Summary
The 2024 Lancet Commission Report on dementia prevention has identified 14 modifiable risk factors that account for approximately 45% of global dementia cases. We used a global multidimensional approach that integrates gender equity considerations, poverty, wealth shocks, income inequality and HIV infection rates to identify additional risk factors beyond those reported in 2024 report. This methodological framework aims to enhance equitable prevention strategies to mitigate the global burden of dementia. We demonstrate that adding four additional risk factors: poverty, wealth shocks, income inequality, and HIV, while also considering the influences of sex and gender will improve the global applicability of the 2024 report. This is important because, despite dementia primarily affecting women, 57% of the risk factors identified in the 2024 report are more prevalent in men. Our analysis suggests that incorporating these four additional factors could potentially increase the proportion of preventable dementia cases to about 65%. This approach would also reshape the understanding of dementia risk, indicating that around 56% of modifiable risks disproportionately impact women. Expanding risk models in this manner is crucial for developing equitable and effective global dementia prevention strategies, particularly in underrepresented regions. We present these considerations as enhancements to the Commission's significant work.
Keywords: Dementia, Risk factors, Global health, 2024 Lancet Commission Report
Introduction
In 2020, the Lancet Commission on Dementia Prevention, Intervention, and Care identified and published 12 modifiable risk factors linked to dementia, spanning various stages of the life course.1 These were categorised as follows: Early life (education); Midlife (hearing loss, traumatic brain injury, hypertension, alcohol misuse, and obesity); and Late-life (smoking, depression, social isolation, physical inactivity, diabetes, and exposure to air pollution). The Commission estimated that by addressing these 12 risk factors, governments could potentially prevent up to 40% of global dementia cases.1
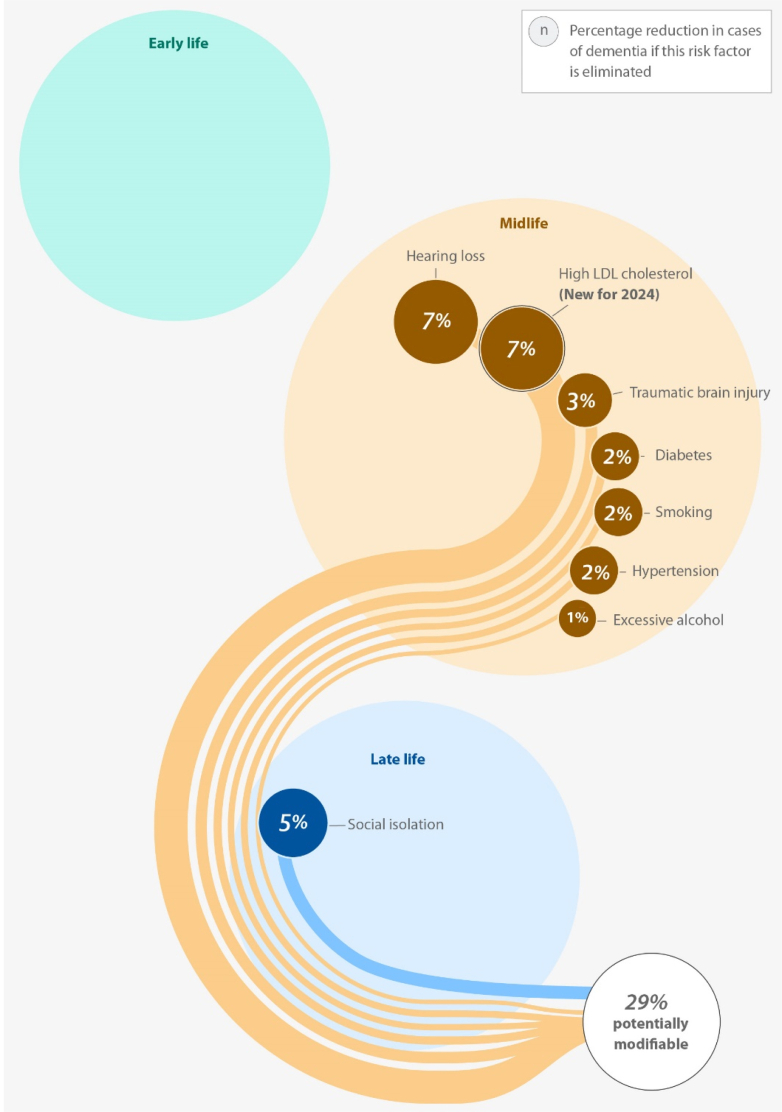
Since the release of the influential 2020 report, meaningful discussions have emerged regarding the applicability and generalisability of these risk factors in global contexts,2 particularly in low-income and middle-income countries (LMICs). Indeed, several groups have noted that many of the datasets used in formulating these estimates were largely derived from high-income country (HIC) populations, raising concerns about the representativeness of these risk factors for diverse global populations.3 In response to these and other evolving scientific insights, the Lancet Commission updated its report in 2024, expanding the list of global dementia risk factors from 12 to 14.4 The updated version included vision loss and elevated low-density lipoprotein (LDL) cholesterol levels.4 This updated analysis suggested that addressing these 14 factors could potentially reduce global dementia cases by approximately 45% [Fig. 1].
Fig. 1.
Risk factors for Dementia according to the 2024 Lancet Commission Report.
Source: Authors' design based on 2024 Lancet Commission Report.4
Nevertheless, while the 2024 update represents a critical advancement,5 there remain concerns about insufficient data from low-income and middle-income countries (LMICs), included in the global analysis. This gap in data coverage underscores the need for caution in universally applying these findings and highlights the importance of further research to identify additional risk factors that may be particularly relevant in underrepresented populations.6
In this personal view paper, we aim to contribute constructively to this ongoing dialogue by examining five key areas that warrant further consideration to strengthen and refine global dementia risk frameworks. First, we discuss potential misalignments between current risk factor estimates from the 2024 report and global gender patterns in dementia prevalence and progression. Second, we identify four critical risk factors that are currently omitted from the 2024 report but may significantly influence dementia risk globally. Third, we propose the inclusion of these risk factors in future reports in order to enhance the comprehensiveness and global applicability of future estimates.
Fourth, we discuss intervention strategies and revised estimates of the proportion of global dementia cases that could be prevented by broadening the dementia risk profile, with the consideration of these additional risk factors. Finally, we suggest models for optimising risk reduction frameworks, ensuring that future research reflects a broader and more inclusive understanding of dementia risk worldwide.
Discrepancy between 2024 report findings and global gender patterns of dementia
Though the 2024 Lancet Commission report provides an important update on dementia risk factors and offers critical insights for prevention strategies worldwide, when examined in the context of global gender patterns of dementia epidemiology, certain gaps warrant careful consideration. Specifically, the gendered distribution of risk factors reported raises questions about how comprehensively the findings reflect established epidemiological evidence and their applicability across diverse global populations, particularly in LMICs. In most areas of the world, dementia disproportionately affects women, both in terms of prevalence and incidence,7 with well-documented differences in disease progression, symptom manifestation, and outcomes.8,9
Global projections indicate that the prevalence of dementia is expected to rise significantly, from 57.4 million cases (95% uncertainty interval: 50.4–65.1) in 2019 to 152.8 million cases (130.8–175.9) by 2050.10 Age-standardised estimates reveal a higher incidence of dementia among women compared to men globally, with a female-to-male ratio of 1.69 (1.64–1.73) in 2019.10 This disparity is anticipated to persist through 2050,10 as dementia risks remain higher in females than in males.8, 9, 10, 11
Furthermore, there are notable geographical variations in the projected increases in dementia cases going to 2050. High-income regions in the Asia Pacific (53% [41–67]) and Western Europe (74% [58–90]) are expected to experience the smallest percentage increases.10 In contrast, the most substantial rises in dementia prevalence are forecasted for North Africa and the Middle East (367% [329–403]) and Eastern sub-Saharan Africa (357% [323–395]).10 These projections highlight the diverse risk profiles that each region must address in relation to dementia.12 However, the 2024 report appears to assert that the 14 identified risk factors will exert a uniform impact globally, irrespective of regional and gender variations. This assertion may lack validity, given the substantial disparities that exist across different regions and between genders. Notably, certain risk factors are more prevalent and disproportionately affect one gender group compared to the other.13 It is essential for the current 2024 report to recognise and incorporate this reality into its analysis.
For example, the 2024 report identifies 14 modifiable risk factors for dementia, 8 of which are more prevalent among males.
-
1.
Hearing Loss: The prevalence among males is 12.2% (9.7%–16.2%), whereas for females, it is 9.8% (7.7%–13.2%).14
-
2.
Elevated LDL Cholesterol: The age-standardised disability-adjusted life years rate is 67.3% (50.8–86.4) for males, compared to 46.5% (32.7–62.4) for females.15
-
3.
Traumatic Brain Injuries: The prevalence in males is 16.7% (15.1–17.9), while in females it is 8.5% (7.1–9.1).16
-
4.
Diabetes: The prevalence among males is 6.5% (6.2–7.0), in contrast to 5.8% (5.4–6.1) in females.17
-
5.
Smoking: Males exhibit a prevalence of 32.6% (32.2–33.1), compared to 6.5% (6.3–6.7) among females.18
-
6.
Hypertension: In males, the prevalence is 24.1% (23.2–25.1), while for females it is 20.1% (19.2–21.1).19
-
7.
Alcohol Consumption: The prevalence for males is 39.1% (36.1–43.1), compared to 25.1% (23.1–27.1) for females.20
-
8.
Social Isolation: The prevalence among males is 13.8% (12.8–14.8), while it stands at 10.9% (10.0–11.8) for females.21,22 Collectively, the eight risk factors account for a concerning 57% of the overall risk share for men [ See Fig. 2].
Fig. 2.
Prevalent male risk factors for dementia derived from the 2024 Report findings.
Source: Authors' design based on 2024 Lancet Commission Report.4
While these findings highlight important male-associated risks, they are difficult to reconcile with robust global epidemiological data indicating that women continue to bear the greater burden of dementia10 and the share of risk factors for dementia is higher in women than in men.8, 9, 10, 11 Therefore, there is a need to recalibrate and expand the established risk factors to better align with global gender-specific epidemiological patterns of dementia risk and rates.3
The 2024 report currently identifies six risk factors that are notably more prevalent among females [Fig. 3]. These factors are as follows:
-
1.
Lower Educational Attainment: Among individuals aged 25–29, males have completed an average of 0.9 additional years of primary schooling globally, whereas females have completed only 0.3 additional years.23
-
2.
Depression: The prevalence of depression is significantly higher among females, with an estimated 35.5 million cases (ranging from 30.0 to 41.8 million) compared to 17.7 million cases for males (ranging from 14.7 to 21.3 million).24
-
3.
Insufficient Physical Activity: The prevalence of insufficient physical activity is observed in 28.7% of global males (with a range of 25.0%–32.6%) and 33.8% of females (with a range of 29.9%–37.7%).25
-
4.
Obesity: The prevalence of obesity is recorded at 43.4% for males (ranging from 42.9% to 43.8%) and 46.7% for females (ranging from 46.2% to 47.8%).26
-
5.
Exposure to Air Pollution: Personal exposure to air pollution is measured at 62 μg/m3 for males (with a range of 58.0–67.6 μg/m3) in contrast to 67 μg/m3 for females (with a range of 62.0–72.1 μg/m3).27
-
6.
Visual Impairment: The prevalence of visual impairment is reported as 3.3% for males (ranging from 3.0% to 3.7%) and 4.1% for females (ranging from 3.7% to 4.6%).28
Fig. 3.
Prevalent female risk factors for dementia derived from the 2024 Report findings.
Source: Authors' design based on 2024 Lancet Commission Report.4
These account for an estimated 43% of the risk share, suggesting, when considering weighted contributions, that men are presented as the larger contributors to global dementia risk; a finding that contrasts with robust epidemiological trends showing higher dementia risks among women.8, 9, 10, 11
Moreso, while the 2024 report's global focus is commendable, there remains a critical need to contextualise gender-specific risk factors within diverse cultural and social realities, especially in majority world populations where gender roles, healthcare access, education, and social determinants of health differ markedly from high-income countries.29,30
For instance, intersecting factors such as gender-based violence, caregiving roles, HIV infections, wealth shocks, poverty, post-traumatic stress disorders, concussions, and systemic educational inequities that disproportionately affect women in LMICs are underexplored yet likely to contribute significantly to dementia risk trajectories.29,30 In light of this, recalibrating and expanding current dementia risk models to fully account for sex- and gender-based differences, while also incorporating geographical diversity, will be essential to ensure that prevention strategies are equitable, globally relevant, and culturally sensitive.3
Dementia risk factors not addressed in the 2024 report
The 2024 report has not considered some critical risk factors associated with dementia despite substantial evidence highlighting their significance. For example, existing literature from diverse geographical contexts unequivocally demonstrates that factors such as poverty, wealth shocks, income inequality, and HIV significantly influence the progression of dementia [see Table 1]. Consequently, our focus on these specific risk factors is informed by contemporary global research that emphasises their substantial impact on dementia prevalence, particularly within the African continent.31 This region is projected to witness the most pronounced increase in dementia cases in the forthcoming decades.10
Table 1.
Global studies show that poverty, wealth shocks, income inequality, and HIV are risk factors that worsen dementia.
| Article | Region/country | Effect size on dementia |
|---|---|---|
| Income inequality | ||
| Sakaniwa et al. (2024)32 | Japan | 1.4 (0.3–1.6) |
| Bodryzlova et al. (2023)33 | Global | 2.1 (1.2–3.7) |
| Li et al. (2024)34 | United Kingdom | 2.3 (1.8–3.0) |
| Lai et al. (2023)35 | United Kingdom | 3.2 (1.8–3.0) |
| Average 2.2 (1.3–3.0) | ||
| Poverty | ||
| Chen et al. (2025)36 | USA | 2.3 (2.0–2.6) |
| Trani et al. (2022)37 | South Africa | 2.3 (1.1–5.0) |
| Trani et al. (2024)38 | Pakistan | 2.0 (1.4–2.9) |
| Klee et al. (2023)39 | United Kingdom | 1.7 (1.4–2.0) |
| Average 2.1 (1.5–3.1) | ||
| HIV | ||
| Lam et al. (2021)40 | USA | 1.6 (1.3–1.9) |
| Shayegi-Nik et al. (2024)41 | Canada | 2.0 (1.2–3.2) |
| Hyle et al. (2024)42 | USA | 2.3 (2.0–2.4) |
| Lee et al. (2024)43 | Malawi | 2.5 (1.4–4.6) |
| Average 2.0 (1.5–3.1) | ||
| Wealth shocks | ||
| Cho et al. (2023)44 | USA | 16.0 (4.0–29.0) |
| Cho et al. (2023)44 | China | 14.0 (7.0–21.0) |
| Cho et al. (2025)45 | USA | 14.0 (6.1–22.0) |
| Cadar et al. (2018)46 | England | 12.6 (10.7–16.9) |
| Average 14.1 (6.8–22.2) | ||
Source: Authors' analysis based on global literature.
Income inequality and dementia
We submit that it is crucial to monitor income inequality, defined as the unequal distribution of income within a population, as measured by the Gini index. This risk factor has the potential to significantly influence the incidence of dementia, as presented in Table 1. For example, research in Japan indicates that reducing income inequality correlates with a significant increase in dementia-free years within an individual's lifespan.32 Conversely, worsening income inequality is linked with the most considerable loss of dementia-free years,32 suggesting that adverse inequalities are the most significant contributor to dementia in late life.47
Poverty and dementia
Poverty is another significant risk factor for dementia across all life stages—early, mid, and late life. This risk factor is also worth monitoring globally, considering it can worsen dementia [See Table 1]. We defined poverty as the situation in which an individual or group lacks the financial resources and essentials needed for a minimum standard of living. Currently, various metrics are used to measure poverty; however, we recommend adopting the multidimensional poverty scale globally48 as a more effective tool for identifying population groups that are more vulnerable early.
For example, during early life, poverty-related malnutrition and micronutrient deficiencies can negatively impact brain development, increasing the likelihood of cognitive decline later in life. Although studies examining the link between early-life poverty and late-life dementia risk often depend on retrospective memories of childhood conditions, meta-analyses have consistently shown that early-life disadvantage, including childhood poverty, is associated with a 1.58–1.64-fold increased risk of dementia.49 Deprivation in adulthood also increases dementia due to biological, environmental, and social dimensions, which progresses cognitive decline leading to pathological ageing.47,50 Therefore, policymakers need to monitor poverty levels across the lifespan.51
HIV and dementia
HIV infection presents a significant risk for developing dementia.31,52 Recent research, especially studies conducted in African nations, has identified HIV as a modifiable risk factor for cognitive decline and dementia.31,50,53 These studies suggest that the interplay between HIV and neurocognitive functioning can lead to severe cognitive deficits, as presented in Table 1. Some studies have suggested that an average CD4 count of 407.8 cells/mm3 is associated with mild dementia.54 Hence, it becomes more important to address the potential neurological complications that may arise from a high viral load, which destroys CD4 cells.55 The recognition of HIV as a modifiable risk factor emphasises the importance of early interventions, routine cognitive assessments, and retroviral therapies that could potentially mitigate the risks of dementia in HIV-positive individuals.
Wealth shock and dementia
Wealth shock is typically defined as a significant decline in a household's financial resources, often quantified as a loss of 75% of their total wealth.44 This experience can be particularly distressing and can impact various aspects of an individual's life, including their mental and emotional well-being.56 Research has shown that such financial downturns can exacerbate health issues, including cognitive decline and dementia.44, 45, 46
When individuals or families undergo a wealth shock, the stress and anxiety associated with financial insecurity can lead to increased levels of depression and isolation.56 These psychological factors can have a cascading effect, worsening dementia.4,44 The loss of financial stability may limit access to healthcare services and support networks that are crucial for managing dementia, potentially accelerating the decline in cognitive functions [See Table 1].
Enhancing the global applicability of the 2024 report
We propose including wealth shocks, income inequality, poverty, and HIV infections as significant risk factors for global dementia. Adding these four risk factors will enhance the alignment of the 2024 findings with the established gender risk profile for global dementia.
For example, income inequality and poverty are also more prevalent among females than males globally.57,58 Estimates show that females spend an average of 3–6 h per day on unpaid work, while males spend 0.5–2 h per day.59 The number of 15–24-year-olds not in education, employment, or training for a job is estimated to be around three times higher for young females (175 million) than young males (63 million).60
The high poverty rate among females contributes to a reduction in grey matter volume in the frontal and temporal cortices, as well as in the hippocampus.61 Income inequality also negatively affects brain measures in females across various global settings, particularly in disadvantaged regions. This impact is observed in both healthy individuals and those with dementia.62
Both poverty and income inequality contribute to increased stress levels, which can lead to neuronal damage and cognitive decline.62 The combined effects of poverty and income inequality have been shown to exacerbate dementia, similar to the negative impact associated with a lack of education.63 According to the 2024 report, this lack of education is estimated to account for 5% of global dementia cases.4
HIV also affects more females than males globally.64 Current global estimates show that there are 18.0 million (16.6–19.4) HIV positive males versus 22.1 million (21.2–23.2) HIV positive females.65 The majority of people living with HIV reside in sub-Saharan Africa (29.1 million, 28.0–30.4), a region expected to have the highest dementia cases globally.10
Indeed, adding HIV as a global risk factor for dementia is crucial, especially considering the increasing number of people living with HIV, which has reached 3.13 million (ranging from 2.0 to 4.2 million) in high-income countries.65 The HIV virus can induce neuroinflammation, leading to cytotoxicity, neuronal cell damage, and cell death.66,67 Studies have linked HIV to an increased risk of developing dementia; however, the precise risk for individuals aging with long-term suppressive antiretroviral therapy remains unclear. Our estimates suggest that this risk is comparable to that associated with diabetes,40,42 with the Lancet Commission report indicating a 2% increased risk of developing dementia.4 Preliminary global studies support this estimation, as outlined in Table 1.
Wealth shocks have been shown to affect different demographics in varying ways, with women experiencing more severe impacts than men globally.68 In 2014, estimates indicated that human capital wealth per capita was $108,655.69 Of this amount, $66,832 was attributed to male earnings, while $41,823 was attributed to female earnings.69 For every dollar in future earnings expected for men, women are projected to earn only 63 cents.69
This means that women worldwide will likely earn slightly less than two-thirds of what men are expected to earn, highlighting a significant level of gender inequality and increasing the risk of wealth shocks for women. Additionally, since women are already at a higher risk of developing dementia due to biological and social factors,8, 9, 10, 11 the cognitive decline exacerbated by wealth shocks is expected to worsen this issue.
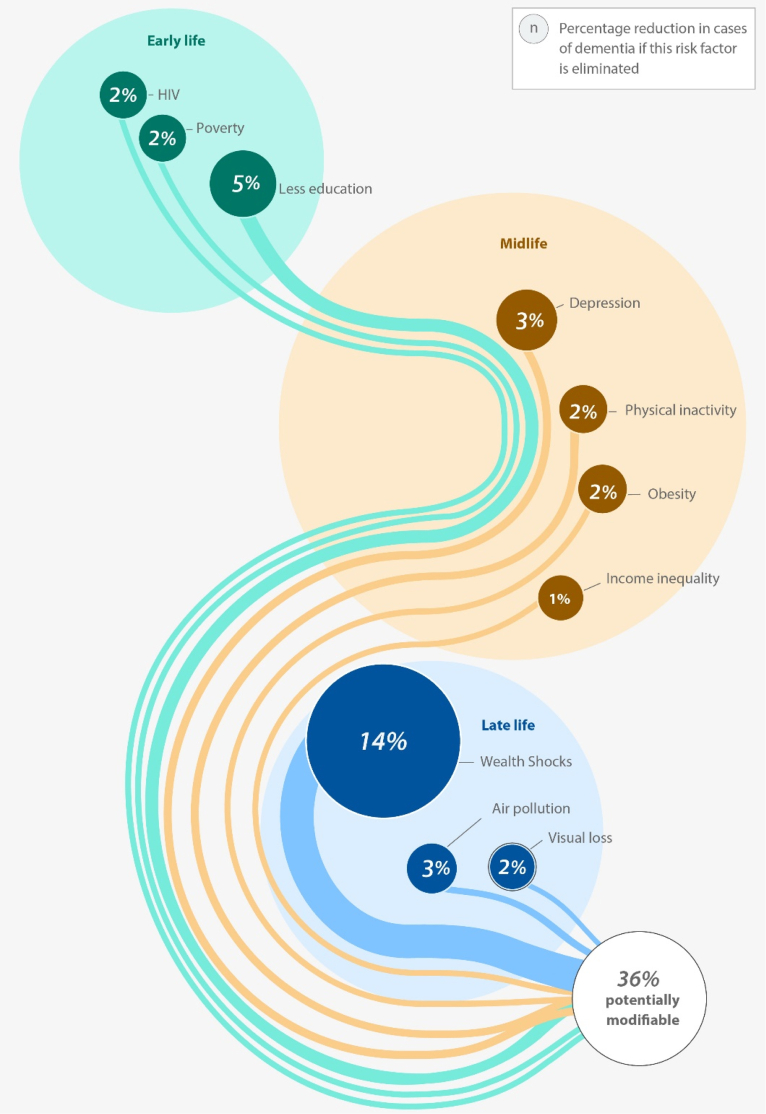
Hence, integrating these four previously omitted risk factors into the 2024 report is warranted from an epidemiological perspective, particularly given the higher risk profile for dementia observed in females relative to males in existing global literature.8, 9, 10, 11 Specifically, by recognising these four risk factors, we illustrate that 10 out of 18 total risk factors for dementia are more prevalent in females [See Fig. 4] than 8 out of 18 risk factors found to affect males. This discrepancy indicates a 56% risk share of dementia within the female population compared to a 44% risk share in males. Such a percentage breakdown is acceptable in global dementia studies.8, 9, 10, 11
Fig. 4.
Expanded Female Risk Factors for Dementia.
Source: Authors' design based on 2024 Lancet Commission Report.4
Broadening the dementia risk profile: addressing socioeconomic risk factors
We call for expanding the dementia risk profile. Our analysis suggests that 45% and 65% of dementia risk may be modified [See Fig. 5]. Our proposal also aligns with epidemiological gender-based findings from global literature.8, 9, 10, 11 Notably, one study conducted in the USA found that 45%–60% of dementia cases could be averted,8 substantiating our claim that up to 65% of global dementia risk may be preventable. Governments must develop progressive policies to address the unique impacts of these four identified risk factors, given their substantial contribution to dementia progression.
Fig. 5.
Expanded Global Risk Factors for Dementia.
Source: Authors' design based on 2024 Lancet Commission Report.4
Poverty risk
Poverty and inadequate access to essential resources represent a global challenge that necessitates urgent recognition in the dementia risk literature. Individuals living in poverty often endure heightened stress levels, restricted access to healthcare, and suboptimal nutritional status—factors that collectively elevate the risk for dementia. Targeted interventions, such as cash transfer programs, social pensions, and school feeding scheme programs, should be scaled up globally,51 considering these measures could potentially avert 2% of dementia cases.
Income inequality
The increasing disparity between the rich and the disadvantaged group has significant implications for mental health and cognitive function, thereby exacerbating the global dementia crisis.70 Communities characterised by high-income inequality frequently confront elevated social isolation and diminished access to cognitively stimulating activities and healthcare services, both of which can precipitate cognitive decline, leading to dementia.70 To address these issues, governments should implement progressive income tax systems, invest in education and job creation, promote financial inclusion, establish minimum living wage policies,71 and enhance social safety nets such as unemployment insurance and food assistance programs. These interventions could play a critical role in averting an estimated 2% of global dementia cases.
Wealth shocks
Sudden financial disruptions—such as job loss, unanticipated medical expenses, or market downturns can induce significant stress and disrupt mental health, leading to cognitive impairment and, ultimately, dementia. Policymakers should consider implementing minimum cash reserves for households, providing loan payment moratoriums for struggling families, and instituting caps on debt-to-income ratios to mitigate the vulnerabilities that can exacerbate wealth shocks. Such social policies could contribute to the prevention of dementia cases linked to wealth shocks.
HIV
Advancements in treatment have enabled individuals living with HIV to enjoy longer lifespans, and the global population of people living with HIV is increasing and ageing. Public health approaches should focus on widespread suppressive antiretroviral coverage to ensure maximal protection of brain health. Ensuring those living with HIV are diagnosed and linked to care is crucial, and current programs may not target older persons who can be perceived to be at lower risk.72 Hence, there is a need for regular cognitive assessment for HIV patients, and early enrolment in antiretroviral therapy remains the best strategy to avert cognitive decline.
Indeed, broadening the dementia risk profile to encompass these additional four risk factors can facilitate a more nuanced understanding of the complexities associated with cognitive decline and dementia. This holistic approach promises to inform the development of more effective public health strategies to ameliorate these risks, ultimately fostering healthier aging populations.
Way forward
Enhancing our understanding of the biological pathways that mediate the effect of the 18 identified modifiable risk factors on dementia is essential for developing more effective prevention and intervention strategies. Improved insight into these pathways will not only help identify actionable targets for intervention but also contribute to the design of personalised and culturally adaptable strategies for dementia prevention worldwide.
Emerging evidence suggests that many of these risk factors are likely to exert their effects through complex immunological, metabolic, and inflammatory cascades, which may give rise to distinct biomarker profiles and cytokine signatures associated with the onset and progression of dementia.73 However, the complex aetiology of these risk factors, compounded by their multifactorial and comorbid nature, especially in low-resource settings where multimorbidity is prevalent, makes it challenging to delineate their precise individual contributions to dementia.74 This underscores the pressing need for robust and reliable precision-based epidemiological modelling to better quantify the unique and interactive contributions of each risk factor. One promising avenue is the application of causal inference models, including instrumental variable (IV) approaches, and vector autoregressive models,75,76 which can account for confounding and estimate risk factor contributions over time. Importantly, regional and culturally specific adaptations of these models are crucial to capture local variations in risk exposure and disease manifestation, ensuring that prevention strategies are both equitable and contextually appropriate.
Regional case studies and the need for culturally adapted modelling
Our expanded risk factors could significantly assist Africa, a region expected to see the most considerable increases in dementia cases in the coming decades. Current literature indicates that HIV is a critical risk factor.31,55 Therefore, our expanded risk factors can guide the continent in prioritising interventions tailored to its specific needs.
Moreover, our expanded model reveals that most of the risk falls within the female population. This underscores the global necessity of monitoring wealth shocks, HIV infections, income inequalities, and poverty, which predominantly affect women worldwide.
In Latin America, studies show that the current Lancet risk factors are not universally applicable in influencing dementia. This indicates that classical risk factors may have weak predictive power for dementia.77 In Latin America, income inequalities stand out as more robust drivers of cognitive decline.78 Consequently, our expanded risk model can help Latin America prioritise the elimination of inequalities, which could aid in averting dementia.79
Lastly, case studies from North Africa highlight the potential dominance of metabolic risk factors in shaping dementia burden. Estimates suggest that metabolic contributors account for up to 28.8% of dementia-related Disability-Adjusted Life Years (DALYs).80 Specific contributors include a high Body Mass Index (BMI), accounting for 18.7%, and elevated fasting blood glucose levels, responsible for 12.4%.80 Additionally, behavioural risk factors, such as tobacco use, contribute an additional 15.4% to the dementia burden.80 While many of these risk factors are acknowledged in the 2024 Lancet Commission report,4 empirical evidence from regional data suggests their actual contribution may be substantially higher than global estimates indicate. Alarmingly, the findings indicate that up to 73% of dementia cases in Northern Africa could potentially be preventable, which is significantly higher than the 45% global preventable fraction reported by the Lancet Commission.4 These regional variations underscore the importance of complementing global estimates with locally informed data and modelling. By doing so, we can robustly improve the accuracy of risk attribution, minimise potential under or over estimation of risk, and ultimately design more effective, culturally sensitive intervention strategies. We recommend that future iterations of the Lancet Commission consider incorporating regional and culturally adapted modelling approaches to enhance the global applicability of dementia risk reduction strategies. This would ensure that interventions are relevant and effective for populations in the majority world, who currently bear a significant and growing proportion of the global dementia burden.
Modelling approaches
To further elucidate the complexities surrounding these risk factors, one recommended approach is the adoption of network analysis modelling81 across the life span.82 This methodology can enhance our understanding of how various risk factors interact and contribute to the development of dementia. Numerous researchers support this viewpoint, suggesting it is crucial to identify the complex biological pathways that mediate the relationship between modifiable risk factors and dementia.83 Such pathways might serve as realistic therapeutic targets for clinical trials. Additionally, exploring Artificial Intelligence-driven models, such as DeepPPI, is a pivotal strategy since these models have been shown to outperform traditional methods in predicting interactions within complex networks.83
Recent studies utilising genomic structural equation modelling to examine dementia alongside 12 modifiable risk factors have revealed high levels of genetic overlap among most dementia risk factors.84 This suggests the existence of overarching shared biological pathways that may elevate dementia risk. These findings illuminate the advantages of employing latent variable modelling (LVM) to streamline data complexity and identify common mechanistic patterns among risk factors. However, it is essential to note that traditional LVM models struggle with highly complex and nonlinear data.85 Consequently, multivariate growth mixture models (GMM) emerge as superior alternatives,86 representing a promising route for a more accurate understanding of dementia risk factors and cognitive decline.
Future research
Future studies in this domain must consider risk factors that may not be immediately reported in the 2024 Lancet report. As discussed, poverty, wealth shocks, income inequality, and HIV infections play critical roles in varying stages of the course of life. They may influence the prevalence and incidence of dementia. These risk factors profoundly affect intellectual and physical developmental trajectories, impacting overall health in later life.
Current surveys of older populations often touch upon histories of malnutrition during childhood. However, understanding the extent, duration, and contextual factors surrounding poverty, wealth shocks, food insecurity, undernutrition, and malnutrition is crucial for comprehending their long-term impacts on dementia.
The bulk of evidence supporting the Lancet Commission's 2020 and 2024 selection of risk factors comes from large longitudinal cohort studies and randomised controlled trials (RCTs) in high-income countries. These types of trials are expensive and require long-term funding commitments from government and private foundations, which is often lacking in low- and middle-income countries. This contributes to a major gap in our ability to effectively model global dementia risk and establish prevention strategies that are relevant for the majority world. New trials in Africa87 and Latin America88 have been launched that promise to fill this gap, but more work is needed.
Addressing these multifaceted risk factors requires a comprehensive, interdisciplinary approach that integrates insights from various fields—including anthropology, biology, economics, public health, neuroscience, and mathematics aimed at advancing innovative strategies to mitigate dementia risk.
Conclusion
Expanding our understanding of dementia risk is crucial for effective prevention. The 2024 Lancet Commission Report underscores the need for a global perspective, recognising that dementia is shaped not only by genetics but also by socioeconomic, environmental, and lifestyle factors. Poverty, limited healthcare access, and educational disparities heighten risk, while environmental exposure, such as air pollution, toxins, and social isolation, further contribute. Promoting healthier habits, including exercise, nutrition, and cognitive engagement, is essential. A collaborative, interdisciplinary approach that integrates research, policy, and lived experiences can drive impactful strategies. Addressing these multifaceted influences will enhance dementia prevention and care worldwide.
Contributors
CM conceptualised the paper and wrote the original draft. AI, CU, AW, CMc, HE, MS, KR, DT, GV, WH, GF, SA, NM, AL, KB, LM, KA, AS, RM, MB, SN, JJ, AKN, MR, JK, KBl and ZM review the draft paper and gave inputs. CM, AI, and CU provided senior oversight of the manuscript and edited the final version. All authors read and approved the final version of the manuscript. CM and AI have the final responsibility for the decision to submit for publication.
Declaration of interests
The authors declare that they have no competing interests.
Acknowledgements
AI is supported by grants from ReDLat, supported by Fogarty International Center (FIC), National Institutes of Health, National Institute on Aging (R01s AG075775, AG057234, AG082056 and AG083799, CARDS-NIH 75N95022C00031), Alzheimer's Association (SG-20-725707), Rainwater Charitable Foundation – The Bluefield project to cure FTD, and Global Brain Health Institute)], ANID/FONDECYT Regular (1250091, 1210195, 1210176, and 1220995); ANID/PIA/ANILLOS ACT210096; FONDEF ID20I10152, and ANID/FONDAP 15150012.
AKN is supported by grants from the National Institute on Aging (U19-AG074865, UG3AG090675); FWO Grant, University of Antwerp, (Ref: G0A0522N).
The funding sources had no role in study design, review, data curation and analysis, interpretation, decision to publish, or preparation of the manuscript.
References
- 1.Livingston G., Huntley J., Sommerlad A., et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet. 2020;396:413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desai R., John A., Saunders R., et al. Examining the lancet commission risk factors for dementia using Mendelian randomization. BMJ Ment Health. 2023;26 doi: 10.1136/bmjment-2022-300555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ribeiro F.S., Crivelli L., Leist A.K. Gender inequalities as contributors to dementia in Latin America and the Caribbean: what factors are missing from research? Lancet Healthy Longev. 2023;4:e284–e291. doi: 10.1016/S2666-7568(23)00052-1. [DOI] [PubMed] [Google Scholar]
- 4.Livingston G., Huntley J., Liu K.Y., et al. Dementia prevention, intervention, and care: 2024 report of the lancet standing commission. Lancet. 2024;404:572–628. doi: 10.1016/S0140-6736(24)01296-0. [DOI] [PubMed] [Google Scholar]
- 5.Avan A., Hachinski V. Increasing risks of dementia and brain health concerns. Lancet Public Health. 2024;9:e414–e415. doi: 10.1016/S2468-2667(24)00123-3. [DOI] [PubMed] [Google Scholar]
- 6.Kiani L. Dementia risk scores in diverse populations. Nat Rev Neurol. 2024;20:692. doi: 10.1038/s41582-024-01039-6. [DOI] [PubMed] [Google Scholar]
- 7.Huque H., Eramudugolla R., Chidiac B., et al. Could country-level factors explain sex differences in dementia incidence and prevalence? A systematic review and meta-analysis. J Alzheimers Dis. 2023;91(4):1231–1241. doi: 10.3233/JAD-220724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang M., Hu J., Weiss J., et al. Lifetime risk and projected burden of dementia. Nat Med. 2025;31:772–776. doi: 10.1038/s41591-024-03340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong J., Harris K., Lipnicki D.M., et al. Cohort studies of memory in an international consortium (COSMIC). Sex differences in dementia risk and risk factors: individual-Participant data analysis using 21 cohorts across six continents from the COSMIC consortium. Alzheimers Dement. 2023;19(8):3365–3378. doi: 10.1002/alz.12962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.GBD 2019 Dementia Forecasting Collaborators, et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the global burden of disease study 2019. Lancet Public Health. 2022;7:e105–e125. doi: 10.1016/S2468-2667(21)00249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moutinho S. Women twice as likely to develop Alzheimer's disease as men — but scientists do not know why. Nat Med. 2025;31:704–707. doi: 10.1038/s41591-025-03564-3. [DOI] [PubMed] [Google Scholar]
- 12.Gong J. Understanding dementia in the Western Pacific: a region-specific approach to prevention. Lancet Reg Health West Pac. 2024;50 doi: 10.1016/j.lanwpc.2024.101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong J., Harris K., Lipnicki D.M., et al. Cohort studies of memory in an international consortium (COSMIC). Sex differences in dementia risk and risk factors: individual-Participant data analysis using 21 cohorts across six continents from the COSMIC consortium. Alzheimers Dement. 2023;19(8):3365–3378. doi: 10.1002/alz.12962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens G., Flaxman S., Brunskill E., et al. Global burden of disease hearing loss expert group. Global and regional hearing impairment prevalence: an analysis of 42 studies in 29 countries. Eur J Public Health. 2013;23(1):146–152. doi: 10.1093/eurpub/ckr176. [DOI] [PubMed] [Google Scholar]
- 15.Zheng J., Wang J., Zhang Y., et al. The global burden of diseases attributed to high low-density lipoprotein cholesterol from 1990 to 2019. Front Public Health. 2022;10 doi: 10.3389/fpubh.2022.891929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biegon A. Considering biological sex in traumatic brain injury. Front Neurol. 2021;12 doi: 10.3389/fneur.2021.576366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.GBD 2021 Diabetes Collaborators, et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the global burden of disease study 2021. Lancet. 2023;402:203–234. doi: 10.1016/S0140-6736(23)01301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai X., Gakidou E., Lopez A. Evolution of the global smoking epidemic over the past half century: strengthening the evidence base for policy action. Tob Control. 2022;31(2):129–137. doi: 10.1136/tobaccocontrol-2021-056535. [DOI] [PubMed] [Google Scholar]
- 19.Zhou B., Perel P., Mensah G., Ezzati M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat Rev Cardiol. 2021;18(11):785–802. doi: 10.1038/s41569-021-00559-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.GBD 2016 Alcohol Collaborators, et al. Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2018;392(10152):1015–1035. doi: 10.1016/S0140-6736(18)31310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Röhr S., Wittmann F., Engel C., et al. Social factors and the prevalence of social isolation in a population-based adult cohort. Soc Psychiatry Psychiatr Epidemiol. 2022;57(10):1959–1968. doi: 10.1007/s00127-021-02174-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naito R., Leong D.P., Bangdiwala S.I., et al. Impact of social isolation on mortality and morbidity in 20 high-income, middle-income and low-income countries in five continents. BMJ Glob Health. 2021;6(3) doi: 10.1136/bmjgh-2020-004124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedman J., York H., Graetz N., et al. Measuring and forecasting progress towards the education-related SDG targets. Nature. 2020;580:636–639. doi: 10.1038/s41586-020-2198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.COVID-19 Mental Disorders Collaborators, et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 2021;398(10312):1700–1712. doi: 10.1016/S0140-6736(21)02143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strain T., Flaxman S., Guthold R., et al. National, regional, and global trends in insufficient physical activity among adults from 2000 to 2022: a pooled analysis of 507 population-based surveys with 5.7 million participants. Lancet Glob Health. 2024;12(8):e1232–e1243. doi: 10.1016/S2214-109X(24)00150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.GBD 2021 Adult BMI Collaborators, et al. Global, regional, and national prevalence of adult overweight and obesity, 1990-2021, with forecasts to 2050: a forecasting study for the global burden of disease study 2021. Lancet. 2025;405(10481):813–838. doi: 10.1016/S0140-6736(25)00355-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shupler M., Hystad P., Birch A., et al. Household and personal air pollution exposure measurements from 120 communities in eight countries: results from the PURE-AIR study. Lancet Planet Health. 2020;4(10):e451–e462. doi: 10.1016/S2542-5196(20)30197-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burton M., Ramke J., Marques A.P., et al. The lancet global health commission on global eye health: vision beyond 2020. Lancet Glob Health. 2021;9(4):e489–e551. doi: 10.1016/S2214-109X(20)30488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mielke M., Aggarwal N.T., Vila-Castelar C., et al. Diversity and disparity professional interest area sex and gender special interest group. Consideration of sex and gender in alzheimer's disease and related disorders from a global perspective. Alzheimers Dement. 2022;18(12):2707–2724. doi: 10.1002/alz.12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vila-Castelar C., Udeh-Momoh C., Aggarwal N., Mielke M. Sex and gender considerations in dementia: a call for global research. Nat Aging. 2023;3(5):463–465. doi: 10.1038/s43587-023-00374-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alemayehu Z., Ayalew B.D., Sime B.L., et al. Dementia in Sub-Saharan Africa: risk factors, public perception, and management approaches. J Med Surg Public Health. 2025;7 [Google Scholar]
- 32.Sakaniwa R., Shirai K., Cador D., et al. Socioeconomic status transition throughout life and risk of dementia. JAMA Netw Open. 2024;7(5) doi: 10.1001/jamanetworkopen.2024.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bodryzlova Y., Kim A., Michaud X., André C., Bélanger E., Moullec G. Social class and the risk of dementia: a systematic review and meta-analysis of the prospective longitudinal studies. Scand J Public Health. 2023;51(8):1122–1135. doi: 10.1177/14034948221110019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li R., Li R., Xie J., et al. Associations of socioeconomic status and healthy lifestyle with incident early-onset and late-onset dementia: a prospective cohort study. Lancet Healthy Longev. 2023;4(12):e693–e702. doi: 10.1016/S2666-7568(23)00211-8. [DOI] [PubMed] [Google Scholar]
- 35.Lai K., Webster C., Kumari S., Gallacher J.E.J., Sarkar C. The associations of socioeconomic status with incident dementia and Alzheimer's disease are modified by leucocyte telomere length: a population-based cohort study. Sci Rep. 2023;13:6163. doi: 10.1038/s41598-023-32974-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen S., Chen S., Hanewald K., et al. Social environment, lifestyle, and genetic predisposition with dementia risk: a long-term longitudinal study among older adults. J Gerontol A Biol Sci Med Sci. 2024;79:128. doi: 10.1093/gerona/glae128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trani J., Moodley J., Maw M., Babulal G. Association of multidimensional poverty with dementia in adults aged 50 years or older in South Africa. JAMA Netw Open. 2022;5(3) doi: 10.1001/jamanetworkopen.2022.4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trani J., Zhu Y., Park S., Babulal G. Is multidimensional poverty associated to dementia risk? The case of older adults in Pakistan. Innov Aging. 2024;8(2) doi: 10.1093/geroni/igae007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klee M., Leist A., Veldsman M., Ranson J., Llewellyn D. Socioeconomic deprivation, genetic risk, and incident dementia. Am J Prev Med. 2023;64(5):621–630. doi: 10.1016/j.amepre.2023.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lam J., Hou C.E., Hojilla J.C., et al. Comparison of dementia risk after age 50 between individuals with and without HIV infection. AIDS. 2021;35(5):821–828. doi: 10.1097/QAD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shayegi-Nik S., Honer W.G., Vila-Rodriguez F., et al. Incidence and contributing factors of dementia among people living with HIV in British Columbia, Canada, from 2002 to 2016: a retrospective cohort study. BMJ Public Health. 2024;2(1) doi: 10.1136/bmjph-2023-000627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hyle E., Wattananimitgul N., Mukerji S.S., et al. Age-associated dementia among older people aging with HIV in the United States: a modeling study. AIDS. 2024;38(8):1186–1197. doi: 10.1097/QAD.0000000000003862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee H., Mlombe Y., Song Y.E., et al. Dementia prevalence and risk factors in people with and without HIV in Malawi: a medical record review. Alzheimers Dement. 2025;21(3) doi: 10.1002/alz.70009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cho T., Yu X., Gross A.L., et al. Negative wealth shocks in later life and subsequent cognitive function in older adults in China, England, Mexico, and the USA, 2012-18: a population-based, cross-nationally harmonised, longitudinal study. Lancet Healthy Longev. 2023;4(9):e461–e469. doi: 10.1016/S2666-7568(23)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cho T., Yu X., Adar S., Choi H., Langa K., Kobayashi L. Changes in marital and health status as risk factors for a subsequent negative wealth shock: a population-based longitudinal study in the United States, 1995-2020. Soc Sci Med. 2025;371 doi: 10.1016/j.socscimed.2025.117905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cadar D., Lassale C., Davies H., Llewellyn D., Batty G., Steptoe A. Individual and area-based socioeconomic factors associated with dementia incidence in England: evidence from a 12-year follow-up in the English longitudinal study of ageing. JAMA Psychiatry. 2018;75(7):723–732. doi: 10.1001/jamapsychiatry.2018.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moguilner S., Baez S., Hernandez H., et al. Brain clocks capture diversity and disparities in aging and dementia across geographically diverse populations. Nat Med. 2024;30:3646–3657. doi: 10.1038/s41591-024-03209-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chan S., Hung W. Measurement and determinants of multidimensional poverty: the case of Hong Kong. J Asian Public Policy. 2024;4:1–21. doi: 10.1080/17516234.2024.2325857. [DOI] [Google Scholar]
- 49.Marden J., Tchetgen Tchetgen E., Kawachi I., Glymour M. Contribution of socioeconomic status at 3 life-course periods to late-life memory function and decline: early and late predictors of dementia risk. Am J Epidemiol. 2017;186(7):805–814. doi: 10.1093/aje/kwx155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Evans A., Hebert L.E., Beckett L.A., et al. Education and other measures of socioeconomic status and risk of incident alzheimer disease in a defined population of older persons. Arch Neurol. 1997;54(11):1399–1405. doi: 10.1001/archneur.1997.00550230066019. [DOI] [PubMed] [Google Scholar]
- 51.Mostert C., et al. Life span policies and macroeconomic transition will help the 21st century brain health revolution in developing countries. Alzheimers Dement. 2025:1–9. doi: 10.1002/alz.14527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Editorial T. For the HIV epidemic to end so must gender inequality. Lancet HIV. 2019;6(7) doi: 10.1016/S2352-3018(19)30198-5. [DOI] [PubMed] [Google Scholar]
- 53.Olajide T., Ogungbemi E., Olajide G., Ogundijo D., Osakuade O., Moshood F. HIV-associated neurocognitive disorders in Africa: challenges, peculiarities, and future directions. AIDS Res Ther. 2024;21(1):88. doi: 10.1186/s12981-024-00677-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brito e Silva E., Caixeta L., Soares V., Sagawa G. HIV-associated dementia in older adults: clinical and tomographic aspects. Int Psychogeriatr. 2011;23(7):1061–1069. doi: 10.1017/S1041610210002474. [DOI] [PubMed] [Google Scholar]
- 55.Nightingale S., Ances B., Cinque P., et al. Cognitive impairment in people living with HIV: consensus recommendations for a new approach. Nat Rev Neurol. 2023;19(7):424–433. doi: 10.1038/s41582-023-00813-2. [DOI] [PubMed] [Google Scholar]
- 56.Mostert C. Macroeconomics and health: understanding the impact of a declining economy on health outcomes of children and young adults in South Africa. SSM Popul Health. 2023;22 doi: 10.1016/j.ssmph.2023.101404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Webster P. Inequality—we should overcome, but will we? J Public Health. 2024;46:303–304. doi: 10.1093/pubmed/fdae128. [DOI] [PubMed] [Google Scholar]
- 58.Boudet A., et al. World Bank; 2021. A global view of poverty, gender, and household composition. World bank policy research working paper 9553.https://openknowledge.worldbank.org/server/api/core/bitstreams/24fb3df7-5812-57ad-9c69-85dfe7f36ce0/content Available at: [Google Scholar]
- 59.Ervin J., Taouk Y., Alfonzo L.F., Hewitt B., King T. Gender differences in the association between unpaid labour and mental health in employed adults: a systematic review. Lancet Public Health. 2022;7:e775–e786. doi: 10.1016/S2468-2667(22)00160-8. [DOI] [PubMed] [Google Scholar]
- 60.Azzopardi P., Hearps S.J.C., Francis K.L., et al. Progress in adolescent health and wellbeing: tracking 12 headline indicators for 195 countries and territories, 1990-2016. Lancet. 2019;393(10176):1101–1118. doi: 10.1016/S0140-6736(18)32427-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blair C., Raver C. Poverty, stress, and brain development: new directions for prevention and intervention. Acad Pediatr. 2016;16(3 Suppl):S30–S36. doi: 10.1016/j.acap.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zugman A., Alliende L.M., Medel V., et al. Country-level gender inequality is associated with structural differences in the brains of women and men. Proc Natl Acad Sci U S A. 2023;120(20) doi: 10.1073/pnas.2218782120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Samuel L., Szanton S.L., Wolff J.L., Ornstein K.A., Parker L.J., Gitlin L.N. Socioeconomic disparities in six-year incident dementia in a nationally representative cohort of U.S. older adults: an examination of financial resources. BMC Geriatr. 2020;20:156. doi: 10.1186/s12877-020-01553-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ogden L., Ogden J., Mthembu P., Williamson N. Impact of HIV on women internationally. Emerg Infect Dis. 2004;10(11):2032–2033. doi: 10.3201/eid1011.040624_01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.GBD 2021 HIV Collaborators, et al. Global, regional, and national burden of HIV/AIDS, 1990-2021, and forecasts to 2050, for 204 countries and territories: the global burden of disease study 2021. Lancet HIV. 2024;11(12):e807–e822. doi: 10.1016/S2352-3018(24)00212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hussain H., Fadel A., Garcia E., et al. HIV and dementia. Microbe. 2024;2 doi: 10.1016/j.microb.2024.100052. [DOI] [Google Scholar]
- 67.Cornea A., Lata I., Simu M., Rosca E.C. Assessment and diagnosis of HIV-associated dementia. Viruses. 2023;15(2):378. doi: 10.3390/v15020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mohapatra S. Gender differentiated economic responses to crises in developing countries: insights for COVID-19 recovery policies. Rev Econ Househ. 2021;19(2):291–306. doi: 10.1007/s11150-020-09512-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wodon T., et al. World Bank Group; Washington, DC: 2020. How large is the gender dividend? Measuring selected impacts and costs of gender inequality (English). The cost of gender inequality notes series.http://documents.worldbank.org/curated/en/669491583160170913 [Google Scholar]
- 70.Gireesh A., Sacker A., McMunn A., Bhatt R., Cadar D. Socioeconomic inequalities linked to the transitioning to neurocognitive disorders and mortality. Sci Rep. 2024;14 doi: 10.1038/s41598-024-74125-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mollalo A., Knox S., Meng J., Benitez A., Lenert L.A., Alekseyenko A.V. Geospatial analysis of the association between medicaid expansion, minimum wage policies, and alzheimer's disease dementia prevalence in the United States. Information. 2024;15(11):688. doi: 10.3390/info15110688. [DOI] [Google Scholar]
- 72.Cysique A., Levin J., Howard C., et al. Fostering healthy cognitive ageing in people living with HIV. Lancet HIV. 2025;12:e71–e80. doi: 10.1016/S2352-3018(24)00248-0. [DOI] [PubMed] [Google Scholar]
- 73.Bodryzlova Y., Mehrabi F., Bosson A., et al. The potential of social policies in preventing dementia: an ecological study using systematic review and meta-analysis. J Aging Soc Policy. 2024;36(5):1004–1025. doi: 10.1080/08959420.2023.2245672. [DOI] [PubMed] [Google Scholar]
- 74.Hu Z., Jiao R., Wang P., et al. Shared causal paths underlying Alzheimer's dementia and type 2 diabetes. Sci Rep. 2020;10:4107. doi: 10.1038/s41598-020-60682-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Newby D., Orgeta V., Marshall C.R., et al. Artificial intelligence for dementia prevention. Alzheimers Dement. 2023;19(12):5952–5969. doi: 10.1002/alz.13463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hiu S., Yong T., Hasoon J., Teare M.D., Taylor J.P., Lin N. Instrumental variables in real-world clinical studies of dementia and neurodegenerative disease: systematic review of the subject-matter argumentation, falsification test, and study design strategies to justify a valid instrument. Brain Behav. 2024;14(1) doi: 10.1002/brb3.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ibanez A., Maito M., Botero-Rodríguez F., et al. Healthy aging meta-analyses and scoping review of risk factors across Latin America reveal large heterogeneity and weak predictive models. Nat Aging. 2024;4(8):1153–1165. doi: 10.1038/s43587-024-00648-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Legaz A., et al. Structural inequality linked to brain volume and network dynamics in aging and dementia across the americas. Nat Aging. 2024;27 doi: 10.1038/s43587-024-00781-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Santamaria-Garcia H., Sainz-Ballesteros A., Hernandez H., et al. Factors associated with healthy aging in Latin American populations. Nat Med. 2023;29(9):2248–2258. doi: 10.1038/s41591-023-02495-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.GBD 2019 North Africa and the Middle East Neurology Collaborators, et al. The burden of 133 neurological conditions in North Africa and the Middle East, 1990-2019: a systematic 134 analysis of the global burden of disease study 2019. Lancet Glob Health. 2024;12(6):e960–e982. doi: 10.1016/S2214-109X(24)00093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Muzio G., O'Bray L., Borgwardt K. Biological network analysis with deep learning. Brief Bioinform. 2021;22:1515–1530. doi: 10.1093/bib/bbaa257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Green R., Lord J., Xu J., et al. Metabolic correlates of late midlife cognitive outcomes: findings from the 1946 British birth cohort. Brain Commun. 2022;4 doi: 10.1093/BRAINCOMMS/FCAB291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Du X., Sun S., Hu C., Yao Y., Yan Y., Zhang Y. DeepPPI: boosting prediction of protein-protein interactions with deep neural networks. J Chem Inf Model. 2017;57:1499–1510. doi: 10.1021/ACS.JCIM.7B00028/SUPPL_FILE/CI7B00028_SI_001.ZIP. [DOI] [PubMed] [Google Scholar]
- 84.Foote I., Jacobs B.M., Mathlin G., et al. The shared genetic architecture of modifiable risk for Alzheimer's disease: a genomic structural equation modelling study. Neurobiol Aging. 2022;117:222–235. doi: 10.1016/J.NEUROBIOLAGING.2022.02.016. [DOI] [PubMed] [Google Scholar]
- 85.Kong X., Jiang X., Zhang B., Yuan J., Ge Z. Latent variable models in the era of industrial big data: extension and beyond. Annu Rev Control. 2022;54:167–199. doi: 10.1016/J.ARCONTROL.2022.09.005. [DOI] [Google Scholar]
- 86.Wang Y., Haaksma M.L., Ramakers I.H.G.B., et al. Cognitive and functional progression of dementia in two longitudinal studies. Int J Geriatr Psychiatry. 2019;34(11):1623–1632. doi: 10.1002/gps.5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Udeh-Momoh C., Maina R., Anazodo U.C., et al. Dementia risk reduction in the African context: multi-National implementation of multimodal strategies to promote healthy brain aging in Africa (the Africa-FINGERS project) Alzheimers Dement. 2024;20(12):8987–9003. doi: 10.1002/alz.14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Crivelli L., Calandri I.L., Suemoto C.K., et al. Latin American initiative for lifestyle intervention to prevent cognitive decline (LatAm-FINGERS): study design and harmonization. Alzheimers Dement. 2023;19(9):4046–4060. doi: 10.1002/alz.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]