Abstract
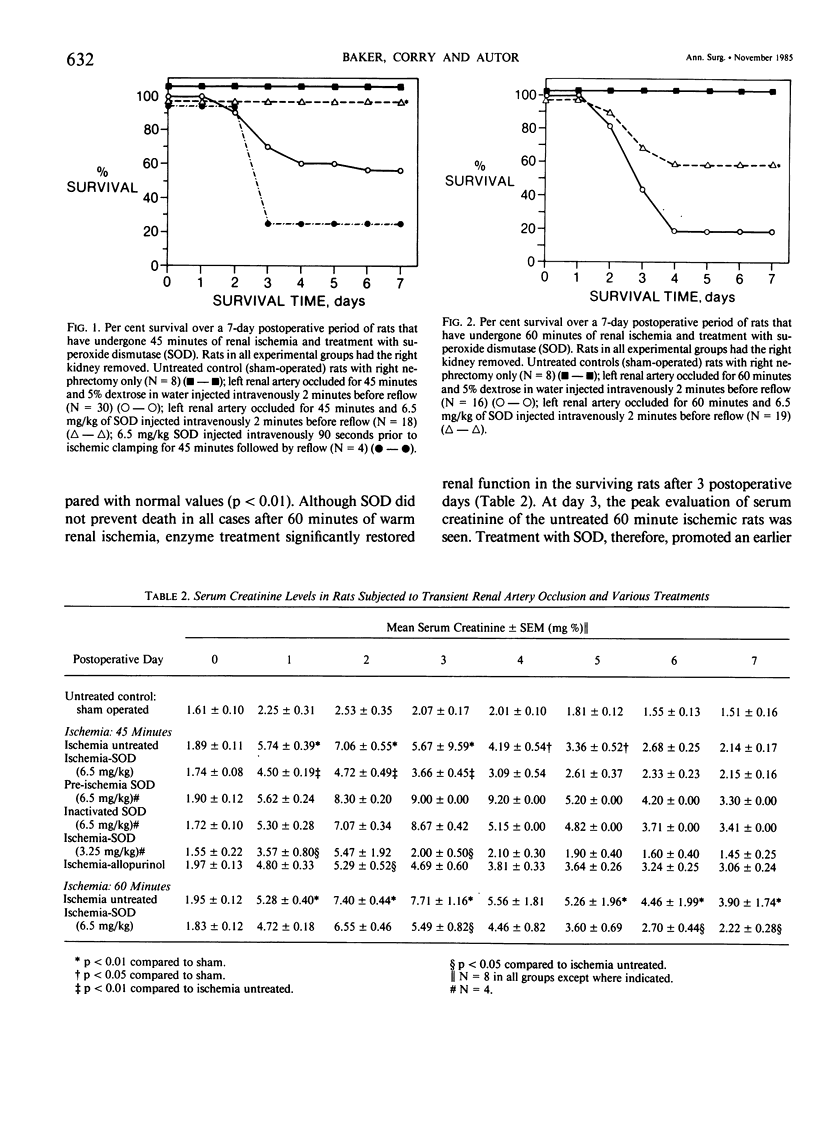
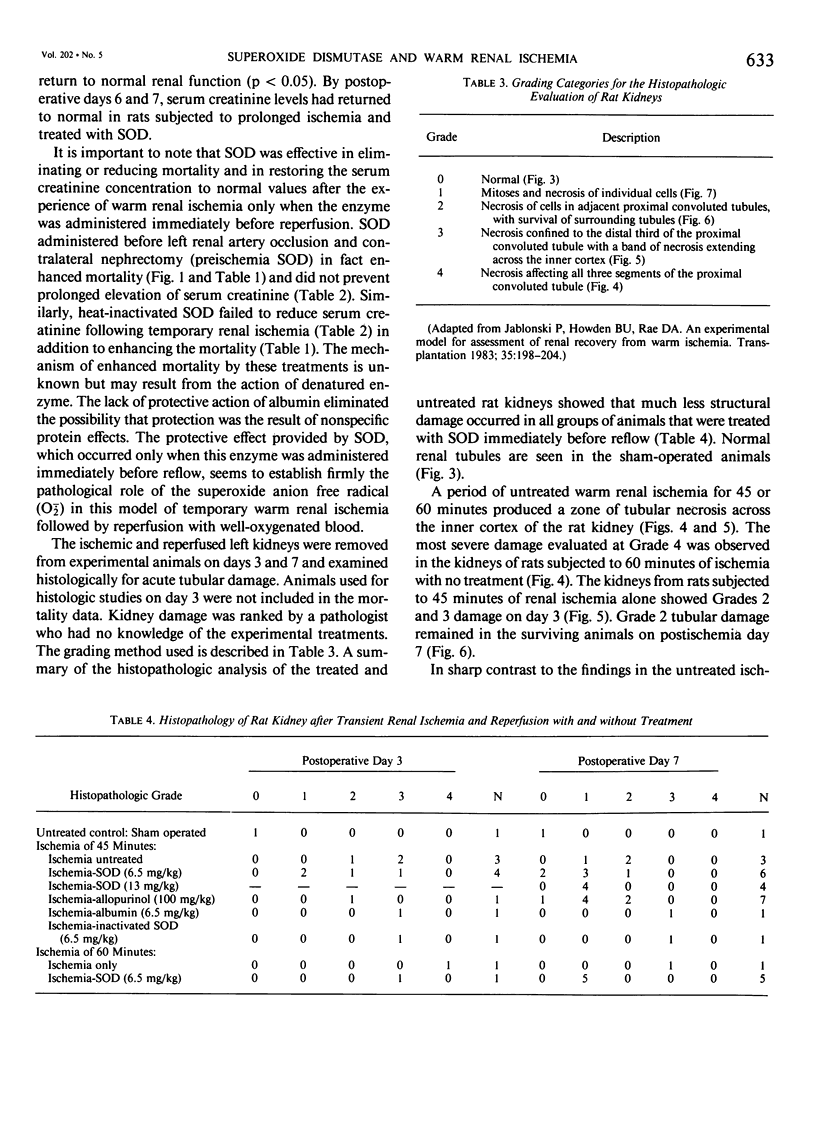
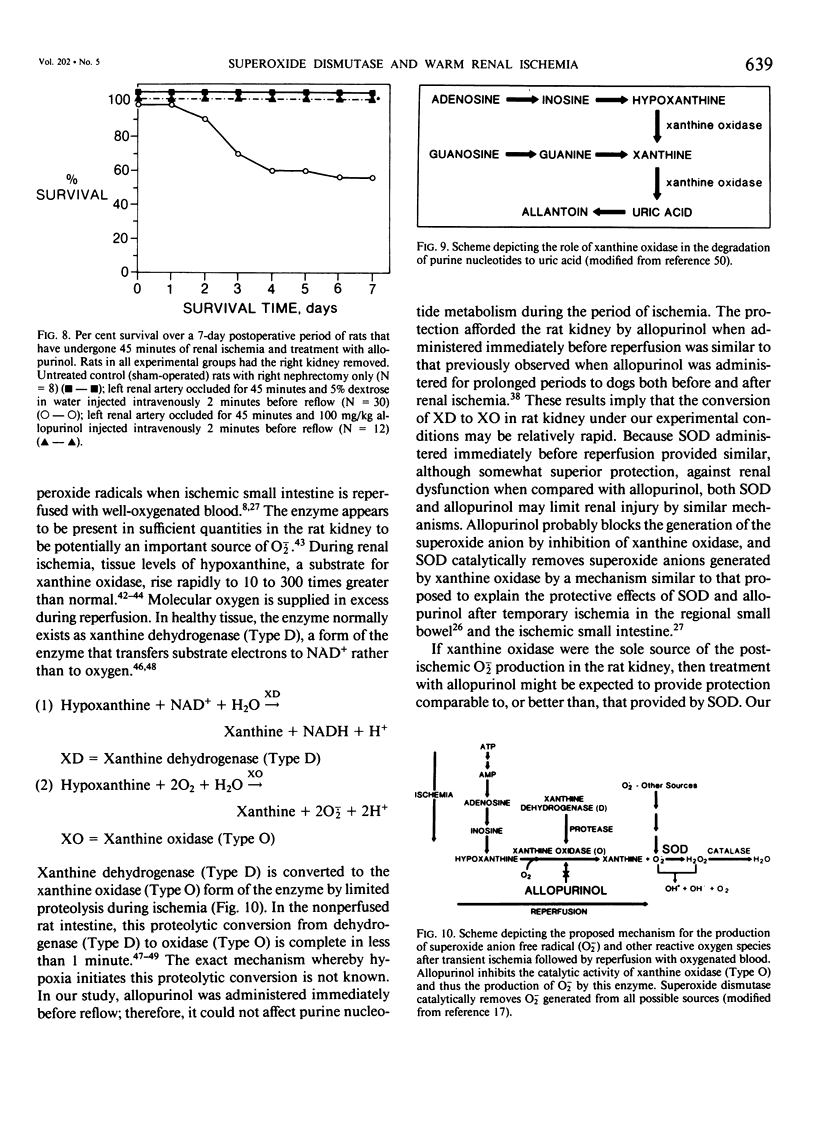
Superoxide anion free radical (O2-.) has been implicated in the pathogenesis of tissue injury consequent to ischemia/reperfusion in several different organs, including heart and bowel. Superoxide dismutase (SOD), an enzyme free radical scavenger specific for O2-., has been used successfully to protect these organs from structural damage during reoxygenation of ischemic tissue. It has been suggested that the catalytic action of xanthine oxidase in injured tissue is an important source of O2-. during reoxygenation. In order to evaluate the potential of SOD to protect against kidney damage resulting from transient ischemia followed by reperfusion with oxygenated blood, a model of warm renal ischemia was studied. LBNF1 rats underwent right nephrectomy and occlusion of the left renal artery for 45 minutes. Survival in the group of ischemic untreated rats (N = 30) was 56% at 7 days and serum creatinine was greatly elevated (p less than 0.01) in rats remaining alive over the full 7-day period. In strong contrast to these results, all of the animals treated with SOD before reperfusion (N = 18) were alive after 7 days similar to sham operated control rats (N = 8). Serum creatinine in the SOD treated rats was significantly elevated only to postoperative day 3 and thereafter returned to normal. Rats treated with inactive SOD (N = 4) or SOD before ischemia (N = 4) had decreased survival rates compared to ischemic untreated animals and prolonged elevation of serum creatinine. When the ischemia time was extended to 60 minutes, only 19% of the untreated animals (N = 16) survived at 7 days whereas nearly 60% of the SOD-treated animals survived (N = 19). Serum creatinine was greatly elevated during the full 7-day observation period in all surviving rats in the untreated ischemic group, whereas serum creatinine returned to normal (p less than 0.05) after 4 days in the surviving rats treated with SOD. To test whether the action of xanthine oxidase contributed to the kidney damage after reoxygenation, 45 min. ischemic rat kidneys were treated with allopurinol. All of the animals treated with allopurinol (N = 12) were alive at 7 days. Serum creatinine values returned to normal after the episode of ischemia and reperfusion but more slowly than after SOD treatment. Histologic evaluation of kidney tissue taken from animals after ischemia alone showed extensive renal tubular damage, which was essentially absent in kidneys from SOD-treated animals.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Autor A. P., Bonham A. C., Thies R. L. Toxicity of oxygen radicals in cultured pulmonary endothelial cells. J Toxicol Environ Health. 1984;13(2-3):387–395. doi: 10.1080/15287398409530505. [DOI] [PubMed] [Google Scholar]
- Battelli M. G., Corte E. D., Stirpe F. Xanthine oxidase type D (dehydrogenase) in the intestine and other organs of the rat. Biochem J. 1972 Feb;126(3):747–749. doi: 10.1042/bj1260747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brophy D., Najarian J. S., Kjellstrand C. M. Acute tubular necrosis after renal transplantation. Transplantation. 1980 Mar;29(3):245–248. doi: 10.1097/00007890-198003000-00016. [DOI] [PubMed] [Google Scholar]
- Bulkley G. B. The role of oxygen free radicals in human disease processes. Surgery. 1983 Sep;94(3):407–411. [PubMed] [Google Scholar]
- Cook J. G. Creatinine assay in the presence of protein. Clin Chim Acta. 1971 May;32(3):485–486. doi: 10.1016/0009-8981(71)90452-9. [DOI] [PubMed] [Google Scholar]
- Crowell J. W., Jones C. E., Smith E. E. Effect of allopurinol on hemorrhagic shock. Am J Physiol. 1969 Apr;216(4):744–748. doi: 10.1152/ajplegacy.1969.216.4.744. [DOI] [PubMed] [Google Scholar]
- Cunningham S. K., Keaveny T. V., Fitzgerald P. Effect of allopurinol on tissue ATP, ADP and AMP concentrations in renal ischaemia. Br J Surg. 1974 Jul;61(7):562–565. doi: 10.1002/bjs.1800610716. [DOI] [PubMed] [Google Scholar]
- Dalsing M. C., Grosfeld J. L., Shiffler M. A., Vane D. W., Hull M., Baehner R. L., Weber T. R. Superoxide dismutase: a cellular protective enzyme in bowel ischemia. J Surg Res. 1983 Jun;34(6):589–596. doi: 10.1016/0022-4804(83)90115-4. [DOI] [PubMed] [Google Scholar]
- DeWall R. A., Vasko K. A., Stanley E. L., Kezdi P. Responses of the ischemic myocardium to allopurinol. Am Heart J. 1971 Sep;82(3):362–370. doi: 10.1016/0002-8703(71)90302-4. [DOI] [PubMed] [Google Scholar]
- Del Maestro R. F., Björk J., Arfors K. E. Increase in microvascular permeability induced by enzymatically generated free radicals. II. Role of superoxide anion radical, hydrogen peroxide, and hydroxyl radical. Microvasc Res. 1981 Nov;22(3):255–270. doi: 10.1016/0026-2862(81)90096-0. [DOI] [PubMed] [Google Scholar]
- Del Maestro R., Thaw H. H., Björk J., Planker M., Arfors K. E. Free radicals as mediators of tissue injury. Acta Physiol Scand Suppl. 1980;492:43–57. [PubMed] [Google Scholar]
- Flamm E. S., Demopoulos H. B., Seligman M. L., Poser R. G., Ransohoff J. Free radicals in cerebral ischemia. Stroke. 1978 Sep-Oct;9(5):445–447. doi: 10.1161/01.str.9.5.445. [DOI] [PubMed] [Google Scholar]
- Flanigan W. J., Ardon L. F., Brewer T. E., Caldwell F. T. Etiology and diagnosis of early post-transplantation oliguria. Am J Surg. 1976 Dec;132(6):808–815. doi: 10.1016/0002-9610(76)90464-5. [DOI] [PubMed] [Google Scholar]
- Flores J., DiBona D. R., Beck C. H., Leaf A. The role of cell swelling in ischemic renal damage and the protective effect of hypertonic solute. J Clin Invest. 1972 Jan;51(1):118–126. doi: 10.1172/JCI106781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Granger D. N., Rutili G., McCord J. M. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981 Jul;81(1):22–29. [PubMed] [Google Scholar]
- Grøgaard B., Parks D. A., Granger D. N., McCord J. M., Forsberg J. O. Effects of ischemia and oxygen radicals on mucosal albumin clearance in intestine. Am J Physiol. 1982 May;242(5):G448–G454. doi: 10.1152/ajpgi.1982.242.5.G448. [DOI] [PubMed] [Google Scholar]
- Guarnieri C., Flamigni F., Caldarera C. M. Role of oxygen in the cellular damage induced by re-oxygenation of hypoxic heart. J Mol Cell Cardiol. 1980 Aug;12(8):797–808. doi: 10.1016/0022-2828(80)90081-4. [DOI] [PubMed] [Google Scholar]
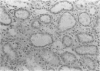
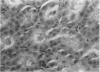
- Jablonski P., Howden B. O., Rae D. A., Birrell C. S., Marshall V. C., Tange J. An experimental model for assessment of renal recovery from warm ischemia. Transplantation. 1983 Mar;35(3):198–204. doi: 10.1097/00007890-198303000-00002. [DOI] [PubMed] [Google Scholar]
- Kjellstrand C. M., Casali R. E., Simmons R. L., Shideman J. R., Buselmeier T. J., Najarian J. S. Etiology and prognosis in acute post-transplant renal failure. Am J Med. 1976 Aug;61(2):190–199. doi: 10.1016/0002-9343(76)90169-8. [DOI] [PubMed] [Google Scholar]
- Kleihues P., Kobayashi K., Hossmann K. A. Purine nucleotide metabolism in the cat brain after one hour of complete ischemia. J Neurochem. 1974 Aug;23(2):417–425. doi: 10.1111/j.1471-4159.1974.tb04374.x. [DOI] [PubMed] [Google Scholar]
- Manson P. N., Anthenelli R. M., Im M. J., Bulkley G. B., Hoopes J. E. The role of oxygen-free radicals in ischemic tissue injury in island skin flaps. Ann Surg. 1983 Jul;198(1):87–90. doi: 10.1097/00000658-198307000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe R., Stevens L. E., Subramamian A., Lattes C., Hashim G. M. Reduction of acute tubular necrosis (ATN) by furosemide and steroids in cadaveric kidney recovery. Am J Surg. 1975 Mar;129(3):246–248. doi: 10.1016/0002-9610(75)90232-9. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Miller W. L., Thomas R. A., Berne R. M., Rubio R. Adenosine production in the ischemic kidney. Circ Res. 1978 Sep;43(3):390–397. doi: 10.1161/01.res.43.3.390. [DOI] [PubMed] [Google Scholar]
- Osswald H., Schmitz H. J., Kemper R. Tissue content of adenosine, inosine and hypoxanthine in the rat kidney after ischemia and postischemic recirculation. Pflugers Arch. 1977 Oct 19;371(1-2):45–49. doi: 10.1007/BF00580771. [DOI] [PubMed] [Google Scholar]
- Paller M. S., Hoidal J. R., Ferris T. F. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984 Oct;74(4):1156–1164. doi: 10.1172/JCI111524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks D. A., Bulkley G. B., Granger D. N., Hamilton S. R., McCord J. M. Ischemic injury in the cat small intestine: role of superoxide radicals. Gastroenterology. 1982 Jan;82(1):9–15. [PubMed] [Google Scholar]
- Parks D. A., Bulkley G. B., Granger D. N. Role of oxygen-derived free radicals in digestive tract diseases. Surgery. 1983 Sep;94(3):415–422. [PubMed] [Google Scholar]
- Roberts J. A., Roth J. K., Jr, Domingue G., Lewis R. W., Kaack B., Baskin G. Immunology of pyelonephritis in the primate model. V. Effect of superoxide dismutase. J Urol. 1982 Dec;128(6):1394–1400. doi: 10.1016/s0022-5347(17)53516-8. [DOI] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatney C. H., Toledo-Pereyra L. H., Lillehei R. C. Experiences with allopurinal in canine endotoxin shock. Adv Shock Res. 1980;4:119–137. [PubMed] [Google Scholar]
- Shlafer M., Kane P. F., Kirsh M. M. Superoxide dismutase plus catalase enhances the efficacy of hypothermic cardioplegia to protect the globally ischemic, reperfused heart. J Thorac Cardiovasc Surg. 1982 Jun;83(6):830–839. [PubMed] [Google Scholar]
- Staub N. C., Schultz E. L., Albertine K. H. Leucocytes and pulmonary microvascular injury. Ann N Y Acad Sci. 1982;384:332–343. doi: 10.1111/j.1749-6632.1982.tb21382.x. [DOI] [PubMed] [Google Scholar]
- Stirpe F., Della Corte E. The regulation of rat liver xanthine oxidase. Conversion in vitro of the enzyme activity from dehydrogenase (type D) to oxidase (type O). J Biol Chem. 1969 Jul 25;244(14):3855–3863. [PubMed] [Google Scholar]
- Vasko K. A., DeWall R. A., Riley A. M. Effect of allopurinol in renal ischemia. Surgery. 1972 May;71(5):787–790. [PubMed] [Google Scholar]
- Waud W. R., Rajagopalan K. V. The mechanism of conversion of rat liver xanthine dehydrogenase from an NAD+-dependent form (type D) to an O2-dependent form (type O). Arch Biochem Biophys. 1976 Feb;172(2):365–379. doi: 10.1016/0003-9861(76)90088-6. [DOI] [PubMed] [Google Scholar]