-3-methylglutaryl-CoA reductase. In: Law JH, Rillings HC, editors. Methods in Enzymology. Vol. 110. New York: Academic Press; 1985. pp. 51–58. [DOI] [PubMed] [Google Scholar]
- Peter MG, Shirk PD, Dahm KH, Röller H. On the specificity of juvenile hormone biosynthesis in the male Cecropia moth. Z Naturforsch. 1981;36:579–585. [Google Scholar]
- Piel J, Donath J, Bandemer K, Boland W. Mevalonate-independent biosynthesis of terpenoid volatiles in plants: induced and constitutive emission of volatiles. Angew Chem Int Ed. 1998;37:2478–2481. doi: 10.1002/(SICI)1521-3773(19981002)37:18<2478::AID-ANIE2478>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Rohmer M. The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat Prod Rep. 1999;16:565–574. doi: 10.1039/a709175c. [DOI] [PubMed] [Google Scholar]
- Rohmer M, Knani M, Simonin P, Sutter B, Sahm H. Isoprenoid biosynthesis in bacteria: a novel pathway for the early steps leading to isopentenyl diphosphate. Biochem J. 1993;295:517–524. doi: 10.1042/bj2950517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk RU, Hildebrandt AC. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can J Bot. 1972;50:199–204. [Google Scholar]
- Schooley DA, Baker FC. Juvenile hormone biosynthesis. In: Kerdut GA, Gilbert LI, editors. Comprehensive Insect Physiology, Biochemistry and Pharmacology. Vol. 7. New York: Pergamon Press; 1985. pp. 363–389. [Google Scholar]
- Schwartz AM, Paskewitz SM, Orth AP, Tesch MJ, Toong YC, Goodman WG. The lethal effects of Cyperus iria on Aedes aegypti. J Am Mosquito Control Assoc. 1998;14:78–82. [PubMed] [Google Scholar]
- Sparagana SP, Bhaskaran G, Barrera P. Juvenile hormone acid methyltransferase activity in imaginal discs of Manduca sexta prepupae. Arch Insect Biochem Physiol. 1985;2:191–202. [Google Scholar]
- Stermer BA, Bianchini GM, Korth KL. Regulation of HMG-CoA reductase activity in plants. J Lipid Res. 1994;35:1133–1140. [PubMed] [Google Scholar]
- Sutherland TD, Feyereisen R. Target of cockroach allatostatin in the pathway of juvenile hormone biosynthesis. Mol Cell Endocrinol. 1996;120:115–123. doi: 10.1016/0303-7207(96)03825-7. [DOI] [PubMed] [Google Scholar]
- Svoboda JA, Kaplanis JN, Robbins WE, Thompson MJ. Recent developments in insect steroid metabolism. Ann Rev Entomol. 1975;20:205–220. doi: 10.1146/annurev.en.20.010175.001225. [DOI] [PubMed] [Google Scholar]
- Teal PEA, Proveaux AT, Heath RR. Analysis and quantitation of insect juvenile hormones using chemical ionization ion-trap mass spectroscopy. Anal Biochem. 2000;277:206–213. doi: 10.1006/abio.1999.4377. [DOI] [PubMed] [Google Scholar]
- Tobe SS, Stay B. Structure and regulation of the corpus allatum. Adv Insect Physiol. 1985;18:305–432. [Google Scholar]
- Toong YC, Schooley DA, Baker FC. Isolation of insect juvenile hormone III from a plant. Nature. 1988;333:170–171. [Google Scholar]
- Versini G, Rapp A, Dalla-serra A, Pichler U, Ramponi M. Methyl trans geranate and farnesoate as markers for Gewürztraminer grape skins and related distillates. Vitis. 1994;33:139–142. [Google Scholar]
- Vögeli U, Chappell J. Inhibition of a plant sesquiterpene cyclase by mevinolin. Arch Biochem Biophys. 1991;288:157–162. doi: 10.1016/0003-9861(91)90178-l. [DOI] [PubMed] [Google Scholar]
- Wang Z, Ding Q, Yagi KJ, Tobe SS. Terminal stages in juvenile hormone biosynthesis in corpora allata of Diploptera punctata: developmental changes in enzyme activity and regulation by allatostatins. J Insect Physiol. 1994;40:217–223. [Google Scholar]
- Wink M. Plant secondary metabolites: biochemistry, function and biotechnology. In: Wink M, editor. Biochemistry of Plant Secondary Metabolism, Annual Plant Reviews. Vol. 2. Sheffield, UK: Sheffield Academic Press; 1999. pp. 1–16. [Google Scholar]
- Xiao XY, Prestwich GD. Minimizing geometric isomerization during oxidation of allylic alcohols to aldehydes. Synth Comm. 1990;20:3125–3130. [Google Scholar]
- Zeidler J, Schwender J, Müller C, Wiesner J, Weidemeyer C, Beck E, Jomaa H, Lichtenthaler HK. Inhibition of the non-mevalonate 1-deoxy-D-xylulose-5-phosphate pathway of plant isoprenoid biosynthesis by fosmidomycin. Z Naturforsch. 1998;53:980–986. [Google Scholar]