Abstract
One challenge for plant biology has been to identify floral stimuli at the shoot apex. Using sensitive and specific gas chromatography-mass spectrometry techniques, we have followed changes in gibberellins (GAs) at the shoot apex during long day (LD)-regulated induction of flowering in the grass Lolium temulentum. Two separate roles of GAs in flowering are indicated. First, within 8 h of an inductive LD, i.e. at the time of floral evocation, the GA5 content of the shoot apex doubled to about 120 ng g−1 dry weight. The concentration of applied GA5 required for floral induction of excised apices (R.W. King, C. Blundell, L.T. Evans [1993] Aust J Plant Physiol 20: 337–348) was similar to that in the shoot apex. Leaf-applied [2H4] GA5 was transported intact from the leaf to the shoot apex, flowering being proportional to the amount of GA5 imported. Thus, GA5 could be part of the LD stimulus for floral evocation of L. temulentum or, alternatively, its increase at the shoot apex could follow import of a primary floral stimulus. Later, during inflorescence differentiation and especially after exposure to additional LD, a second GA action was apparent. The content of GA1 and GA4 in the apex increased greatly, whereas GA5 decreased by up to 75%. GA4 applied during inflorescence differentiation strongly promoted flowering and stem elongation, whereas it was ineffective for earlier floral evocation although it caused stem growth at all times of application. Thus, we conclude that GA1 and GA4 are secondary, late-acting LD stimuli for inflorescence differentiation in L. temulentum.
Plants of Lolium temulentum remain vegetative when grown in short days (SD), but flower after exposure of their leaves to a single long day (LD). The leaf gibberellin (GA) content increases in LD (Gocal et al., 1999) and applied GAs can cause flowering in noninductive SD (Evans, 1964; Pharis et al., 1987; Evans et al., 1990). Thus, GAs mimic LD responses and they could be a transmissible endogenous floral stimulus in this LD plant. A number of other LD plants but not all (for summary, see Metzger, 1995) flower in response to GA, and recent genetic and molecular studies with Arabidopsis support such a role for endogenous GAs in flowering in LD (Wilson et al., 1992; Weigel and Nilsson, 1995; Blásquez et al., 1997). Furthermore, where there are effects of LD exposure on stem elongation there are clear increases in the GA content of leaves, petioles, and shoot tips (Talon and Zeevaart, 1990; Talon et al., 1991; Zeevaart et al., 1993).
Inflorescence initiation in L. temulentum after one LD precedes any acceleration of stem elongation. Therefore, if GAs were to play a role endogenously in floral evocation, they should have little effect on stem elongation. From application studies, we previously identified a number of GAs, including GA5, that meet this criterion of inducing flowering but with little or no effect on stem elongation (Evans et al., 1990, 1994a, 1994b). However, in a broad context, three lines of evidence should be obtained to confirm a role for GAs in floral evocation. First, exposure to LD should increase the levels of florally active endogenous GAs in the shoot apex. Second, there should be evidence that inhibitors of GA biosynthesis block flowering. Third, there should be molecular/biochemical linkages between GA and floral initiation at the apex. Elsewhere, we and others have addressed the latter two issues (Evans, 1969; Evans et al., 1994a; Weigel and Nilsson, 1995; Blásquez et al., 1997; Gocal et al., 1999).
Considering the requirement for change in GA content, not only did the endogenous content of several GAs increase in leaves of L. temulentum soon after exposure to two or more florally inductive LD (Gocal et al., 1999), but bioassayable GA-like activity at the shoot apex increased within 8 h of the end of the LD (Pharis et al., 1987), i.e. at the time when floral evocation occurs (McDaniel et al., 1991). Furthermore, GAs do act at the shoot apex because plants of L. temulentum flower in SD when GA3 is applied either to the leaf, near to the shoot apex of intact plants (Evans, 1964), or to cultured apices excised from plants in SD (King et al., 1993). The latter study was particularly persuasive because, without added GA, the excised shoot apex continued to grow vegetatively and only formed leaves.
Here, we examine changes in the spectrum of GAs and in their content in the shoot apex of L. temulentum following exposure of the leaf to florally inductive LD. High-resolution (HR)-mass spectrometry (MS) and selected reaction monitoring (SRM)-MS provided the specificity and high sensitivity required for measurements of GAs (Moritz and Olsen, 1995). These MS methods allowed various precursors, active GAs, and their catabolites to be measured simultaneously. The high sensitivity of MS meant that femtogram amounts of individual GAs could be detected in batches of 40 shoot apices (about 200 μg total dry weight). The selectivity of HR-MS and SRM-MS coupled with the use of deuterated GAs as internal standards allowed analyses with minimal purification and, thereby, minimal losses.
RESULTS
Shoot Apex GAs
Due to the low shoot apex tissue amounts, the identification of GAs was based on HR-selected ion monitoring (SIM). Identity was certain when both the ratio of the ions (at a resolution of 10,000) and the retention time matched the standard. The GAs identified included GAs 1, 3, 4, 5, 6, 8, 9, 19, 20, 24, and 34, many of these having also been identified by full-scan MS in leaves of L. temulentum (Gocal et al., 1999). In general, on a dry weight basis, the shoot apex contained up to 50-fold more GA than the leaf.
It was not possible, routinely, to analyze all the GAs we had identified both because of restrictions on data collection (e.g. GA3 and GA6) and, sometimes, because of the presence of contaminating ions (e.g. GA20). As a consequence, we quantified only the C-13 hydroxylated GAs 1, 5, and 19 and the non-C-13 hydroxylated GAs 4, 9, 24, and 34. Aside from GA5, these GAs are metabolites of two parallel biosynthetic pathways, with several possible cross-links between them, GA5 being a potential intermediate in the conversion of GA20 to GA3 (Hedden and Kamiya, 1997). In leaves of L. temulentum, we previously identified GA3 and GA20 (Gocal et al., 1999), but GA5 was not detected with certainty because we only obtained a small peak and a match to three fragment ions (A. Poole, unpublished data). GA1 and GA4 were detected in leaves of L. temulentum and especially after exposure to repeated LD (Gocal et al., 1999), but they were hardly detectable in vegetative or pre-inflorescence-stage apices, their content increasing later.
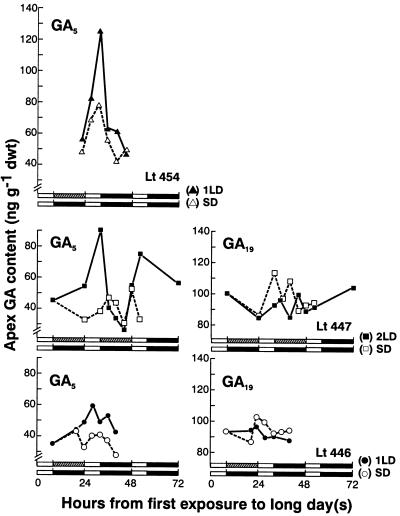
Of the seven GAs analyzed in these vegetative apices, GA19 was the most abundant and GA1 and GA4 were the least abundant. As an indication of reproducibility between experiments, for SD shoot apices collected over four matched experiments, the average content of GA19 was 96.3 ± 2.0 ng g−1 dry weight (n = 15), and by visual inspection (Fig. 1) there appears to be no diurnal trend.
Figure 1.
Diurnal changes in three separate experiments in the GA5 and GA19 content (ng g−1 dry weight [dwt]) of the shoot apex of L. temulentum of vegetative plants in SD (white symbols) and for plants exposed to 1 or 2 florally inductive LD (black symbols). The LD low-irradiance day length extension is shown as a stippled bar, darkness as a black bar, and daylight as a white bar.
Early LD-Induced Changes in GAs at the Shoot Apex
There was an early increase in the GA5 content of the shoot apex following exposure to florally inductive LDs (Fig. 1). By the end of the 8-h high-light period after the 1st LD, there was up to a 2-fold increase in apex GA5 content (91 vs 38 ng g−1 dry weight, experiment Lt447; 126 vs 74 ng g−1 dry weight, experiment Lt454), but with a smaller increase in a further experiment (Fig. 1, Lt446).
Apex dry weight was essentially unchanged over this 1st d (see later, Fig. 2) and, based on estimates of water content of the L. temulentum apex (Rijven and Evans, 1967), the endogenous GA5 concentration in the shoot apex after one LD reached a maximum of about 6 × 10−8 m. This concentration approaches the threshold of 10−7 m required in the medium for GA5 to induce inflorescence initiation in shoot apices of L. temulentum excised from plants in noninductive SD (King et al., 1993).
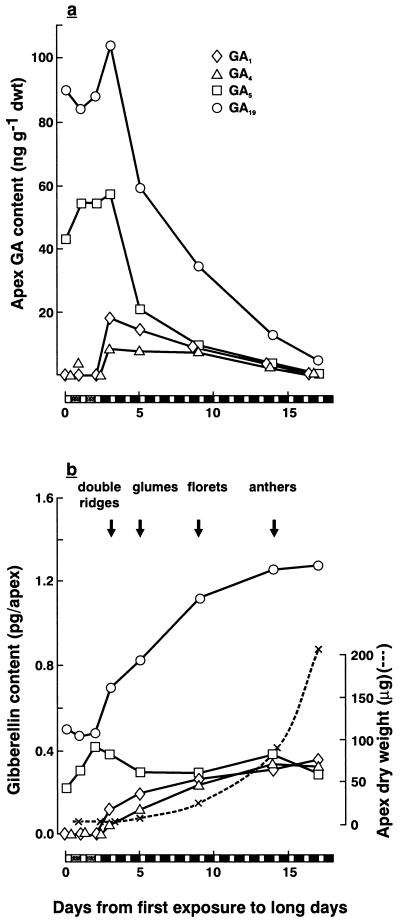
Figure 2.
Changes during inflorescence development in the content of various GAs in the shoot apex of L. temulentum exposed to 2 LD (Expt. Lt 447). Harvests were at the start (8:30 am) of each daily 8-h sunlight exposure. a, GA content is shown as ng g−1 dry weight; b, GA content is shown as ng per apex, together with apex dry weights (×).
For vegetative SD apices, there was some diurnal increase in GA5 content in one experiment (Lt454) but not in the other two (Fig. 1). In contrast to GA5, the content of GA19 in the same apices showed relatively little variation, with no obvious distinction between SD and LD treatments (Fig. 1). GA24 content was relatively unchanged or increased by between 30% and 90%, little or no GA1 or GA4 could be detected, and GA9 content dropped 3-fold (data not shown).
Longer term trends in GA content are shown in Figure 2 for daily samples taken at the start of each day (8:30 am) up to the stage of floret formation at 18 d. As the inflorescence differentiated, GA content per gram dry weight declined (Fig. 2a), but for any one GA the total content per apex remained high or increased up to 10 d (Fig. 2b). Due to increased apex weight associated with inflorescence differentiation (40-fold dry weight increase by the last harvest in Fig. 2b), the late decline in GA content per gram dry weight shown in Figure 2a therefore represents, in part, a late “dilution” of the GAs. It is noteworthy that apex dry weight was constant over the first 3 d after LD exposure (Fig. 2b) and did not change diurnally (data not shown). Thus, the early changes in Figure 1 in GA5 content (“concentration” in Fig. 2a or “amount” in Fig. 2b) relate solely to its metabolism.
To confirm the evidence in Figure 2a of a fall in GA5 content by 4 or 5 d after the start of the LD, three experiments were carried out with daily harvests at the diurnal maxima at 4:30 pm. All confirmed the findings in Figure 2 in that there was a clear doubling in the content of GA5 by 56 h from the start of the LD followed by a fall (data not shown).
Changes at Later Times in GAs at the Shoot Apex
Three days after the start of two LD, the content of GA1 and GA4 increased, whereas that of GA5 and GA19 began to decrease 1 d later (Fig. 2). These findings were confirmed in a further experiment (not shown).
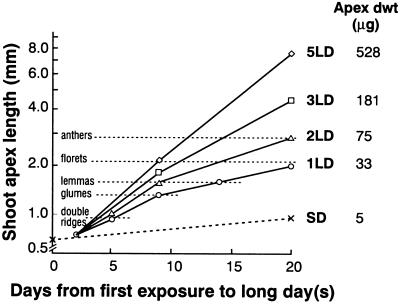
Although inflorescence initiation and flowering of L. temulentum results from exposure to only 1 LD, repeated cycles accelerate floral development, as shown in Figure 3. This enhancement was evident soon after the LD exposure and was matched by the earlier appearance of floral organs (Fig. 3) and by a faster increase in apex dry weight (right-side column in Fig. 3). Plants kept in SD remained vegetative with a final apex length of less than 1.0 mm. Such results confirm the increased flowering response with extra LD (Evans, 1960) and the close relation between shoot apex length and mass previously reported (Rijven and Evans, 1967), which we have used to establish the weight of our batches of apices harvested for GA analysis (see “Materials and Methods”).
Figure 3.
Effect of increasing numbers of LD on shoot apex length (mm) for L. temulentum (Lt449). Measurements continued until 21 d after the first exposure to up to 5 LD. The error bars were smaller than the symbols. For each daylength treatment, apex dry weight (μg) was determined at 21 d on batches of 20 apices. Plants held in SDs remained vegetative and apex dry weight and length increased relatively little over 3 weeks. These dry weights along with values at earlier harvests were used in deriving a relationship between apex length and weight (see “Materials and Methods”).
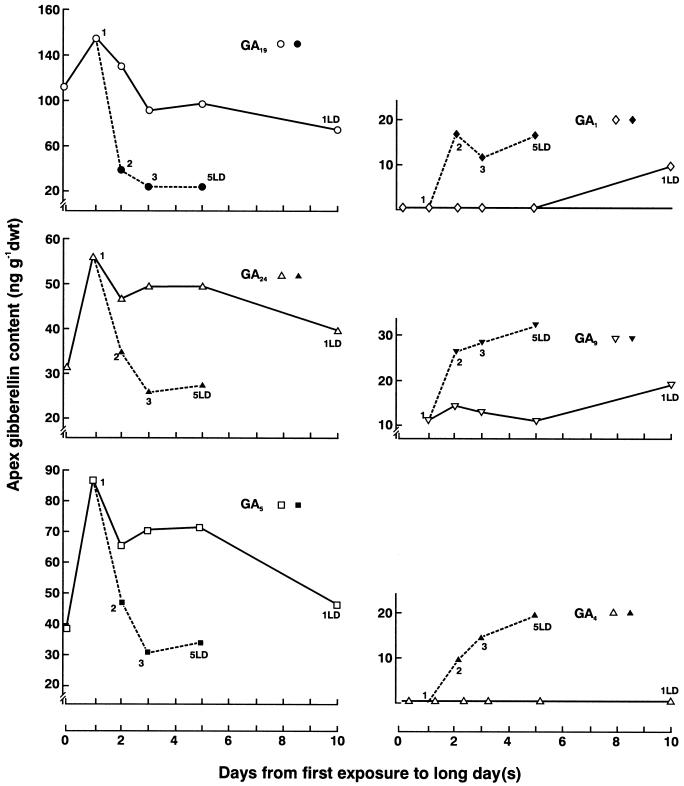
In parallel with enhanced flowering response from extra LD (Fig. 3), increasing the number of LD cycles resulted in large changes in the GA content of the shoot apex as shown in Figure 4. Although a single LD exposure led to a small increase in GA1 from an initially non-detectable level in the vegetative shoot apex, this occurred only after 10 d. Increasing the number of LD cycles greatly increased the content of GA1, GA4, and GA9 in the apex and these changes occurred much earlier (Figs. 2 and 4). Such increases in GA content with multiple LD cycles match those we previously detected in the leaf for GA1, GA4, and GA9 (Gocal et al., 1999). In contrast, multiple LD cycles led to dramatic decreases in the GA19, GA24, and GA5 content of the apex (Fig. 4). The changes in GAs potentially reflect precursor/product conversion of GA19 to GA1, and of GA24 to GA9 and then to GA4. Where complete data were available from three replicate experiments, the content of GA24 had changed little after 2 LD (1.6-fold to 39.0 ± 4.3 ng g−1 dry weight), but GA9 had increased 7-fold to 24.3 ± 7.0 ng g−1 dry weight, and GA4 increased 23-fold to 9.3 ± 5.0 ng g−1 dry weight, whereas GA34 showed little change (2.3-fold increase to 25.9 ± 8.1 ng g−1 dry weight).
Figure 4.
Effect on L. temulentum shoot apex GA content of exposure to a single LD or to up to 5 LD (Expt Lt 443). Apices were harvested at 8:30 am each day for up to 10 d after the single LD or at 8:30 am on the day of ending the exposure to 1, 2, 3, or 5 LD. Where multiple LD cycles were imposed prior to harvest (filled symbol), their number is shown next to the symbol.
Changes in GA content due to LD exposure may indicate regulation by day length at two steps in GA biosynthesis. The increases in GA9 (7-fold) following 2 LD could result from increased activity of a GA 20-oxidase enzyme. The even greater increase in GA4 (23-fold) may indicate an added activation of biosynthesis involving a 3β-hydroxylase enzyme capable of converting GA9 to GA4. The activity of the 2β-hydroxylase that converts GA4 to GA34 may not have changed.
Response to Applied GAs
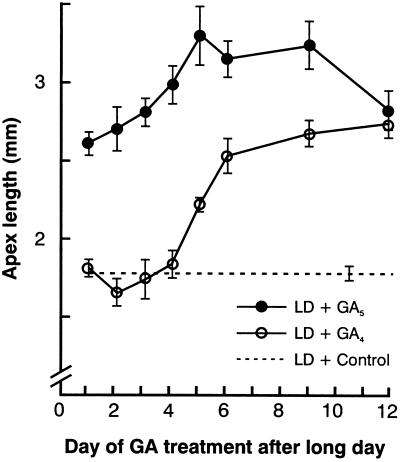
The physiological relevance for flowering of the early increase in endogenous GA5 at the apex and of the later increase in GA4 was examined by applying these GAs to L. temulentum plants induced to flower by one LD. Flowering was promoted by a GA5 application at the time of the LD (Fig. 5), which supports a physiological role for the early rise at the apex in the content of GA5 (Fig. 1). In sharp contrast, applications of GA4 at the time of the LD were ineffective for flowering. Four days later, however, at the time when endogenous GA4 levels had increased at the apex (Figs. 2 and 4), GA4 became florally promotive. There was apparently no restriction on GA uptake and transport to the apex because at all times of application both GA4 and GA5 caused growth of the stem immediately below the apex (data not shown).
Figure 5.
Effect on flowering of a single application to the leaf of 25 μg of GA4 or GA5. The earliest treatment was 6 h after the start of the main light period prior to the single LD extension. Flowering was determined at 21 d and the values are the mean and se.
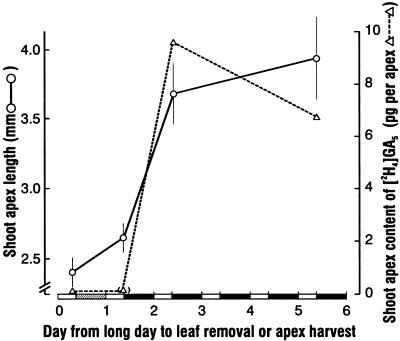
To establish the timing of GA5 export from the leaf blade and of import of the intact (unmetabolized) molecule into the shoot apex, we applied either GA5 or [2H4] GA5 to the leaf. Varying the time of removal of the GA5-treated leaf blade showed that for flowering, export from the treated blade had begun by 30 h from the start of the LD and approached completion by 48 h (Fig. 6). A matching export profile was obtained when stem length was measured on the same plants (not shown). In parallel treatments but involving [2H4] GA5 applied to the leaf blade, its arrival at the shoot apex reached a peak at 48 h (Fig. 6). We refer to this peak time because assay sensitivity becomes a problem in defining the threshold for [2H4] GA5 arrival at the apex. Nevertheless, the match in time between apex arrival of [2H4] GA5 and leaf export of GA5 indicates probable physiological relevance. We detected no [2H4] GA6 at the apex although it is a logical metabolite of [2H4] GA5. Thus, when applied, GA5 can be transported from the leaf to the apex within 1 to 2 d and without chemical alteration.
Figure 6.
Timing of GA5 export from a treated leaf to the shoot apex as shown either by increase in the floral response of the shoot apex with increasing time until removal of the GA-treated leaf blade, or by arrival at the shoot apex of [2H4] GA5 following its application to the leaf. In both treatments, a dose of 25 μg of GA was applied to the leaf blade 6 h after the start of the main light period prior to the single LD extension. Values for floral apex length are the means and se. Plants not treated with GA5 flowered to the same extent as those with the treated leaf removed at the start. There was only a trace of [2H4] GA5 detected at the apex after 24 h, but it is shown in brackets because there was uncertainty about quantitation of the gas chromatography (GC)-MS peak for the [2H2] GA5 internal standard.
The kinetics of [2H4] GA5 import by the apex cannot be used to determine the relationship between applied GA5 and flowering response. However, we could ascertain if there was such a relationship by varying the amount of [2H4] GA5 applied to the leaf. As shown in Table I, based on the one study we performed, there was a reasonable correlation between the amount of [2H4] GA5 at the shoot apex after 48 h and the flowering response after 21 d (r = 0.92; n = 4). Given the lesser amount of endogenous GA5 than [2H4] GA5 in the apex, there was apparently a distinction between the physiologically active and total pools of GA5 and [2H4] GA5.
Table I.
Effect on apex GA content and on flowering of various doses of [2H4] GA5 applied once to the leaf blade 6 h after the start of a LD exposure
| [2H4] GA5 Applied to the Leaf | [2H4] GA5 Detected at the Apex | Flowering Response (Shoot Apex Length) |
|---|---|---|
| μg | pg | mm |
| 0 | 0 | 2.69 ± 0.07 |
| 1 | 2.3 | 2.73 ± 0.16 |
| 5 | 6.5 | 2.98 ± 0.21 |
| 25 | 9.5 | 3.85 ± 0.43 |
The apex was harvested 48 h later.
A separate issue is that only 9.5 pg of the 25 μg of [2H4] GA5 applied to the leaf had reached the shoot apex 48 h later (Table I, Fig. 6), i.e. less than one part in 1 million applied. Such limited import agrees with our earlier studies in which comparable low proportions of leaf-applied radiolabeled sulfate or phosphate reached the shoot apex (Evans and Rijven, 1967). Such limited import no doubt reflects the minute size of the shoot apex as well as potential for compartmentalization and metabolism of a compound during its uptake and transport.
DISCUSSION
GAs as LD Floral Stimuli
We have detected a number of GAs in the minute shoot apex (about 5 μg dry weight) of the grass L. temulentum and established that a florally inductive LD leads to an early doubling in GA5 levels and, several days later, to large increases in GA1 and GA4.
Using highly sensitive and specific MS, we detected GAs at sub-picogram levels. Furthermore, our repeated checks of reliability of the analyses by spiking samples with the GA of interest, and our inclusion of deuterated internal standards for each GA, made these assays specific and reproducible across independent experiments. Previous GC-MS-based studies have not focused on the shoot apex alone, nor has it been considered that different GAs may participate at different stages of floral development. The most relevant report, that of Talon et al. (1991), was focused on the response of subapical tissue that comprised the major portion of their shoot tip samples. Our samples excluded nonapical tissue and were “apically enriched” in that the floral apex of this grass includes up to 24 spikelet sites.
GAs Associated with Floral Evocation
Our previous studies have provided considerable detail on the timing of floral evocation at the shoot apex of L. temulentum. After reaching the critical day length of approximately 16 h, the LD floral stimulus in L. temulentum is translocated down the leaf blade and sheath at 1 to 2 cm h−1 (Evans and Wardlaw, 1966) to reach the shoot apex early on the day after the LD. Experiments with shoot apices excised from such floral plants at various times (McDaniel et al., 1991; King et al., 1993) indicate that floral evocation is virtually complete by the end of that day.
Thus, the close matching in the timing of increase in GA5 that we see at the shoot apex makes it a strong candidate for the LD (photoperiodic) stimulus, a claim we base on the following grounds: (a) During the light period following the LD, the endogenous GA5 doubled at the apex; (b) the GA5 concentration was close to that required for flowering in vitro; (c) the timing of the doubling in endogenous GA5 coincides with the timing of floral evocation; (d) applied GA5 was transported intact from the leaf to the shoot apex (Fig. 6), where it then regulated flowering. This GA5 was imported in physiologically meaningful amounts as increasing leaf application led to parallel increases in both the amount of GA5 transported to the apex and in flowering (Table I); and (e) applied GA5 acts without causing extensive stem elongation, a characteristic of the response to a single LD but not of the response to multiple LD cycles or to other candidate GAs such as GA1, GA3, or GA4 (Evans et al., 1990, 1994a, 1994b).
Considering (a) and (b) above, a doubling in GA5 content of the shoot apex would be biologically important because floral response increased 3-fold with a 3-fold increase in the GA5 dose supplied to excised shoot apices cultured in vitro (King et al., 1993). We have not measured the GA content of cultured apices, but the shoot apex concentration of endogenous GA5 reached 6 × 10−8 m, a value close to the 10−7 m GA5 threshold required for flowering of cultured apices (King et al., 1993). In addition, other endogenous GAs are likely contributors to bioactivity and our preliminary studies indicate a role for GA6. The latter GA is florally effective, causes flowering with little or no stem elongation, and the shoot apex content of GA6 also doubles at the time of floral evocation (R.W. King, L.N. Mander, T. Moritz, R.P. Pharis, and L.T. Evans, unpublished data). As an aside, roles for GA1, GA3, or GA4 in floral evocation can be excluded based on their inherent growth activity and the poor floral activity of GA1 and GA4 (Evans et al., 1990, 1994a, 1994b). Furthermore, for these latter two GAs, inhibitor studies also excluded any early role at the time of the LD. An inhibitor capable of blocking the 3β-hydroxylase enzyme did not inhibit flowering although stem elongation was inhibited (Evans et al., 1994a).
Although exogenously supplied GA5 is transported from the leaf to the shoot apex of L. temulentum, it remains to be shown that the leaf is the source of GA5 for the shoot apex because detection of GA5 in leaf extracts of L. temulentum has proven technically difficult. The shoot apex itself could be the site of GA5 formation via a single step conversion of GA20 to GA5 (Hedden and Kamiya, 1997), GA20 being an endogenous transported form of GA as suggested for pea (Pisium sativum; Proebsting et al., 1992). In support of this scenario, the content of GA20 in leaves of L. temulentum does increase with exposure to 2 LD (Gocal et al., 1999). However, when GA20 was applied to the leaf of vegetative plants, it had little or no floral activity compared with GA5 (Evans et al., 1990). Furthermore, the recent report of Sakamoto et al. (2001) of high levels of expression of a GA 2-oxidase gene in procambium at the base of the vegetative shoot apex of rice (Oryza sativa), but not in florally evoked apices, suggests that prior to floral evocation there will be rapid catabolism of any GA20 arriving at the shoot apex. Given such uncertainties, GA5 is either a primary LD floral stimulus in L. temulentum or its content increases directly at the shoot apex in response to a transported stimulus. Either way, it is GA5 at the shoot apex that our findings suggest is important for LD-induced floral evocation.
GAs Associated with Inflorescence Development
For the first time, our study shows that GAs are important at the beginning of inflorescence differentiation. This claim is based on our evidence of a dramatic increase at inflorescence differentiation in the shoot apex content of the 3β-hydroxylated GA1 and GA4. Furthermore, at this time applied GA4 first becomes florally active (Fig. 5), being inactive for earlier floral evocation (Pharis et al., 1987; Evans et al., 1990). In addition, the involvement of GA1 and GA4 in inflorescence initiation is supported by our earlier studies with inhibitors of GA20 3β-hydroxylation. We found (Evans et al., 1994a) that several days after the inductive LD, flowering and stem elongation were both inhibited by application of Trinexapac-ethyl (ethyl-[3-oxido-4-cyclopropionyl-5-oxo]oxo-3-cyclohexenecarboxylate). With L. temulentum, such acylcyclohexanediones block the 3β-hydroxylation of GA20 to GA1 and cause GA20 to accumulate (Junttila et al., 1997).
For both the L. temulentum shoot apex (this study) and the leaf (Gocal et al., 1999), exposure to multiple LD causes increases in GA1 and GA4 levels. By contrast, despite the increase in the shoot apex content of GA5 after a single LD, its content in the apex declining with exposure to multiple LD. Why the spectrum of GAs at the apex shifts over time is a matter for speculation but, for GA1 and GA4 which are readily 2β-hydroxylated, a reduced expression of GA 2-oxidase activity at inflorescence differentiation, as reported in rice (Sakamoto et al., 2001), would allow an increase in apical GA1 and GA4 at this time. In a converse manner, in the vegetative shoot apex and at floral evocation these GAs would be degraded but there could be a buildup of GA5 because of its lesser susceptibility to 2-oxidase activity due to its ring A C2-3 double bond.
Of the two actions of GAs proposed to control flowering of L. temulentum, it is the late changes in GA1 and GA4 at floral differentiation that are most similar to GA changes that occur just before the onset of rapid stem elongation (bolting) of dicots where GA1 levels can increase dramatically in shoots and petioles (Talon and Zeevaart, 1990; Talon et al., 1991; Zeevaart et al., 1993; Zanewich and Rood, 1995; for review, see Metzger, 1995). However, unlike dicots where GA biosynthesis inhibitors may block bolting but not flowering (Cleland and Zeevaart, 1970), with L. temulentum GA biosynthesis inhibitors inhibit stem elongation but may either promote or inhibit flowering (Evans et al., 1994a). Given our evidence of complexity not only in the spectrum of active GAs, but in their timing of action and in the response of flowering to inhibitors of GA biosynthesis, it would be interesting to examine for dicots early shoot apex changes during flowering and for GAs other than GA1 or GA4.
Overall, despite recent speculative claims to the contrary (Colasanti and Sunderasan, 2000; Samach and Coupland, 2000), our studies show that, for L. temulentum at least, GAs may serve as LD flowering signals. Also, our findings provide a novel and more dynamic view than has been considered previously to explain the role of GAs in floral evocation and inflorescence differentiation.
MATERIALS AND METHODS
Plant Materials and Growing Conditions
Plants of Lolium temulentum strain Ceres were grown vegetatively in 8-h SDs in sunlit controlled-environment cabinets as described previously (Evans et al., 1990). Floral induction by LD involved one or more exposures to 16-h extensions of the 8-h day using light from incandescent lamps at a low photon flux density (10 μmol m−2 s−1). Three weeks later, the flowering response was scored for both stage of morphogenesis and apex length, these two measures being closely related (Evans et al., 1990). At this time stem length was also measured. Timing from the LD is taken from the start of the 8-h main photoperiod of the first LD.
All GA applications were made to the uppermost expanded leaf blade in a 10-μL drop of 95% (v/v) ethanol:water, the controls being treated with 95% (v/v) ethanol:water. The GAs were pure samples supplied by Prof. Lewis Mander (Research School of Chemistry, Australian National University, Canberra). In one study, to assess export, the GA5-treated leaf blade was cut off at various times after application. Some metabolism/transport studies with GA5 utilized a sample of [15,15,17,17-2H4] GA5 and, on GC-MS, this form was readily distinguished from a deutero [17,17-2H2] GA5 internal standard as well as from GA5 but all were matched for relevant ions and their abundance.
GA Analysis
The first four experiments were carried out at 1-month intervals as duplicate pairs, and 2 years later a fifth set of harvests was made. Shoot apices were harvested as batches of 40 for each sampling time/treatment and, over all experiments, 4,320 apices were used. At dissection, they were immediately frozen in a microcentrifuge tube and each batch of 40 apices was freeze dried. To account for differences in apex weight especially over time, we derived apex dry weights from measured apex lengths based on a linear relationship that exists after log transformation of length and dry weight (r2 = 0.988: n = 14). The flowering response to LD exposure was similar for all experiments, and particularly for each of the paired duplicates in the four initial experiments (data not shown). Plants held in SD remained vegetative.
For GA assay, homogenized tissue was extracted with 500 μL of 80% (v/v) aqueous MeOH at 4°C, with 30 pg of [17,17-2H2] GAs added as internal standards to give a deutero:protio ratio close to 1.0. The GAs were partitioned 3× at pH 2.8 into an equal volume of ethyl acetate (etOAc). The extract was applied in EtOAc to a pre-equilibrated 100-mg aminopropyl Isolute cartridge (Sorbent AB, Västra Frölunda, Sweden). The cartridge was washed with 3 mL of EtOAc, then eluted with 5 mL of 0.2 m formic acid that was run directly onto a pre-equilibrated 100-mg C18 Isolute cartridge (Sorbent AB) and the GAs were then eluted with 2 mL of 80% (v/v) MeOH.
The samples were methylated with ethereal diazomethane and, after evaporation, dissolved in MeOH and loaded onto a 100-mg Isolute aminopropyl cartridge. The methylated GAs were eluted with 3 mL MeOH, which was reduced to dryness and trimethylsilylated in 10 μL pyridine and 10 μL N-methyl-N-trimethylsilyltrifluoroacetamide at 70° for 30 min. Samples were injected in dichloromethane in the splitless mode into a 5890 GC (Hewlett-Packard, Sydney) equipped with a 30-m × 0.25-mm i.d. fused silica capillary column with a chemically bonded 0.25 mm DB-5 MS stationary phase (J&W Scientific, Folsom, CA). The injector temperature was 270°C. The column temperature was held at 50° for 2 min, then increased by 20°C min−1 to 220°C, and by 4°C min−1 to 270°C. The column effluent was introduced into the ion source of a JEOL JMS-SX/SX102A four-sector tandem mass spectrometer of B1E1-B2E2 geometry (JEOL, Tokyo). The interface and the ion source temperatures were 270°C and 250°C, respectively. Ions were generated with 70 eV at an ionization current of 600 μA. Measurements involving HR-SIM were performed with an accelerating voltage switching from 10 KV and a resolution of 7,000 to 10,000.
Perfluorokerosene was used as a reference compound after choosing a suitable lock mass. The dwell time was 50 ms. For each GA, the ions recorded for HR-MS and SRM-MS are shown in Table II along with their deuterated analogs. In the SRM mode, the acceleration voltage was 10 kV and the precursor ions were selected by magnetic switching. The daughter ions formed in the first field-free region were detected by switching the magnetic field and the electrostatic field simultaneously. The dwell time was 100 ms, and specific reactions for the different GAs were recorded according to Moritz and Olsen (1995).
Table II.
Ions detected for quantification of specific GAs and the deuterated analogues used as internal standards
| GA | Ions HR-SIM | SRM |
|---|---|---|
| GA1 | 506.2520; 448.2101 | 506–448 |
| [2H2]-GA1 | 508.2645; 450.2227 | 506–207 |
| GA3 | 504.2364; 370.1961 | 504–370 |
| [2H2]-GA3 | 506.2489; 372.2086 | 506–372 |
| GA4 | 284.1776; 224.1565 | 418–390 |
| [2H2]-GA4 | 286.1902; 226.1691 | 420–392 |
| GA5 | 416.2020; 299.1645 | 416–299 |
| [2H2]-GA5 | 418.2145; 301.1770 | 418–301 |
| GA6 | 432.1968; 303.1417 | 432–303 |
| [2H2]-GA6 | 434.2091; 305.1540 | 432–207 |
| GA9 | 298.1570; 270.1620 | 298–270 |
| [2H2]-GA9 | 300.1690; 272.1740 | 300–272 |
| GA19 | 434.2489; 375.2355 | 434–375 |
| [2H2]-GA19 | 436.2614; 377.2481 | 436–377 |
| GA20 | 418.2176; 375.2355 | 418–375 |
| [2H2]-GA20 | 420.2301; 377.2481 | 420–377 |
| GA24 | 314.1789; 286.1930 | 314–286 |
| [2H2]-GA24 | 316.1914; 288.2055 | 316–288 |
| GA34 | 506.2520; 289.1440 | 506–289 |
| [2H2]-GA34 | 508.2645; 291.1565 | 508–291 |
Ions used for HR-SIM measurements and detected transitions for SRM measurements are shown. The specific ions were detected in the time range when the specific GA eluted on the GC.
Reliability of analysis was checked on several occasions by adding GAs-MeTMS or [17,17-2H2]GAs-MeTMS to previously analyzed samples, and then re-analyzing the samples. Increased intensity of the GA peak at the right retention time, and lack of chromatography changes at the GA peak, indicated reliability of the analysis.
ACKNOWLEDGMENTS
Cheryl Blundell is thanked for her excellent technical support, Lewis Mander for supplying deuterated GA internal standards, and Andrew Poole for support with GA analysis.
Footnotes
This work was supported by the Department of Tourism and Industry, Australia (to R.W.K.) and by the Human Frontiers Science Program (grant no. RG0303/1997–M to T.M.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010378.
LITERATURE CITED
- Blásquez MA, Soowal LN, Lee I, Weigel D. LEAFY expression and flower initiation in Arabidopsis. Development. 1997;124:3835–3844. doi: 10.1242/dev.124.19.3835. [DOI] [PubMed] [Google Scholar]
- Cleland CF, Zeevart JAD. Gibberellins in relation to flowering and stem elongation in the long day plant Silene armeria. Plant Physiol. 1970;46:392–400. doi: 10.1104/pp.46.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colasanti J, Sunderasan V. “Florigen” enters the molecular age: long-distance signals that cause plants to flower. Trends Biochem Sci. 2000;25:236–240. doi: 10.1016/s0968-0004(00)01542-5. [DOI] [PubMed] [Google Scholar]
- Evans LT. The influence of environmental conditions on inflorescence development in some long day grasses. New Phytol. 1960;59:163–174. [Google Scholar]
- Evans LT. Inflorescence initiation in Lolium temulentum L.: V. The role of auxins and gibberellins. Aust J Biol Sci. 1964;17:10–23. [Google Scholar]
- Evans LT. Inflorescence initiation in Lolium temulentum L.: XIII. The role of gibberellins. Aust J Biol Sci. 1969;22:773–786. [Google Scholar]
- Evans LT, King RW, Chu A, Mander LN, Pharis RP. Gibberellin structure and florigenic activity in Lolium temulentum L., a long-day plant. Planta. 1990;182:97–106. doi: 10.1007/BF00239990. [DOI] [PubMed] [Google Scholar]
- Evans LT, King RW, Mander LN, Pharis RP. The relative significance for stem elongation and flowering in Lolium temulentum of 3β-hydroxylation of gibberellins. Planta. 1994a;192:130–136. [Google Scholar]
- Evans LT, King RW, Mander LN, Pharis RP, Duncan KA. The differential effects of C-16,17 dihydro gibberellins and related compounds on stem elongation and flowering in Lolium temulentum L. Planta. 1994b;193:107–114. [Google Scholar]
- Evans LT, Rijven AHGC. Inflorescence initiation in Lolium temulentum L.: XI Early increases in the incorporation of 32P and 35S by shoot apices during induction. Aust J Biol Sci. 1967;20:1033–1042. [Google Scholar]
- Evans LT, Wardlaw IF. Inflorescence initiation in Lolium temulentum L.: IV Translocation of the floral stimulus in relation to that of assimilates. Planta. 1966;68:310–326. doi: 10.1007/BF00386331. [DOI] [PubMed] [Google Scholar]
- Gocal GFW, Poole AT, Gubler F, Watts RJ, Blundell C, King RW. Long-day up-regulation of a GAMYB gene during L. temulentum inflorescence formation. Plant Physiol. 1999;119:1271–1278. doi: 10.1104/pp.119.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden P, Kamiya Y. Gibberellin biosynthesis: enzymes, genes and their regulation. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:431–460. doi: 10.1146/annurev.arplant.48.1.431. [DOI] [PubMed] [Google Scholar]
- Junttila O, King RW, Poole A, Kretschmer G, Pharis RP, Evans LT. Regulation in Lolium temulentum of the metabolism of gibberellin A20 and gibberellin A1 by 16,17-dihydro GA5 and by the growth retardant, LAB 198 999. Aust J Plant Physiol. 1997;24:359–369. [Google Scholar]
- King RW, Blundell C, Evans LT. The behavior of shoot apices of Lolium temulentum L. in vitro as the basis of an assay system for florigenic extracts. Aust J Plant Physiol. 1993;20:337–348. [Google Scholar]
- McDaniel CN, King RW, Evans LT. Floral determination and in vitro floral differentiation in isolated shoot apices of Lolium. temulentum L. Planta. 1991;185:9–16. doi: 10.1007/BF00194508. [DOI] [PubMed] [Google Scholar]
- Metzger JD. Hormones and reproductive development. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 617–648. [Google Scholar]
- Moritz T, Olsen JE. Comparison between high-resolution selected ion monitoring, selected reaction monitoring, and four-sector tandem mass spectrometry in quantitative analysis of gibberellins in milligram amounts of plant tissue. Anal Chem. 1995;67:1711–1716. [Google Scholar]
- Pharis RP, Evans LT, King RW, Mander LN. Gibberellins, endogenous and applied, in relation to flower induction in the long-day plant Lolium temulentum L. Plant Physiol. 1987;84:1132–1138. doi: 10.1104/pp.84.4.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proebsting WM, Hedden P, Lewis MJ, Croker SJ, Proebsting LN. Gibberellin concentration and transport in genetic lines of pea. Plant Physiol. 1992;100:1354–1360. doi: 10.1104/pp.100.3.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijven AHGC, Evans LT. Inflorescence initiation in Lolium temulentum L.: IX. Some chemical changes at the shoot apex at induction. Aust J Biol Sci. 1967;20:1–12. [PubMed] [Google Scholar]
- Sakamoto T, Kobaysashi M, Itoh H, Tagiri A, Kayano T, Tanaka H, Iwahori S, Matsuoka M. Expression of a gibberellin 2-oxidase gene around the shoot apex is related to phase transition in rice. Plant Physiol. 2001;125:1508–1516. doi: 10.1104/pp.125.3.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samach A, Coupland G. Time measurement and the control of flowering in plants. Bioessays. 2000;22:38–47. doi: 10.1002/(SICI)1521-1878(200001)22:1<38::AID-BIES8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Talon M, Tadeo FR, Zeevaart JAD. Cellular changes induced by exogenous and endogenous gibberellins in shoot tips of the long-day plant Silene armeria. Planta. 1991;185:487–493. doi: 10.1007/BF00202957. [DOI] [PubMed] [Google Scholar]
- Talon M, Zeevaart JAD. Gibberellins and stem growth as related to photoperiod in Silene armeria L. Plant Physiol. 1990;92:1094–1100. doi: 10.1104/pp.92.4.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel D, Nilsson O. A developmental switch sufficient for flower initiation in diverse plants. Nature. 1995;377:495–500. doi: 10.1038/377495a0. [DOI] [PubMed] [Google Scholar]
- Wilson RN, Heckman JW, Somerville CR. Gibberellin is required for flowering in Arabidopsis thaliana under short days. Plant Physiol. 1992;100:403–408. doi: 10.1104/pp.100.1.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanewich KP, Rood SB. Vernalization and gibberellin physiology of winter canola: endogenous gibberellin (GA) content and metabolism of [3H]GA1 and [3H]GA20. Plant Physiol. 1995;108:615–621. doi: 10.1104/pp.108.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart JAD, Gage DA, Talon M. Gibberellin A1 is required for stem elongation in spinach. Proc Nat Acad Sci USA. 1993;90:7401–7405. doi: 10.1073/pnas.90.15.7401. [DOI] [PMC free article] [PubMed] [Google Scholar]