Abstract
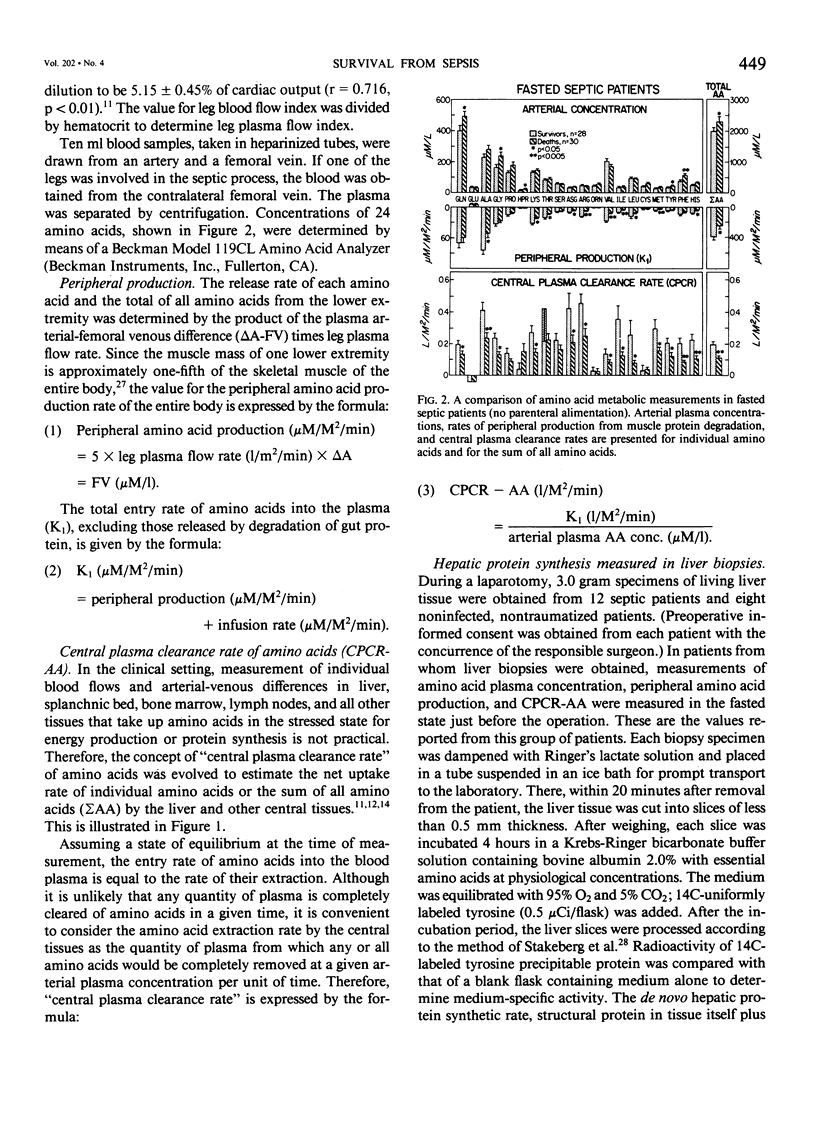
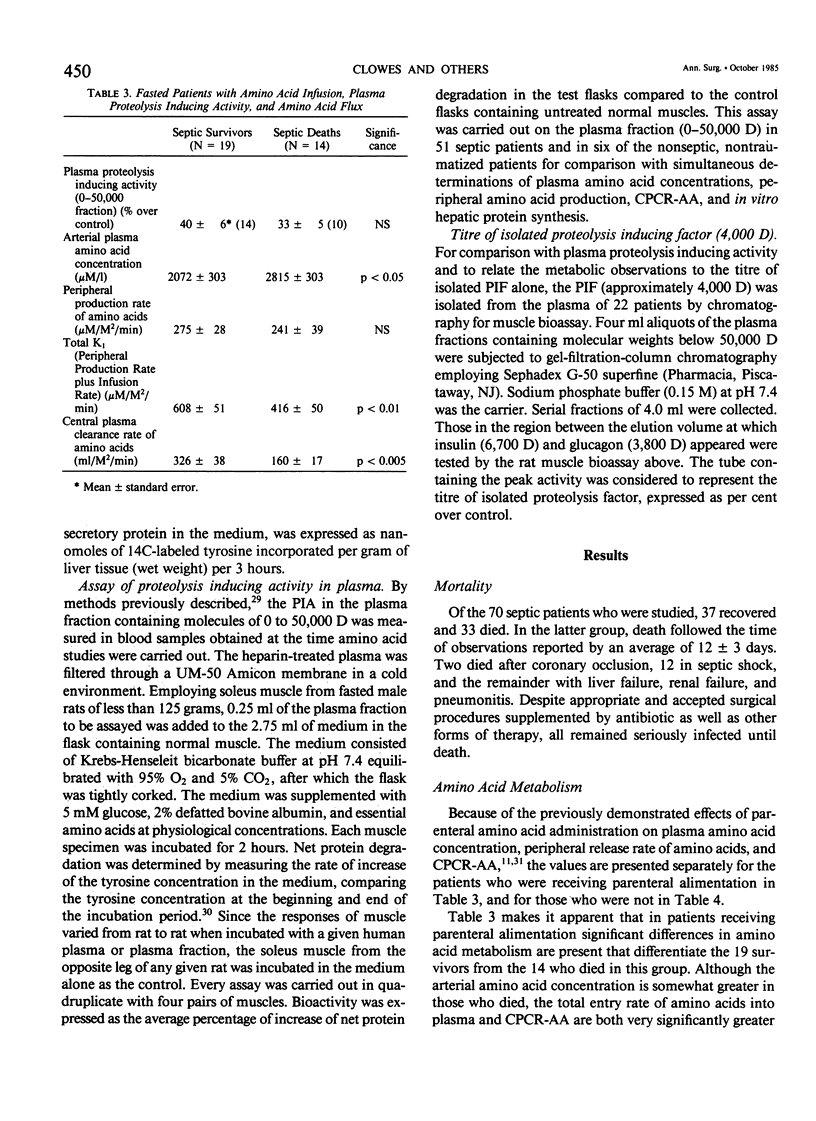
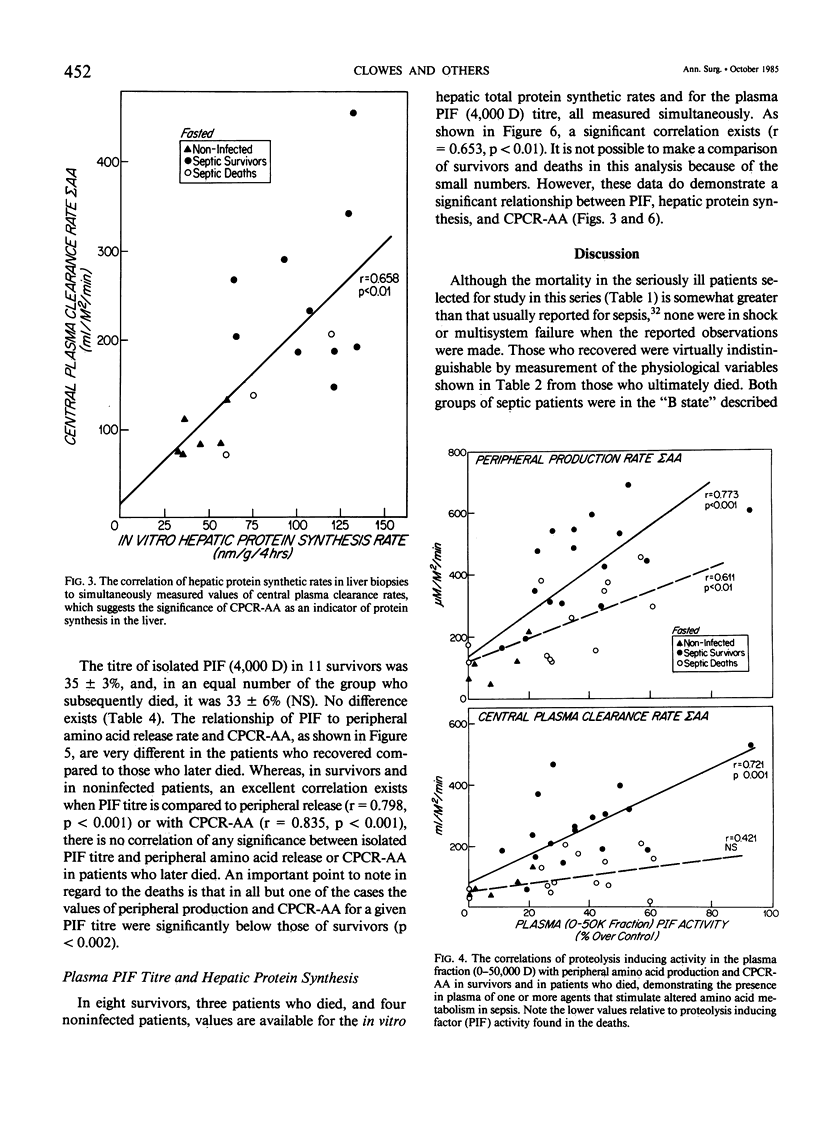
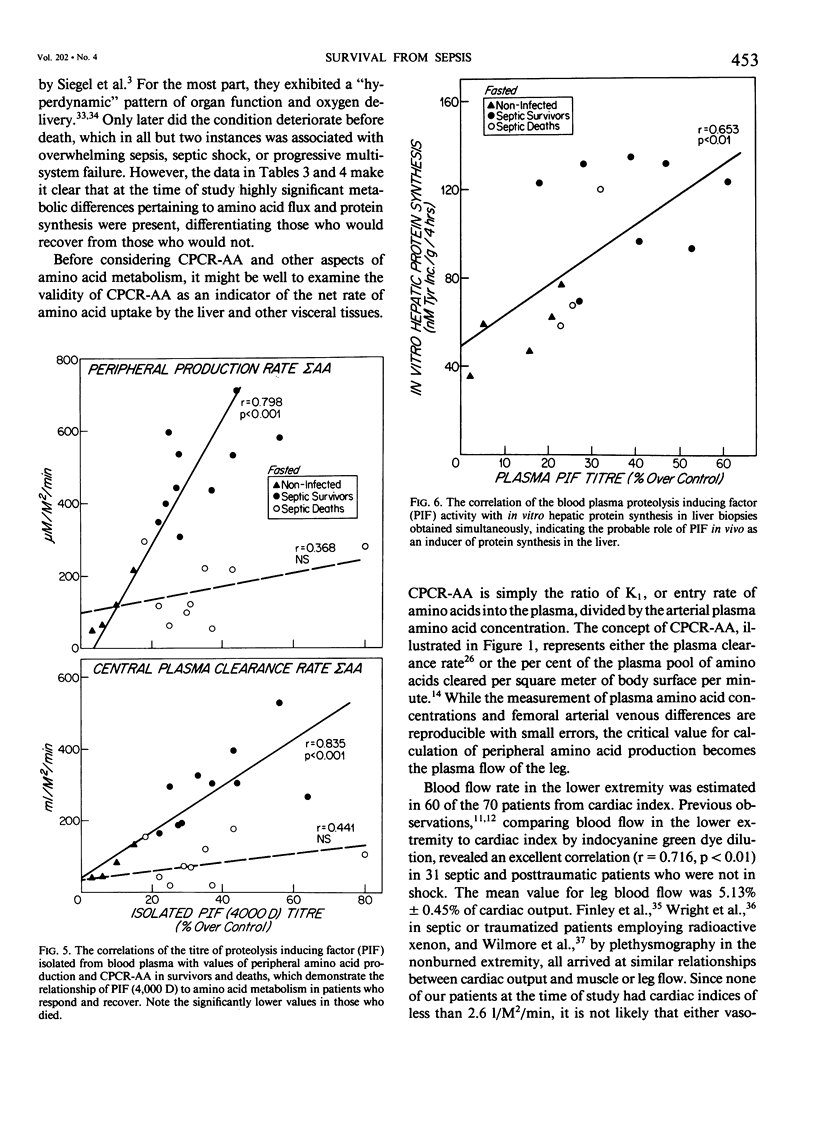
Amino acid (AA) arterial blood plasma concentrations, K1 (peripheral production + infusion rates), and central plasma clearance rates (K1 divided by arterial concentration) (CPCR-AA) were measured in 70 seriously septic patients. All of these people were in the "hyperdynamic" state at the time of observation. Thirty-seven recovered and 33 died. In addition, 10 noninfected, nontraumatized patients about to undergo laparotomy were studied. In 31 patients receiving parenteral alimentation, CPCR-AA was 326 +/- 38 in survivors and 160 +/- 17 ml/M2/min in the deaths (p less than 0.005). In 58 patients studied, while fasted with no intravenous amino acid infusion, values for CPCR-AA were: survivors 202 +/- 22 (28) and deaths 112 +/- 16 (30) ml/M2/min (p less than 0.002). The CPCR-AA in 10 noninfected patients was only 68 +/- 11 ml/M2/min. CPCR-AA in 19 patients correlated with hepatic protein synthetic rates in liver biopsies obtained simultaneously (r = 0.658, p less than 0.01), which shows that CPCR-AA is an indicator of visceral protein synthesis. To study the regulation of amino acid metabolism by synthesis. To study the regulation of amino acid metabolism by proteolysis inducing factor (PIF), the proteolysis inducing activity (PIA) of the plasma fraction (0-50,000 D) was measured 55 times in conjunction with metabolic studies. No significant differences existed in PIA between survivors and deaths. However, in those patients who recovered, PIA was significantly correlated to both peripheral amino acid production (r = 0.773, p less than 0.001) and to CPCR-AA (r = 0.721, p less than 0.001). This observation demonstrates the presence of one or more circulating agents affecting amino acid flux. PIA measured simultaneously in vivo correlated with in vitro protein synthetic rate in incubated liver biopsies (r = 0.653, p less than 0.01). PIF (4,000 D), isolated by chromatography, in patients without amino acid infusion was 35 +/- 3% in survivors and 33 +/- 6% in deaths (N.S.) and only 9 +/- 3% over control in noninfected patients. In patients who recovered, PIF titre was strongly correlated with peripheral amino acid production (r = 0.798, p less than 0.001) and with CPCR-AA (r = 0.835, p less than 0.001). However, values for patients who later died were significantly less for a given PIF titre. Thus, it is concluded that survival from sepsis is, in part, dependent on a significantly elevated CPCR-AA and hepatic protein synthesis, both of which appear to be related to the blood plasma PIF titre.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baracos V., Rodemann H. P., Dinarello C. A., Goldberg A. L. Stimulation of muscle protein degradation and prostaglandin E2 release by leukocytic pyrogen (interleukin-1). A mechanism for the increased degradation of muscle proteins during fever. N Engl J Med. 1983 Mar 10;308(10):553–558. doi: 10.1056/NEJM198303103081002. [DOI] [PubMed] [Google Scholar]
- Beisel W. R. Magnitude of the host nutritional responses to infection. Am J Clin Nutr. 1977 Aug;30(8):1236–1247. doi: 10.1093/ajcn/30.8.1236. [DOI] [PubMed] [Google Scholar]
- Bessey P. Q., Watters J. M., Aoki T. T., Wilmore D. W. Combined hormonal infusion simulates the metabolic response to injury. Ann Surg. 1984 Sep;200(3):264–281. doi: 10.1097/00000658-198409000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill G. F., Jr Starvation in man. N Engl J Med. 1970 Mar 19;282(12):668–675. doi: 10.1056/NEJM197003192821209. [DOI] [PubMed] [Google Scholar]
- Cerra F. B., Siegel J. H., Coleman B., Border J. R., McMenamy R. R. Septic autocannibalism. A failure of exogenous nutritional support. Ann Surg. 1980;192(4):570–580. doi: 10.1097/00000658-198010000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christou N. V., McLean A. P., Meakins J. L. Host defense in blunt trauma: interrelationships of kinetics of anergy and depressed neutrophil function, nutritional status, and sepsis. J Trauma. 1980 Oct;20(10):833–841. doi: 10.1097/00005373-198010000-00003. [DOI] [PubMed] [Google Scholar]
- Clowes G. H., Jr, George B. C., Villee C. A., Jr, Saravis C. A. Muscle proteolysis induced by a circulating peptide in patients with sepsis or trauma. N Engl J Med. 1983 Mar 10;308(10):545–552. doi: 10.1056/NEJM198303103081001. [DOI] [PubMed] [Google Scholar]
- Clowes G. H., Jr, Heideman M., Lindberg B., Randall H. T., Hirsch E. F., Cha C. J., Martin H. Effects of parenteral alimentation on amino acid metabolism in septic patients. Surgery. 1980 Oct;88(4):531–543. [PubMed] [Google Scholar]
- Clowes G. H., Jr, Martin H., Walji S., Hirsch E., Gazitua R., Goodfellow R. Blood insulin responses to blood glucose levels in high output sepsis and spetic shock. Am J Surg. 1978 Apr;135(4):577–583. doi: 10.1016/0002-9610(78)90040-5. [DOI] [PubMed] [Google Scholar]
- Clowes G. H., Jr, McDermott W. V., Williams L. F., Loda M., Menzoian J. O., Pearl R. Amino acid clearance and prognosis in surgical patients with cirrhosis. Surgery. 1984 Oct;96(4):675–685. [PubMed] [Google Scholar]
- Clowes G. H., Jr, Randall H. T., Cha C. J. Amino acid and energy metabolism in septic and traumatized patients. JPEN J Parenter Enteral Nutr. 1980 Mar-Apr;4(2):195–205. doi: 10.1177/014860718000400225. [DOI] [PubMed] [Google Scholar]
- Clowes G. H., Jr, Vucinic M., Weidner M. G. Circulatory and metabolic alterations associated with survival or death in peritonitis: clinical analysis of 25 cases. Ann Surg. 1966 Jun;163(6):866–885. doi: 10.1097/00000658-196606000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellinger E. P., Wertz M. J., Meakins J. L., Solomkin J. S., Allo M. D., Howard R. J., Simmons R. L. Surgical infection stratification system for intra-abdominal infection. Multicenter trial. Arch Surg. 1985 Jan;120(1):21–29. doi: 10.1001/archsurg.1985.01390250015003. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Clowes G. H., Jr, Gordon A. H., Saravis C. A., Wolff S. M. Cleavage of human interleukin 1: isolation of a peptide fragment from plasma of febrile humans and activated monocytes. J Immunol. 1984 Sep;133(3):1332–1338. [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Finley R. J., Duff J. H., Holliday R. L., Jones D., Marchuk J. B. Capillary muscle blood flow in human sepsis. Surgery. 1975 Jul;78(1):87–94. [PubMed] [Google Scholar]
- Fulks R. M., Li J. B., Goldberg A. L. Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975 Jan 10;250(1):290–298. [PubMed] [Google Scholar]
- Keller G. A., West M. A., Wilkes L. A., Cerra F. B., Simmons R. L. Modulation of hepatocyte protein synthesis by endotoxin-activated Kupffer cells. II. Mediation by soluble transferrable factors. Ann Surg. 1985 Apr;201(4):429–435. doi: 10.1097/00000658-198504000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner I. The phenomenon of the acute phase response. Ann N Y Acad Sci. 1982;389:39–48. doi: 10.1111/j.1749-6632.1982.tb22124.x. [DOI] [PubMed] [Google Scholar]
- Loda M., Clowes G. H., Jr, Dinarello C. A., George B. C., Lane B., Richardson W. Induction of hepatic protein synthesis by a peptide in blood plasma of patients with sepsis and trauma. Surgery. 1984 Aug;96(2):204–213. [PubMed] [Google Scholar]
- Loda M., Clowes G. H., Jr, Dinarello C. A., George B. C., Lane B., Richardson W. Induction of hepatic protein synthesis by a peptide in blood plasma of patients with sepsis and trauma. Surgery. 1984 Aug;96(2):204–213. [PubMed] [Google Scholar]
- Long C. L., Birkhahn R. H., Geiger J. W., Betts J. E., Schiller W. R., Blakemore W. S. Urinary excretion of 3-methylhistidine: an assessment of muscle protein catabolism in adult normal subjects and during malnutrition, sepsis, and skeletal trauma. Metabolism. 1981 Aug;30(8):765–776. doi: 10.1016/0026-0495(81)90022-6. [DOI] [PubMed] [Google Scholar]
- Long C. L., Schiller W. R., Blakemore W. S., Geiger J. W., O'Dell M., Henderson K. Muscle protein catabolism in the septic patient as measured by 3-methylhistidine excretion. Am J Clin Nutr. 1977 Aug;30(8):1349–1352. doi: 10.1093/ajcn/30.8.1349. [DOI] [PubMed] [Google Scholar]
- MOORE F. D. Metabolism in trauma: the meaning of definitive surgery: the wound, the endocrine glands, and metabolism. Harvey Lect. 1956;(SERIES):74–99. [PubMed] [Google Scholar]
- MacLean L. D., Meakins J. L., Taguchi K., Duignan J. P., Dhillon K. S., Gordon J. Host resistance in sepsis and trauma. Ann Surg. 1975 Sep;182(3):207–217. doi: 10.1097/00000658-197509000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIrvine A. J., O'Mahony J. B., Saporoschetz I., Mannick J. A. Depressed immune response in burn patients: use of monoclonal antibodies and functional assays to define the role of suppressor cells. Ann Surg. 1982 Sep;196(3):297–304. doi: 10.1097/00000658-198209000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamy R. H., Birkhahn R., Oswald G., Reed R., Rumph C., Vaidyanath N., Yu L., Cerra F. B., Sorkness R., Border J. R. Multiple systems organ failure: I. The basal state. J Trauma. 1981 Feb;21(2):99–114. doi: 10.1097/00005373-198102000-00003. [DOI] [PubMed] [Google Scholar]
- Pearl R. H., Clowes G. H., Jr, Hirsch E. F., Loda M., Grindlinger G. A., Wolfort S. Prognosis and survival as determined by visceral amino acid clearance in severe trauma. J Trauma. 1985 Aug;25(8):777–783. doi: 10.1097/00005373-198508000-00008. [DOI] [PubMed] [Google Scholar]
- Powanda M. C., Beisel W. R. Hypothesis: leukocyte endogenous mediator/endogenous pyrogen/lymphocyte-activating factor modulates the development of nonspecific and specific immunity and affects nutritional status. Am J Clin Nutr. 1982 Apr;35(4):762–768. doi: 10.1093/ajcn/35.4.762. [DOI] [PubMed] [Google Scholar]
- Rosenblatt S., Clowes G. H., Jr, George B. C., Hirsch E., Lindberg B. Exchange of amino acids by muscle and liver in sepsis. Arch Surg. 1983 Feb;118(2):167–175. doi: 10.1001/archsurg.1983.01390020023004. [DOI] [PubMed] [Google Scholar]
- Sganga G., Siegel J. H., Brown G., Coleman B., Wiles C. E., 3rd, Belzberg H., Wedel S., Placko R. Reprioritization of hepatic plasma protein release in trauma and sepsis. Arch Surg. 1985 Feb;120(2):187–199. doi: 10.1001/archsurg.1985.01390260051008. [DOI] [PubMed] [Google Scholar]
- Stakeberg H., Gustafson A., Scherstén T. Incorporation rate of leucine into proteins in human liver slices. Eur J Clin Invest. 1974 Dec 5;4(6):393–398. doi: 10.1111/j.1365-2362.1974.tb00411.x. [DOI] [PubMed] [Google Scholar]
- Wannemacher R. W., Jr Key role of various individual amino acids in host response to infection. Am J Clin Nutr. 1977 Aug;30(8):1269–1280. doi: 10.1093/ajcn/30.8.1269. [DOI] [PubMed] [Google Scholar]
- Wilmore D. W., Aulick L. H., Mason A. D., Pruitt B. A., Jr Influence of the burn wound on local and systemic responses to injury. Ann Surg. 1977 Oct;186(4):444–458. doi: 10.1097/00000658-197710000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmore D. W. Hormonal responses and their effect on metabolism. Surg Clin North Am. 1976 Oct;56(5):999–1018. doi: 10.1016/s0039-6109(16)41029-7. [DOI] [PubMed] [Google Scholar]
- Wilmore D. W., Long J. M., Mason A. D., Jr, Skreen R. W., Pruitt B. A., Jr Catecholamines: mediator of the hypermetabolic response to thermal injury. Ann Surg. 1974 Oct;180(4):653–669. doi: 10.1097/00000658-197410000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C. J., Duff J. H., McLean A. P., MacLean L. D. Regional capillary blood flow and oxygen uptake in severe sepsis. Surg Gynecol Obstet. 1971 Apr;132(4):637–644. [PubMed] [Google Scholar]
- Young V. R., Havenberg L. N., Bilmazes C., Munro H. N. Potential use of 3-methylhistidine excretion as an index of progressive reduction in muscle protein catabolism during starvation. Metabolism. 1973 Nov;23(2):1429–1436. doi: 10.1016/0026-0495(73)90257-6. [DOI] [PubMed] [Google Scholar]
- van Gool J., Boers W., Sala M., Ladiges N. C. Glucocorticoids and catecholamines as mediators of acute-phase proteins, especially rat alpha-macrofoetoprotein. Biochem J. 1984 May 15;220(1):125–132. doi: 10.1042/bj2200125. [DOI] [PMC free article] [PubMed] [Google Scholar]


