Summary
Background
In 2022, the rapid spread of the highly transmissible Omicron variant precipitated a dramatic surge in infections and a subsequent societal shutdown in Shanghai. It offers the opportunity to examine the impact of COVID-19 pandemic on cognitive decline among community-dwelling older adults.
Methods
The Shanghai Aging Study enrolled 3792 community residents aged ≥ 50 from 2010 to 2012 in central Shanghai. Demographics, medical history, ApoE genotyping, and plasma phosphorylated tau 217 (p-tau217), phosphorylated tau 181 (p-tau181), and neurofilament light chain (NfL) were collected at baseline. Cognitive function assessment and MRI scans were conducted at baseline and follow-up visits from 2014 to 2024. Study periods were defined as Wave 1 (Jan.2010–Dec.2012, baseline, pre-pandemic), Wave 2 (Jan.2014–Mar.2022, pre-pandemic), and Wave 3 (Jun.2022–Dec.2024, post-pandemic). Event study, difference-in-differences (DID), and linear mixed-effects models were employed to evaluate the pandemic’s impact on cognitive trajectories and brain structural changes.
Findings
We observed steeper age-related declines of the Mini-Mental State Examination (MMSE) during Wave 3. The event study model adjusting for age, sex, and education, showed significant MMSE decline in Wave 3, but not in Wave 2. Declines were more pronounced in individuals with high baseline plasma p-tau217, p-tau181, and NfL, ApoE-ε4 carriers, those with multi-comorbidities, or long-term medication use. The DID and linear mixed-effects models with individual-specific follow-up data revealed accelerated declines in global cognition, executive and language function, and structural brain atrophy from Wave 2–3, compared with Wave 1–2.
Interpretation
The COVID-19 pandemic significantly accelerated cognitive and brain structural changes in community-dwelling older adults, particularly among those with pre-existing AD pathology or health vulnerabilities. These findings provide evidence of COVID-19 pandemic-related cognitive decline and highlight the need for mechanistic research into how direct and indirect stressors converge to drive cognitive impairment.
Funding
National Natural Science Foundation of China, Tianqiao and Chrissy Chen Institute, China National Postdoctoral Committee Fund, UCL: Wellcome Early-Career Award and Royal Society.
Keywords: COVID-19 pandemic, Cognitive decline, Brain structure changes, Community-based, Chinese population
Research in context.
Evidence before this study
We searched PubMed for studies conducted between Jan 1, 2020, and April 30, 2025, using the search terms ([“Cognition” OR “cognitive” OR “dementia” OR “Alzheimer” OR “structural brain changes” OR “neuroimaging”] AND [“COVID” OR “COVID-19”] AND “pandemic” AND “longitudinal” OR “cohort”). The date range was dictated by the timeline of the COVID-19 pandemic. Most existing studies were based on hospital cohorts following the SARS-CoV-2 infection while lacking pre-pandemic cognitive data, particularly those among community-based older adults. The few cohort studies comparing pre- and post-pandemic cognitive performances have yielded inconsistent findings and lacked objective neuroimaging data on brain structural changes.
Added value of this study
This study, based on data from a longitudinal cohort with continuous assessments of cognitive function and brain imaging among community-dwelling older adults in Shanghai, utilized event study, DID, and linear mixed-effects model to examine the impact of the COVID-19 pandemic on cognitive function and decline, and brain structural changes. We found that older adults who experienced the COVID-19 pandemic had a greater decline in cognitive function, affecting including general cognition, executive function, and language function, as well as greater reductions in volume and cortical thickness across multiple AD-related ROIs. This effect was more pronounced among individuals with high pre-existing AD pathology, and those with physical health conditions.
Implications of all the available evidence
Our findings suggest that the COVID-19 pandemic significantly accelerated cognitive decline and brain structural changes in community-dwelling older adults in Shanghai, with those having pre-existing AD pathology, and with physical health conditions being particularly vulnerable. These findings provide evidence of COVID-19-pandemic-related cognitive decline and highlight the need for mechanistic research into how direct and indirect stressors converge to drive cognitive impairment.
Introduction
The COVID-19 pandemic has reshaped numerous aspects of public health and societal dynamics, imposing unprecedented challenges on vulnerable older adults.1, 2, 3, 4, 5 Although the immediate focus was respiratory and acute medical complications following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, emerging evidence suggests lasting effects on cognitive health and brain structure.6, 7, 8, 9, 10, 11, 12, 13, 14 However, most existing studies were based on hospital cohorts following the SARS-CoV-2 infection – for instance Liu et al. demonstrated an accelerated cognitive decline among COVID-19 survivors in Wuhan11 – while lacking pre-pandemic cognitive data, particularly from community-dwelling older adults. Additionally, the pandemic’s indirect consequences, i.e., health needs inaccessibility and prolonged social isolation, may have synergistical impact on longitudinal cognitive trajectories and brain morphology.6,8 Recent population-based studies,15, 16, 17, 18, 19 such as the UK Biobank, PROTECT,15 and Nurses’ Health Study,18 have yielded inconsistent findings. They predominantly featured Western cohorts and left a notable gap in data representativeness from Chinese community-based population.
As one of China’s most dynamic metropolises, Shanghai has faced substantial public health challenges due to its pronounced population aging.20,21 In response to the sporadic COVID-19 cases from January 2020 onwards,22,23 Shanghai adopted a ‘precise prevention (精准防控 in Chinese)’ strategy.24 However, the pattern of the COVID-19 pandemic in Shanghai differed significantly from the global trend.5,22,24 The swift spread of the highly transmissible Omicron variant in early 2022 precipitated an abrupt shift to an unprecedented citywide lockdown in April and May 2022,23, 24, 25, 26, 27 which significantly disrupted daily life and social engagement of local residents, particularly the older adults. By the end of 2022, as most infections manifested quite mild symptoms, the city loosened interventions and achieved a major policy transition.28 The evolving pattern of public health measures in these distinct periods provides the context for examining whether the COVID-19 pandemic accelerated cognitive decline among community-dwelling older adults.
To address this question, we utilized the Shanghai Aging Study (SAS), a longitudinal, community-based cohort that includes comprehensive assessments of cognitive function and brain structure via magnetic resonance imaging (MRI) from 2010 to 2024. Encompassing both pre-pandemic and post-pandemic periods, this cohort offers the opportunity to examine the pandemic’s impact over time. This study aimed to verify the hypotheses that the COVID-19 pandemic accelerated the cognitive decline and brain atrophy in community-dwelling older adults, and that those with higher Alzheimer’s disease (AD) pathology burden or other health conditions became more vulnerable during the pandemic.
Methods
Study participants
The SAS,21 as an ongoing community-based cohort study aims to evaluate the prevalence, incidence, and risk factors for dementia and mild cognitive impairment (MCI) in older adults resided in the Jing’ansi Community of Shanghai, China. During 2010–2012, 3792 registered residents aged ≥50 years were enrolled at baseline, with participants excluded if they: (a) had severe schizophrenia or intellectual disabilities as documented in their medical records; (b) had severe impairments in vision, hearing, or speech that hindered participation in neuropsychological assessments. Detailed descriptions of the study design and procedures of the SAS cohorts have been published elsewhere.21 Between 2018 and 2021, additional 302 residents aged ≥50 years were recruited from the same community using the identical inclusion and exclusion criteria. In this study, we included participants from both the baseline cohort and those recruited during 2018–2021. Enrolled study participants were invited for follow-up interviews from March 2014 to December 2024. Death certificates of study participants were obtained from the database of the local Bureau of Public Security and Center of Disease Control.29
COVID-19 pandemic pattern and observational waves
As indicated in Fig. 1, from January 2020 to December 2021, no more than 300 COVID cases were reported sporadically in Shanghai.23 During April and May 2022 the number of COVID cases was surging: over 58,000 residents were tested positive.26 It implemented a strict lockdown policy: over 24 million residents in all 216 sub-districts of Shanghai complied with home quarantine; daily necessities were delivered door-to-door by government agencies and volunteers, and several rounds of massive PCR tests were conducted.25 In November 2022, tracing applications and PCR tests were removed; markets, transport systems, and social services were operated as normal.28
Fig. 1.
COVID-19 pandemic pattern in Shanghai.
Regarding the pattern of the COVID-19 pandemic in Shanghai, this study proposed the key window period (“shock window”) related to the COVID-19 pandemic: April to May 2022. We define January 2010 to December 2012 as the pre-pandemic Wave 1, January 2014 to March 2022 as the pre-pandemic Wave 2, and June 2022 to December 2024 as the post-pandemic Wave 3 (Fig. 2).
Fig. 2.
Observational waves and the paradigm of study design.
Neuropsychological assessments and clinical diagnosis
Each participant underwent a comprehensive battery of neuropsychological tests at baseline and follow-up interviews. The detailed description and normative data of neuropsychological tests have been published previously.21,30
The global cognition was assessed by the Mini-Mental State Examination (MMSE). During June to October 2022, due to COVID-19-related public health restrictions, follow-up assessments were conducted via telephone using the Telephone Interview for Cognitive Status 40-item version (TICS-40).31 To ensure comparability with MMSE scores, TICS-40 results were converted to MMSE-equivalent scores using established crosswalk methodologies.31,32
The domain-specific cognitive functions, including memory, attention, language, executive function, and visuospatial were assessed by 1) the Modified Common Objects Sorting Test (MCOST) -categorization for executive function; 2) the Stick Test for spatial construction function; 3) the MCOST-category naming for language function; 4) the Auditory Verbal Learning Test -delayed memory for verbal delayed memory in participants with ≥6 years of education, or the Modified Fuld Object Memory Evaluation for immediate and delayed memory in participants with <6 years of education; 5) Trail Making Test A for attention in participants with ≥6 years of education, or the Renminbi Test in participants with <6 years of education. Domain-specific cognitive scores were standardized into z-scores.21,30
An expert panel, consisting of two neurologists, one neuropsychologist, and one neuroepidemiologist, conducted a consensus diagnosis of cognitive status based on all available medical, neurological, and neuropsychological examinations. Dementia was diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, 4th edition criteria.33 Only those who were not diagnosed with dementia were considered for a diagnosis of MCI, which was defined according to Petersen’s criteria.34 Those without dementia and MCI were defined as having normal cognition.
MRI acquisition
At baseline, MRI scans were performed using a 1.5-T General Electric scanner. Diffusion-weighted imaging was performed using a single-shot echo-planar imaging technique enhanced by simultaneous multi-slice technology, with repetition time = 8000 ms, echo time = 80 ms, using 27 diffusion directions, a b-value = 1000 s/mm2, and acquisition at a single b = 0 s/mm2, field of view = 230 mm, flip angle = 90°, and slice thickness/gap = 6/6 mm. MRI scans were conducted using various imaging protocols over different time points in follow-ups. Detailed specifications of the imaging equipment and acquisition parameters are provided in the Supplementary Table S1.
For T1-weighted (T1w) MRI scans, we used FreeSurfer (version 7.2.0) for the automated segmentation and parcellation of the brain MRI data (Supplementary Methods in detail). Gray matter volume and cortical thickness were extracted from predefined AD signature regions of interest (ROIs), including the medial temporal lobe, inferior parietal lobe, inferior frontal lobe, inferior temporal lobe, temporal pole, precuneus, supramarginal gyrus, superior parietal lobe, and superior frontal lobe.35,36 Additionally, hippocampal gray matter volume was analyzed as a structural MRI marker of AD-related neurodegeneration.35,36 ROI volumes and cortical thickness metrics were computed by averaging left- and right-hemisphere measures across participants. Total intracranial volume was then included as a covariate in all regression models to adjust for between-subject differences in head size.
Covariates
Besides age, sex, and years of education, participants were interviewed for the comorbidities and long-time medications use (taken for more than one year) at baseline confirmed by their medical records. Individuals were defined as have multi-comorbidities if they had two or more of conditions: diabetes, dyslipidemia, hypertension, cardiovascular or cerebrovascular diseases, respiratory diseases, metabolic diseases, eye diseases, arthritis or osteoporosis, cancer, kidney disease, hepatitis, and tuberculosis. Participants were grouped into five-year birth cohorts based on the year of birth (e.g., individuals born between 1940 and 1945 were defined as the 1940 birth cohort).
Overnight fasting blood samples were collected, centrifuged, and stored at −80 °C at baseline, and were used for biomarker assays. EDTA plasma phosphorylated tau 217 (p-tau217), phosphorylated tau 181 (p-tau181), and neurofilament light chain (NfL) were quantified using an ultra-sensitive single-molecule array (Simoa) technology (Quanterix, MA, USA) on the automated Simoa HD-X platform (GBIO, Hangzhou, China). The ALZpath Simoa p-tau217 v2 (Cat No: 104371), p-tau181 v2 (Cat No: 103714), and NfL (Cat No: 103186) assay kits were used according to the technical notes.37 High level of baseline plasma p-tau217, p-tau181, and NfL were defined as ≥0.315 pg/mL, ≥1.882 pg/mL, and ≥15.187 pg/mL, respectively, according to their median values of all tested samples.
ApoE genotyping was conducted by the TaqMan single nucleotide polymorphism method. ApoE-ε4 positive was indicated by the presence of at least one ε4 allele.
Statistical analysis
Categorical variables were expressed as frequency (%), while continuous variables were expressed as mean (standard deviation). Welch and post hoc Games–Howell test were used to test the difference of continuous variables, while chi-square and post hoc z test (FDR method for adjusting multiple comparisons) were adopted to compare the difference of categorical variables. We used linear regression model to examine the association between cognitive function and age across all assessment years and waves, adjusting for sex and education years.
For the panel data of Waves 1, 2, and 3, we employed the event study approach38 to assess the impacts of COVID-19 pandemic on the global cognition (MMSE) of the participants. We considered Waves 1 to 2 as pre-pandemic periods and Wave 3 as post-pandemic period. If the pandemic had an effect on cognition, the performance of participants should exhibit a gap from Wave 1/2 to Wave 3, adjusting for all other factors including age at wave, sex, and education years.
The individual-specific longitudinal data included global and domain-specific cognition measures, as well as brain imaging data collected during Wave 1–2 (pre-pandemic period) and Wave 2–3 (across-pandemic period). For each period, differences in cognitive performance were observed between the ‘pre’ and ‘post’ periods. This context allowed us to apply the difference-in-differences (DID) approach39, 40, 41 to analyze the effect of the pandemic on cognitive performance by comparing the pre-to-post differences of two periods. Subsequently, a linear mixed-effect model, incorporating random intercepts for each participant to account for repeated measures over time, was fitted to the same sample used in the DID analysis to further examine whether participants who experienced the pandemic exhibited an accelerated rate of cognitive decline. The follow-up duration was defined as the interval between two assessments for each participant.
To assess the robustness of our results, we performed a sensitivity analysis excluding participants who died before April 2022, thereby minimizing selection bias from individuals who did not experience the pandemic or contribute post-pandemic follow-up data.
To assess whether the association between the pandemic and cognitive function varied across subgroups, we repeated event study and DID analytical procedures within different birth cohorts and subgroups defined by plasma AD biomarker profile, ApoE-ε4 status, multimorbidity, and long-term medication use, and we tested each factor by adding an interaction term in the models. Detailed statistical methods and model specifications are provided in the Supplementary Materials.
Statistical analyses and visualization were conducted in R (version 4.4.1) utilizing packages dplyr, lme4, emmeans, ggplot2, forcats, patchwork, and fsbrain.
Ethics approval
This cohort study was approved by the Medical Ethics Committee of Huashan Hospital, Fudan University (No. 2009-195). All participants and/or their legally authorized representatives provided their written informed consent.
Role of the funding source
The funders (National Natural Science Foundation of China, Tianqiao and Chrissy Chen Institute, China National Postdoctoral Committee Fund, UCL: Wellcome Early-Career Award and Royal Society) had no role in study design, data collection, data analysis, interpretation or writing of the manuscript.
Results
Characteristics of participants
At Wave 1 (January 2010–December 2012), 3792 community-dwelling older adults in Shanghai were enrolled at the baseline. By Wave 2 (January 2014–March 2022), 2120 participants (including 302 newly recruited individuals) were assessed, and by Wave 3 (June 2022–December 2024), cognitive assessments were completed for 990 participants. MMSE scores at Wave 3 were significantly lower than in earlier waves. Among all participants, 904 participants died before the COVID-19 pandemic. Detailed demographic characteristics, plasma biomarker profiles, ApoE-ε4 status, baseline multimorbidity burden, long-term medication use, anxiety symptoms, and cognitive function across waves were presented in Table 1.
Table 1.
Characteristics of participants at each wave.
| Wave1a |
Wave2a |
Wave3a |
P value | |
|---|---|---|---|---|
| (N = 3792) | (N = 2123) | (N = 990) | ||
| Age at each wave, years, mean (SD) | 70.5 (8.8) | 74.8 (7.9) | 79.2 (6.9) | <0.001 |
| Sex, male, n (%) | 1685 (44.4) | 934 (44.1) | 413 (41.7) | 0.305 |
| Education, years, median (IQR)b | 12.0 (6.0) | 12.0 (6.0) | 12.0 (6.0) | <0.001 |
| ApoE-ε4 carrier status, n (%)b | 616 (17.8) | 296 (16.7) | 143 (15.4) | 0.191 |
| With multi-comorbidities, n (%)b | 2655 (70.0) | 1377 (65.0) | 654 (66.1) | <0.001 |
| Long-term medication history, n (%)b | 3144 (82.9) | 1586 (74.8) | 789 (79.8) | <0.001 |
| Plasma p-tau217, pg/mL, median (IQR)b | 0.3 (0.2) | 0.3 (0.2) | 0.3 (0.2) | <0.001 |
| Plasma p-tau181, pg/mL, median (IQR)b | 1.9 (1.0) | 1.9 (1.0) | 1.8 (0.9) | 0.437 |
| Plasma NfL, pg/mL, median (IQR)b | 15.2 (9.2) | 15.3 (9.3) | 13.9 (7.3) | <0.001 |
| MMSE scores assessed at each wave, median (IQR) | 29.0 (3.0) | 28.0 (2.0) | 26.9 (4.6) | <0.001 |
| Follow-up years, median (IQR) | / | 5.2 (1.3) | 12.0 (1.4) | <0.001 |
Continuous variables were compared with the Wilcoxon rank sum test, and categorical variables were compared with Pearson’s chi-squared test or Fisher’s exact test. Continuous variables with a normal distribution were presented as mean ± SD, while continuous variables with non-normal distributions are reported as median (IQR).
ApoE, apolipoprotein E; MMSE, Mini-Mental State Examination; NfL, neurofilament light chain; SD, standard deviation; IQR, interquartile ranges.
We define January 2010 to December 2012 as the pre-pandemic Wave 1, January 2014 to March 2022 as the pre-pandemic Wave 2, and June 2022 to December 2024 as the post-pandemic Wave 3. At Wave 2, an additional 302 residents were newly recruited using the same inclusion and exclusion criteria as described in methods.
The education years, conditions of multi-comorbidities, and long-time medications taken were all assessed at baseline. ApoE genotyping and plasma p-tau217, p-tau181, and NfL were quantified using blood samples collected at baseline. Plasma p-tau217 was measured in 1708 participants, p-tau181 in 1935 participants, and NfL in 1930 participants. The medians and interquartile ranges presented in the table were calculated based on the respective subsets of participants who had baseline measurements for each biomarker.
Cognitive function trajectory across study waves
MMSE scores declined with age across all assessment years, but the rate of decline was markedly steeper in post-COVID-19 period (2022 and 2023–2024). The Wave 3 assessment revealed a steeper age-related decline than Waves 1 and 2 (Supplementary Fig. S2).
In the event study model adjusted for age at wave, sex, and years of education, MMSE scores in Wave 3 (β [95% CI]: −0.459 [–0.688, −0.230], P < 0.001) were significantly lower than in Wave 1, whereas no significant change emerged between Wave 2 (β [95% CI]: 0.047 [–0.120, 0.214], P = 0.582) and Wave 1. In sensitivity analyses excluding participants who deceased before the pandemic, the estimated effects were more pronounced: Wave 3 changes reached β = −0.957 ([95% CI]: [–1.170, −0.745], P = 0.001), but Wave 2 (β [95% CI]: −0.036 [–0.195, 0.122], P = 0.653) showed no significant difference from Wave 1 (Table 2). After adjusting for multi-comorbidies and long-term drug use at baseline, the association persisted (Supplementary Table S2). Notably, the estimated impact of the pandemic on MMSE was comparable to 12 years of aging (annual β [95% CI]: −0.080 [–0.089, −0.070], change in the MMSE per year of age, estimated at Wave 1) and approximately tenfold greater than the baseline effect of ApoE-ε4 carriage (ApoE-ε4 carriers showed lower MMSE scores relative to ApoE-ε4 non-carriers in the Wave 1 assessment, β [95% CI]: −0.092 [−0.279, 0.094]).
Table 2.
Changes of MMSE scores at observational waves.
| Variable | Model 1 |
Model 2 |
|||
|---|---|---|---|---|---|
| β [95% CI] | P value | β [95% CI] | P value | ||
| Event study model | Wave2 (Pre COVID-19) | 0.047 [−0.120, 0.214] | 0.582 | −0.036 [−0.195, 0.122] | 0.653 |
| Wave3 (Post COVID-19) | −0.459 [−0.688, −0.230] | <0.001 | −0.957 [−1.170, −0.745] | <0.001 | |
| Age | −0.158 [−0.167, −0.149] | <0.001 | −0.126 [−0.135, −0.117] | <0.001 | |
| Sex | −0.022 [−0.174, 0.129] | 0.772 | 0.009 [−0.131, 0.149] | 0.903 | |
| Education year | 0.293 [0.274, 0.312] | <0.001 | 0.255 [0.236, 0.273] | <0.001 | |
| DID model | Treat | 0.007 [−0.239, 0.254] | 0.953 | −0.029 [−0.258, 0.200] | 0.804 |
| Post | −0.564 [−0.740, −0.387] | <0.001 | −0.304 [−0.475, −0.133] | <0.001 | |
| Treat∗Post | −0.462 [−0.763, −0.161] | 0.003 | −0.818 [−1.090, −0.545] | <0.001 | |
| Age | −0.147 [−0.159, −0.134] | <0.001 | −0.132 [−0.144, −0.120] | <0.001 | |
| Sex | −0.081 [−0.262, 0.100] | 0.381 | −0.072 [−0.245, 0.101] | 0.412 | |
| Education year | 0.254 [0.231, 0.278] | <0.001 | 0.241 [0.218, 0.264] | <0.001 | |
Model 1 was conducted using entire samples, while Model 2 excluding individuals have deceased before pandemic (March 2022).
DID, difference-in-differences; CI, confidence interval.
As shown in Fig. 3, the estimated effect size became stronger, with a greater decline observed in MMSE scores among individuals with high baseline levels of blood biomarkers, including p-tau217, p-tau181, and NfL compared with those with low baseline biomarker levels. ApoE-ε4 carriers also demonstrated greater decline compared with non-carriers. Furthermore, older adults with MCI, multi-comorbidities, or a history of long-term medication use had significantly lower cognitive function after the pandemic. Moreover, older adults with conditions such as eyes diseases, cancer, and cardiovascular or cerebrovascular exhibited the most pronounced COVID-19-related effects (Supplementary Fig. S3). Interaction analyses further substantiated these findings (P for interaction < 0.05).
Fig. 3.
Estimated effects of the COVID-19 pandemic in various subgroups. Event study analyses were performed separately within subgroups defined by plasma AD biomarker profile, ApoE-ε4 status, multimorbidity, and long-term medication use (A), and different birth cohorts (B) as detailed in the Methods, with Mini-Mental State Examination scores treated as the independent variable.
Moreover, the impacts of pandemic were more pronounced in earlier birth cohorts, except those individuals born in the 1960s who showed no significant cognitive changes associated with the pandemic (Fig. 3B).
Longitudinal changes of global and domain-specific cognition
We assessed 1821 participants at Wave 1 and Wave 2, yielding a median follow-up interval of 5.33 years, while 757 participants were evaluated at Wave 2 and Wave 3 over a median interval of 6.81 years. Those who were followed from Wave 2 to Wave 3 exhibited lower MMSE scores at both their initial (Wave 2) and follow-up (Wave 3) assessments than participants followed from Wave 1 to Wave 2 at the corresponding time points (P < 0.001).
The DID analysis demonstrated that participants followed across Wave 2–3 experienced significantly faster MMSE decline than those across Wave 1–2 (β [95% CI]: −0.462 [–0.763, −0.161]), and the estimated effect size became stronger after excluding pre-pandemic deceased participants (β [95% CI]: −0.818 [–1.090, −0.545]). After adjusting for multi-comorbidies and long-term drug use at baseline, the association persisted (Supplementary Table S2). Executive (β [95% CI]: −0.625 [−0.839, −0.411]) and language (β [95% CI]: −0.202 [−0.346, −0.058]) functions declined significantly across Wave 2–3, whereas other domain assessments didn’t show significant decline (Supplementary Table S3).
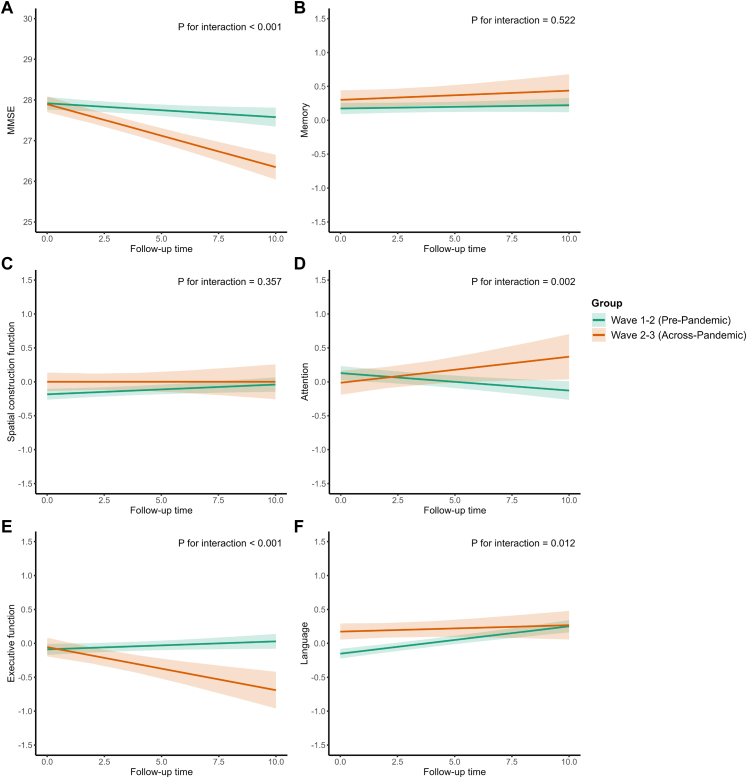
Linear mixed-effects models demonstrated a faster overall decline in MMSE, executive, and language domains (interaction P < 0.05) but a slower decline in attention among participants assessed across Wave 2–3, relative to those assessed across Wave 1–2 (shown in Fig. 4).
Fig. 4.
Longitudinal trajectories of global and domain-specific cognition among individuals in pre-pandemic and across pandemic. Linear mixed-effects models with random intercepts for each participant with repeated measurements were fitted and the Treat ∗ Follow_Up_Year interaction item was to estimate differential cognitive trajectories attributable to the COVID-19 pandemic (P for interaction < 0.05 indicating statistical significance). The follow-up duration was defined as the interval between two assessments for each participant. The participant’s global cognition (A) was assessed using the Mini-Mental State Examination (MMSE). The domain-specific cognitive functions were assessed by (B) the Auditory Verbal Learning Test- delayed memory for verbal delayed memory in participants with ≥6 years of education, or the Modified Fuld Object Memory Evaluation for immediate and delayed memory in participants with <6 years of education; (C) the Stick Test for spatial construction function; (D) Trail Making Test A for attention in participants with ≥6 years of education, or the Renminbi Test in participants with <6 years of education; (E) the Modified Common Objects Sorting Test (MCOST) -categorization for executive function; (F) the MCOST-category naming for language function. The domain-specific cognitive functions were standardized using z-scores.
Longitudinal changes of brain structures
One hundred and ninety-three participants underwent MRI at Waves 1 and 2 (median follow-up: 6.72 years), while 45 participants underwent MRI at Waves 2 and 3 (median follow-up: 5.28 years).
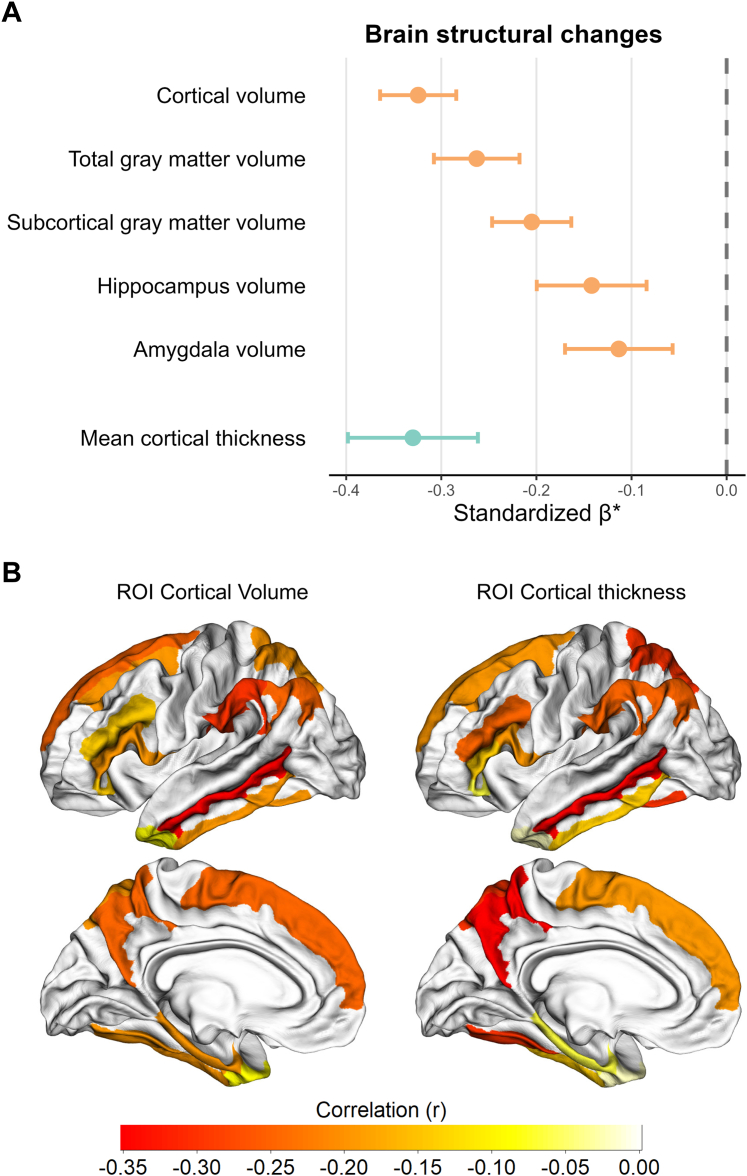
The DID model demonstrated significantly greater reductions in the volume of cortical structures, including total gray matter, subcortical gray matter, hippocampus, amygdala, and AD-related ROIs, as well as in mean cortical thickness and the cortical thickness of these ROIs (P corrected by FDR for DID interaction item < 0.05; Fig. 5 and Supplementary Table S4) among participants assessed across Wave 2–3 compared to those assessed from Wave 1–2.
Fig. 5.
Brain structural changes among individuals experienced the pandemic compared with those before pandemic. Brain structural metrics were evaluated using a DID regression framework adjusting for age at each wave, sex, and years of education. Standardized β-coefficients and their 95% confidence intervals were computed at each region of interest to quantify pandemic-related effects. Effect sizes were expressed as partial correlation coefficients (r) and visualized both in a forest plot and on a brain atlas; statistical maps were thresholded at P < 0.05 following false-discovery-rate correction. (A) Standardized β-coefficients for cortical volume, total gray matter volume, subcortical gray matter volume, hippocampus volume, amygdala volume, and mean cortical thickness. (B) Regions of interest visualized on a brain atlas.
Discussion
This study used a longitudinal cohort with continuous cognitive and brain imaging assessments among community-dwelling older adults in Shanghai, and encompassed both pre- and post-pandemic periods, thereby providing an opportunity to evaluate the impact of the COVID-19 pandemic on cognitive trajectories over time. We found that compared with those in pre-pandemic period, older adults in post-pandemic waves exhibited worse cognitive function. The estimated impact of the pandemic was comparable to twelve years of aging and approximately tenfold of ApoE-ε4 carriage. Older adults who experienced the COVID-19 pandemic had a greater decline in cognitive function affecting including global cognition, executive, and language function, as well as greater reductions in volume and cortical thickness across multiple AD-related ROIs. Notably, this impact was more pronounced among earlier birth cohorts and those with high baseline plasma AD biomarker, ApoE-ε4, MCI, multi-comorbidities, and a history of long-term medication use at baseline.
Several studies have reported the association between the COVID-19 pandemic and SARS-CoV-2 infection with cognitive impairment among older adults at the population level.9,12, 13, 14,42, 43, 44 Longitudinal analyses comparing pre-pandemic and pandemic-era cognitive trajectories have revealed an accelerated decline during the first pandemic year-with persistent effects into the second-as demonstrated by the PROTECT study.15 Similarly, research incorporating both pre- and post-pandemic assessments has found that COVID-19-positive older adults experienced a greater decline in global cognitive function.45 In contrast, another prospective longitudinal study of women aged 51–76 reported no significant cognitive change before and during the pandemic.18 These discrepancies can be attributed to variability in the intensity of pandemic-related disruptions across different regions, disparities in public health policies, and differences in lockdown measures affecting community-dwelling older adults.15,18,45 Given the pervasive impact of the COVID-19 pandemic, which involved nearly all communities in Shanghai. It was hardly feasible to identify a routine control group unexposed to the pandemic.
To address this challenge, since our longitudinal and continuous data contained assessments before and after the pandemic, we employed DID and mixed-effects models to compare cognitive decline and brain imaging outcomes between Wave 1–2 (pre-pandemic) and Wave 2–3 (across pandemic), which enabled us to track the individual trajectories at the population level during pandemic. Besides, the ways of defining “shock window” can also influence the estimates of effect. In Shanghai, the rapid spread of the highly transmissible Omicron variant in early 2022 led to an abrupt and unprecedented citywide lockdown in April and May,27 providing a sharply defined window to evaluate the pandemic’s impact. Moreover, all participants from SAS cohort were exclusively from Shanghai’s central urban districts,21 and ensured a homogeneous exposure context, and by incorporating both pre-pandemic and post-pandemic cognitive and neuroimaging assessments of the identical individuals, we enhanced the validity and robustness of our estimates regarding the pandemic’s effects on cognitive decline and brain structural changes.
Multiple investigations of post-acute SARS-CoV-2 infection and COVID-19-related hospitalization have identified domain-specific deficits, most notably in working memory,9,12,44, 45, 46 among older adults. A large population-based cohort likewise demonstrated significant acute-phase declines in executive function and working memory during the pandemic, with the persistence of working-memory impairment for at least one year after infection.15 Our study did not find selective working-memory impairment; instead, executive and language function deficits were most pronounced, and a likely reason was that lockdowns and psychosocial stressors, rather than direct viral neurotoxicity, affected daily functioning the most in our setting. The unexpected slight improvement in attention might reflect pandemic-induced hypervigilance and anxiety-driven arousal-phenomena linked to enhanced sustained-attention performance of older adults under stress.47,48 During Shanghai’s April to May 2022 lockdown, prolonged online grocery panic-buying demanded continuous monitoring of rapidly updating e-commerce feeds, likely improving simple sustained-attention task scores.47,49 Some longitudinal studies have found no domain-specific impairments using simple tests like Cogstate Brief Battery or TICS-40, possibly because stringent pandemic restrictions hindered the administration of complex neuropsychological tests.13,18 Thus, future research should integrate multimodal biomarkers with remote, digital cognitive assessments, such as mobile-app-based testing, to capture fine-grained trajectories of domain-specific decline in community-dwelling older adults.
Our subgroup analyses revealed that the deleterious cognitive effects of the COVID-19 pandemic were most pronounced among participants with high baseline plasma biomarkers of AD and neurodegenerative pathology, including p-tau217, p-tau181, and NfL. Furthermore, we observed that pandemic exposure was associated with accelerated volume loss and cortical thinning in hallmark AD-related regions, such as the hippocampus, entorhinal cortex, orbitofrontal cortex, and parahippocampal gyrus, which were consistent with previous studies.42,50,51 Douaud et al. found longitudinal gray matter reductions in olfactory and limbic regions in predominantly mild SARS-CoV-2 cases within the UK Biobank cohort, and recent multicenter imaging studies have confirmed significant decreases in orbitofrontal and medial temporal volumes among COVID-19 survivors.12,51 Moreover, SARS-CoV-2 infection has been associated with changes of plasma biomarkers, including reduced Aβ42:Aβ40 ratios and increased p-tau181, implicating accelerated amyloid pathology in post-pandemic neurodegeneration.52 These findings indicate that the COVID-19 pandemic may act as a potent “second hit” that exacerbated underlying AD and neurodegenerative pathology and precipitated steeper cognitive and structural decline in vulnerable community-dwelling older adults.6,53
Although previous research focusing on SARS-CoV-2’s neurological impact has largely emphasized direct viral effects and immune-mediated injury, emerging evidence indicates that SARS-CoV-2 is rarely neuroinvasive.1,54, 55, 56, 57 Instead, its neurological sequelae are chiefly attributable to para- and postinfectious immune responses.6,53,57,58 However, indirect consequences of the pandemic, i.e., prolonged social isolation, heightened psychological distress, and disrupted access to routine healthcare, may synergize with these immune-mediated phenomena to exacerbate cognitive decline among older adults, which were largely overlooked in previous studies. Evidence has confirmed that social isolation and loneliness significantly accelerate global cognitive aging and increase dementia risk, particularly in community-based older cohorts.59,60 Moreover, stringent lockdown measures in China were associated with reduced outpatient visits and medical expenditures among older adults, disproportionately affecting those with multimorbidity who required frequent monitoring. Our results further indicate that the cognitive impact of the pandemic was more pronounced in the oldest participants and those with preexisting chronic diseases or mental health conditions, underscoring the compounded vulnerability of these subgroups when encountering both biological insults and service disruptions. The Nurses’ Health Study did not find significant association between COVID-19 and neurocognitive impairment among older, more affluent white women with nursing backgrounds, likely due to protective social determinants.8,18 Although events like the COVID-19 pandemic are rare, public health crises frequently arise particularly in conjunction with natural disasters such as floods and heat waves. Under such circumstances the older adults often encounter the shortage of resource and lack of medical support and therefore become more vulnerable and experience more severe cognitive decline.61,62 However, most of such concerns can be overcome by advanced planning. Hence it is urgent and meaningful for the government, the medical institutes, and non-governmental organizations, etc. to identify the needs of targeted older adults and take precautions for relevant resource and service provision before another potential crisis occurs.63
The results of our study should be interpreted with caution due to several limitations. First, we lacked individual-level COVID-19-related data such as confirmed infection dates, vaccination and serological status, detailed exposure to specific lockdown measures, and metrics of healthcare access, which precluded clear separation of the pandemic’s direct viral effects from its indirect social and healthcare disruptions, and hence limited causal inference. Future studies with such data could implement a comparison between participants followed prior to the pandemic, unexposed to SARS-CoV-2, and those followed during the pandemic, thereby enhancing the interpretability of the findings. Second, although our research designs offered a solution in the absence of a traditional control group, it remained susceptible to selection bias. Over the long follow-up, community-dwelling older adults with better cognitive and physical health were more likely to participate in all waves and thus be included in both reference and treatment groups. Conversely, those who experienced the greatest pandemic-related cognitive decline or died following infection were lost to follow-up, likely leading to an underestimation of the pandemic’s impact. Third, by defining pre- and post-pandemic waves using a clear-cut timeline in Shanghai, our estimated pandemic effects may capture certain time-varying factors such as rising cardiovascular and cerebrovascular burdens that might worsen cognitive decline and dementia, or lagged impacts of contemporaneous health policies. While our post-pandemic follow-up spanned only two years, the insight into longer-term trajectories can be limited. Furthermore, the older age of participants in later observational waves may have contributed to the observed rate of cognitive decline. Future studies with more directly comparable groups are warranted to further substantiate these findings. Moreover, participants in the SAS have resided predominantly within Jing’an district, and were generally of higher educational and socioeconomic status, with accessibility to better community-based care during the pandemic, potentially leading to an underestimation of its impact, while this impact would be more pronounced for less advantaged populations across China. Finally, our longitudinal MRI data spanned 2010 to 2024 and involved multiple scanner models and acquisition protocols due to hardware upgrades and protocol changes, a common challenge in longitudinal studies. Although we employed some techniques to harmonize them, residual measurement heterogeneity may persist, potentially confounding the interpretation of true biological changes. Addressing these limitations is vital to elucidate the pandemic’s long-term neurological impacts in future studies.
Conclusion
Our findings demonstrated that the COVID-19 pandemic significantly accelerated cognitive decline and brain atrophy in community-dwelling older adults in Shanghai; those with pre-existing AD pathology and with health conditions being particularly vulnerable. These findings provide evidence of COVID-19-pandemic-related cognitive decline and highlight the need for mechanistic research into how direct and indirect stressors converge to drive cognitive deterioration.
Contributors
X. Zhou, H. Deng, and D. Ding conceptualized the study and drafted the first version of the manuscript. X. Zhou and H. Deng performed all statistical analyses and produced all figures. H. Shao and T. Yang conducted the preprocessing of MRI data. Y. Cao, Y. Chen, and Q. Zhao contributed to revising the manuscript. All authors were involved in manuscript revisions and approval of the final draft. X. Zhou and D. Ding verified the data and had full access to the raw data. All authors had final responsibility for the decision to submit for publication.
Data sharing statements
Individual de-identified participant data that underlie this reported study are available as per the SAS protocol up to 10 years after the study end date. Investigators wishing to access the data require approval through the SAS committee, which can be sought by applying through the SAS with a full analysis proposal. Investigators will need to sign a data access agreement. Approved requests will be able to access data from a secure web link for up to 5 years subject to approval. For further information, contact dingding@huashan.org.cn.
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work the authors used ChatGPT and Grok to improve the readability and language of the manuscript. Following the use of these tools, the authors thoroughly reviewed and edited the content as needed and take full responsibility for the final content of the publication.
Declaration of interests
We declare no competing interests.
Acknowledgements
Ding Ding was supported by the National Natural Science Foundation of China (82173599, 82473701), and Tianqiao and Chrissy Chen Institute collaborative project (HIM-202420027). Hanzhi Deng was supported by the National Natural Science Foundation of China (72403055) and China National Postdoctoral Committee Fund (2022 “Yinjin Xiangmu”). Qianhua Zhao was supported by National Natural Science Foundation of China (82071200, 82371429), Shanghai Hospital Development Center (SHDC2020CR4007), MOE Frontiers Center for Brain Science (JIH2642001/028), and STI2030-Major Projects 2021ZD0200800. Yuntao Chen is supported by research fellowship to UCL: Wellcome Early-Career Award (227639/Z/23/Z) and Royal Society (IEC/NSFC/242134). Hanyu Shao and Tao Yang were supported by Tianqiao and Chrissy Chen Institute.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2025.101697.
Appendix A. Supplementary data
References
- 1.Peluso M.J., Ely E.W. How long COVID could lift the fog on neurocognitive disorders. Nature. 2024;634(8032) doi: 10.1038/d41586-024-03047-4. [DOI] [PubMed] [Google Scholar]
- 2.Crook H., Raza S., Nowell J., Young M., Edison P. Long covid-mechanisms, risk factors, and management. BMJ. 2021;374 doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- 3.Nalbandian A., Desai A.D., Wan E.Y. Post-COVID-19 condition. Annu Rev Med. 2023;74:55–64. doi: 10.1146/annurev-med-043021-030635. [DOI] [PubMed] [Google Scholar]
- 4.Aiyegbusi O.L., Hughes S.E., Turner G., et al. Symptoms, complications and management of long COVID: a review. J R Soc Med. 2021;114(9):428–442. doi: 10.1177/01410768211032850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization Trends in COVID-19 cases, world (Jan 2020 to present) https://data.who.int/dashboards/covid19/cases
- 6.Zhao S., Toniolo S., Hampshire A., Husain M. Effects of COVID-19 on cognition and brain health. Trends Cogn Sci. 2023;27(11):1053–1067. doi: 10.1016/j.tics.2023.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crivelli L., Palmer K., Calandri I., et al. Changes in cognitive functioning after COVID-19: a systematic review and meta-analysis. Alzheimers Dement. 2022;18(5):1047–1066. doi: 10.1002/alz.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vance D.E., Fazeli P.L., Lokken K. New reflections on COVID-19's implications for neurocognition. JAMA Netw Open. 2025;8(4) doi: 10.1001/jamanetworkopen.2025.5539. [DOI] [PubMed] [Google Scholar]
- 9.Hampshire A., Azor A., Atchison C., et al. Cognition and memory after covid-19 in a large community sample. N Engl J Med. 2024;390(9):806–818. doi: 10.1056/NEJMoa2311330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Webber C., Myran D.T., Milani C., et al. Cognitive decline in long-term care residents before and during the COVID-19 pandemic in ontario, Canada. JAMA. 2022;328(14):1456–1458. doi: 10.1001/jama.2022.17214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y.H., Chen Y., Wang Q.H., et al. One-year trajectory of cognitive changes in older survivors of COVID-19 in Wuhan, China: a longitudinal cohort study. JAMA Neurol. 2022;79(5):509–517. doi: 10.1001/jamaneurol.2022.0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wood G.K., Sargent B.F., Ahmad Z.-U.-A., et al. Posthospitalization COVID-19 cognitive deficits at 1 year are global and associated with elevated brain injury markers and gray matter volume reduction. Nat Med. 2024;31(1):245–257. doi: 10.1038/s41591-024-03309-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y.H., Wu Q.X., Wang Q.H., et al. Tracking cognitive trajectories in older survivors of COVID-19 up to 2.5 years post-infection. Nat Aging. 2024;4(9):1186–1193. doi: 10.1038/s43587-024-00667-3. [DOI] [PubMed] [Google Scholar]
- 14.Aderinto N., Olatunji G., Kokori E., et al. COVID-19 and cognitive impairment: a review of the emerging evidence. Discover Mental Health. 2025;5(1):56. doi: 10.1007/s44192-025-00189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corbett A., Williams G., Creese B., et al. Cognitive decline in older adults in the UK during and after the COVID-19 pandemic: a longitudinal analysis of PROTECT study data. Lancet Healthy Longev. 2023;4(11):e591–e599. doi: 10.1016/S2666-7568(23)00187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung J., Kim S., Kim B., et al. Accelerated cognitive function decline in community-dwelling older adults during COVID-19 pandemic: the Korean frailty and aging cohort study (KFACS) Int J Environ Res Public Health. 2022;19(17) doi: 10.3390/ijerph191710666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li C., Hua R., Gao D., Zheng F., Xie W. Cognitive decline before and during COVID-19 pandemic among older people with multimorbidity: a longitudinal study. J Am Med Dir Assoc. 2023;24(4):419–425.e10. doi: 10.1016/j.jamda.2023.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang S., Menor A., Chibnik L.B., et al. COVID-19 pandemic-related exposures and cognitive function in middle-aged women. JAMA Netw Open. 2025;8(4) doi: 10.1001/jamanetworkopen.2025.5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hausman H.K., Dai Y., O'Shea A., et al. The longitudinal impact of the COVID-19 pandemic on health behaviors, psychosocial factors, and cognitive functioning in older adults. Front Aging Neurosci. 2022;14 doi: 10.3389/fnagi.2022.999107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H., Qin D., Fang L., Liu H., Song P. Addressing healthy aging in China: practices and prospects. Biosci Trends. 2024;18(3):212–218. doi: 10.5582/bst.2024.01180. [DOI] [PubMed] [Google Scholar]
- 21.Ding D., Zhao Q., Guo Q., et al. The Shanghai Aging Study: study design, baseline characteristics, and prevalence of dementia. Neuroepidemiology. 2014;43(2):114–122. doi: 10.1159/000366163. [DOI] [PubMed] [Google Scholar]
- 22.Commission SMH News releases (24 Jan 2020 to 17 Mar 2020) https://wsjkw.sh.gov.cn/xwfb/index.html
- 23.Commission SMH Monthly report of infectious diseases (Jan & Feb 2020) https://wsjkw.sh.gov.cn/yqxx/index.html
- 24.Wikipedia contributors . 2025. COVID-19 pandemic in Shanghai.https://en.wikipedia.org/w/index.php?title=COVID-19_pandemic_in_Shanghai&oldid=1278741773 [Google Scholar]
- 25.Commission SMH News releases (31 Mar 2022 to 1 Jun 2022) https://wsjkw.sh.gov.cn/xwfb/index.html
- 26.Commission SMH Monthly report of infectious diseases (Apr & May 2022) https://wsjkw.sh.gov.cn/yqxx/index.html
- 27.Yang H., Nie H., Zhou D., Wang Y., Zuo W. The effect of strict lockdown on Omicron SARS-CoV-2 variant transmission in Shanghai. Vaccines (Basel) 2022;10(9) doi: 10.3390/vaccines10091392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Commission SMH News releases (7 Dec 2022 to 26 Dec 2022) https://wsjkw.sh.gov.cn/xwfb/index.html
- 29.Ding D., Wu W., Xiao Z., Luo J., Zhao Q., Hong Z. Two-decade changes in dementia mortality in a Chinese community. Aging Health Res. 2024;4(1) [Google Scholar]
- 30.Ding D., Zhao Q., Guo Q., et al. Prevalence of mild cognitive impairment in an urban community in China: a cross-sectional analysis of the Shanghai Aging Study. Alzheimers Dement. 2015;11(3):300–309.e2. doi: 10.1016/j.jalz.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Hlávka J.P., Yu J.C., Lakdawalla D.N. Crosswalk between the mini-mental state examination and the telephone interview for cognitive status (TICS-27/30/40) Alzheimers Dement. 2022;18(11):2036–2041. doi: 10.1002/alz.12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao Z., Wu W., Ma X., et al. Olfactory function, neurofilament light chain, and cognitive trajectory: a 12-year follow-up of the Shanghai Aging Study. Alzheimers Dement. 2023;15(4) doi: 10.1002/dad2.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diagnostic and statistical manual of mental disorders. 4th ed. American Psychiatric Publishing, Inc.; Arlington, VA, US: 1994. [Google Scholar]
- 34.Petersen R.C. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 35.Moscoso A., Grothe M.J., Ashton N.J., et al. Longitudinal associations of blood phosphorylated Tau181 and neurofilament light chain with neurodegeneration in alzheimer disease. JAMA Neurol. 2021;78(4):396–406. doi: 10.1001/jamaneurol.2020.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turney I.C., Lao P.J., Rentería M.A., et al. Brain aging among racially and ethnically diverse middle-aged and older adults. JAMA Neurol. 2023;80(1):73–81. doi: 10.1001/jamaneurol.2022.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao Z., Wu W., Ma X., et al. Plasma p-tau217, p-tau181, and NfL as early indicators of dementia risk in a community cohort: the Shanghai Aging Study. Alzheimers Dement (Amst) 2023;15(4) doi: 10.1002/dad2.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller D.L. An introductory guide to event study models. J Econ Perspect. 2023;37(2):203–230. [Google Scholar]
- 39.Card D., Krueger A.B. Minimum wages and employment: a case study of the fast-food industry in New Jersey and Pennsylvania. Am Econ Rev. 1994;84(4):772–793. [Google Scholar]
- 40.Goodman-Bacon A., Marcus J. Using difference-in-differences to identify causal effects of COVID-19 policies. Surv Res Methods. 2020;14(2):153–158. [Google Scholar]
- 41.Fu H., Tsuei S., Zheng Y., et al. Effects of comprehensive smoke-free legislation on smoking behaviours and macroeconomic outcomes in Shanghai, China: a difference-in-differences analysis and modelling study. Lancet Public Health. 2024;9(12):e1037–e1046. doi: 10.1016/S2468-2667(24)00262-7. [DOI] [PubMed] [Google Scholar]
- 42.Guillén N., Pérez-Millan A., Falgàs N., et al. Cognitive profile, neuroimaging and fluid biomarkers in post-acute COVID-19 syndrome. Sci Rep. 2024;14(1) doi: 10.1038/s41598-024-63071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu E., Xie Y., Al-Aly Z. Long-term neurologic outcomes of COVID-19. Nat Med. 2022;28(11):2406–2415. doi: 10.1038/s41591-022-02001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hampshire A., Chatfield D.A., Am M.P., et al. Multivariate profile and acute-phase correlates of cognitive deficits in a COVID-19 hospitalised cohort. eClinicalMedicine. 2022;47 doi: 10.1016/j.eclinm.2022.101417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shyam S., Gómez-Martínez C., Ni J., et al. COVID-19 and cognitive decline in older adults with high-cardiovascular risk: a post hoc analysis. Aging Dis. 2024;16(4):2373–2382. doi: 10.14336/AD.2024.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rizzi G., Pacifico D., Sabatini S., et al. SARS-CoV-2 infection and cognition in community-dwelling and nursing home residents in southern Switzerland. Brain Behav Immun Health. 2024;35 doi: 10.1016/j.bbih.2023.100701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldstein Ferber S., Shoval G., Zalsman G., Mikulincer M., Weller A. Between action and emotional survival during the COVID-19 era: sensorimotor pathways as control systems of transdiagnostic anxiety-related intolerance to uncertainty. Front Psychiatry. 2021;12 doi: 10.3389/fpsyt.2021.680403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding S., Lei Q., Wu W., et al. Changes in lifestyle, mood, and disease management among community-dwelling older adults during the COVID-19 pandemic in China. Aging Health Res. 2022;2(1) doi: 10.1016/j.ahr.2022.100059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Omar N.A., Nazri M.A., Ali M.H., Alam S.S. The panic buying behavior of consumers during the COVID-19 pandemic: examining the influences of uncertainty, perceptions of severity, perceptions of scarcity, and anxiety. J Retailing Consum Serv. 2021;62 [Google Scholar]
- 50.Liu X., Yan W., Lu T., Han Y., Lu L. Longitudinal abnormalities in brain structure in COVID-19 patients. Neurosci Bull. 2022;38(12):1608–1612. doi: 10.1007/s12264-022-00913-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Douaud G., Lee S., Alfaro-Almagro F., et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604(7907):697–707. doi: 10.1038/s41586-022-04569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duff E.P., Zetterberg H., Heslegrave A., et al. Plasma proteomic evidence for increased β-amyloid pathology after SARS-CoV-2 infection. Nat Med. 2025;31(3):797–806. doi: 10.1038/s41591-024-03426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Monje M., Iwasaki A. The neurobiology of long COVID. Neuron. 2022;110(21):3484–3496. doi: 10.1016/j.neuron.2022.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Radke J., Meinhardt J., Aschman T., et al. Proteomic and transcriptomic profiling of brainstem, cerebellum and olfactory tissues in early- and late-phase COVID-19. Nat Neurosci. 2024;27(3):409–420. doi: 10.1038/s41593-024-01573-y. [DOI] [PubMed] [Google Scholar]
- 55.Eisenstein M. Do infections have a role in Alzheimer's disease? Nature. 2025;640(8059):S8–S10. doi: 10.1038/d41586-025-01104-0. [DOI] [PubMed] [Google Scholar]
- 56.Bauer L., Laksono B.M., de Vrij F.M.S., Kushner S.A., Harschnitz O., van Riel D. The neuroinvasiveness, neurotropism, and neurovirulence of SARS-CoV-2. Trends Neurosci. 2022;45(5):358–368. doi: 10.1016/j.tins.2022.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Michael B.D., Dunai C., Needham E.J., et al. Para-infectious brain injury in COVID-19 persists at follow-up despite attenuated cytokine and autoantibody responses. Nat Commun. 2023;14(1):8487. doi: 10.1038/s41467-023-42320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dunai C., Collie C., Michael B.D. Immune-mediated mechanisms of COVID-19 neuropathology. Front Neurol. 2022;13 doi: 10.3389/fneur.2022.882905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guarnera J., Yuen E., Macpherson H. The impact of loneliness and social isolation on cognitive aging: a narrative review. J Alzheimers Dis Rep. 2023;7(1):699–714. doi: 10.3233/ADR-230011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang A.R., Roth D.L., Cidav T., et al. Social isolation and 9-year dementia risk in community-dwelling Medicare beneficiaries in the United States. J Am Geriatr Soc. 2023;71(3):765–773. doi: 10.1111/jgs.18140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu Y., Liu Y., Jiang H. Geriatric health care during the COVID-19 pandemic: managing the health crisis. Clin Interv Aging. 2022;17:1365–1378. doi: 10.2147/CIA.S376519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vigezzi G.P., Bertuccio P., Amerio A., et al. Older adults' access to care during the COVID-19 pandemic: results from the LOckdown and LifeSTyles (LOST) in lombardia project. Int J Environ Res Public Health. 2022;19(18) doi: 10.3390/ijerph191811271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Werner R.M., Hoffman A.K., Coe N.B. Long-term care policy after covid-19 - solving the nursing home crisis. N Engl J Med. 2020;383(10):903–905. doi: 10.1056/NEJMp2014811. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.