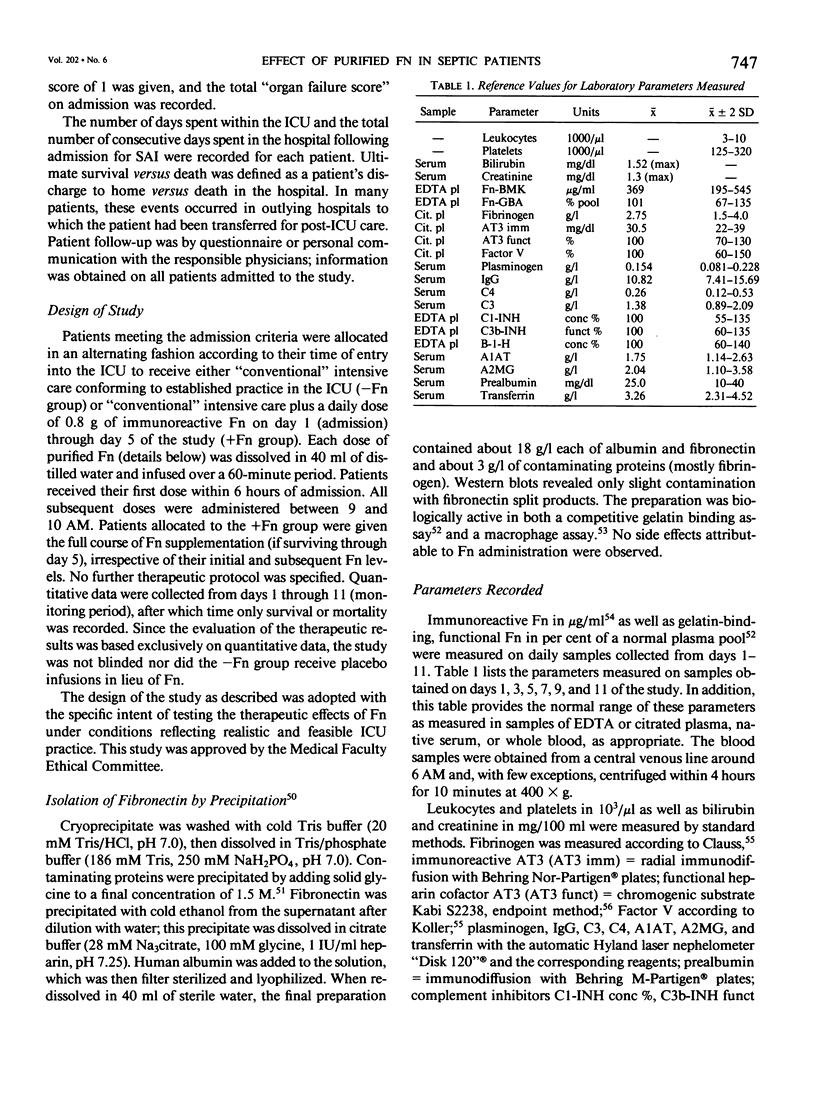
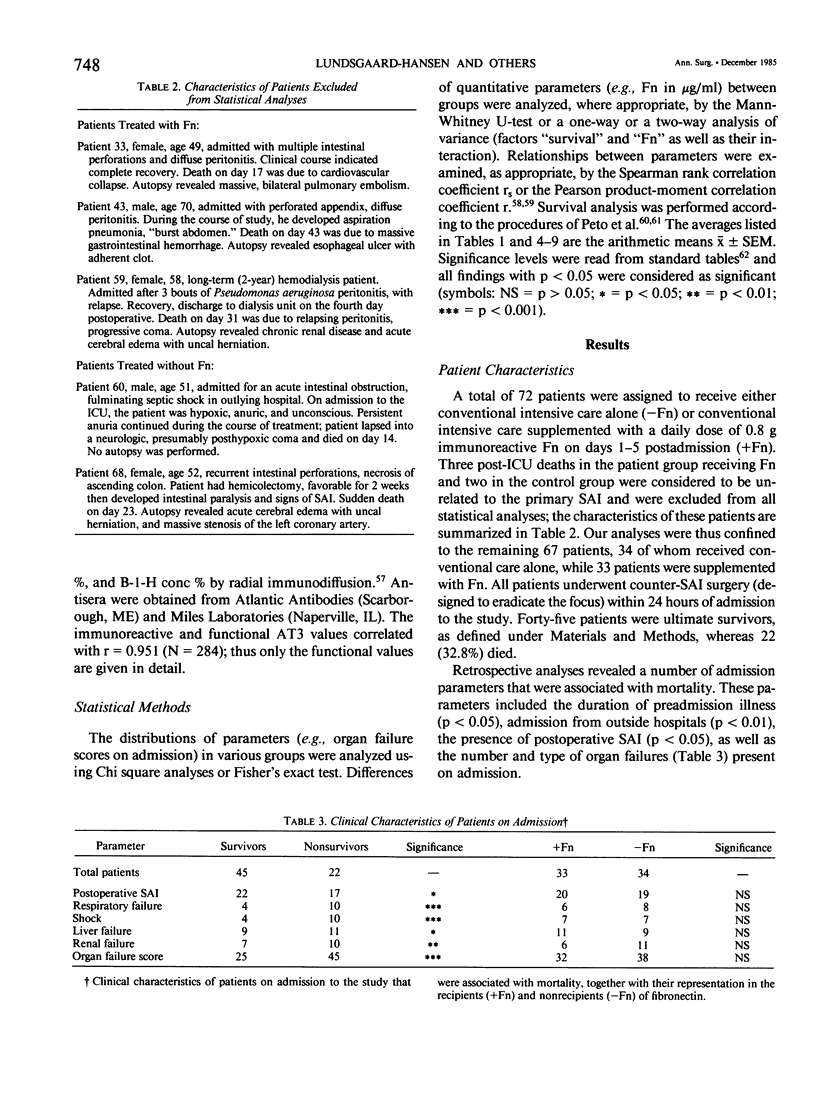
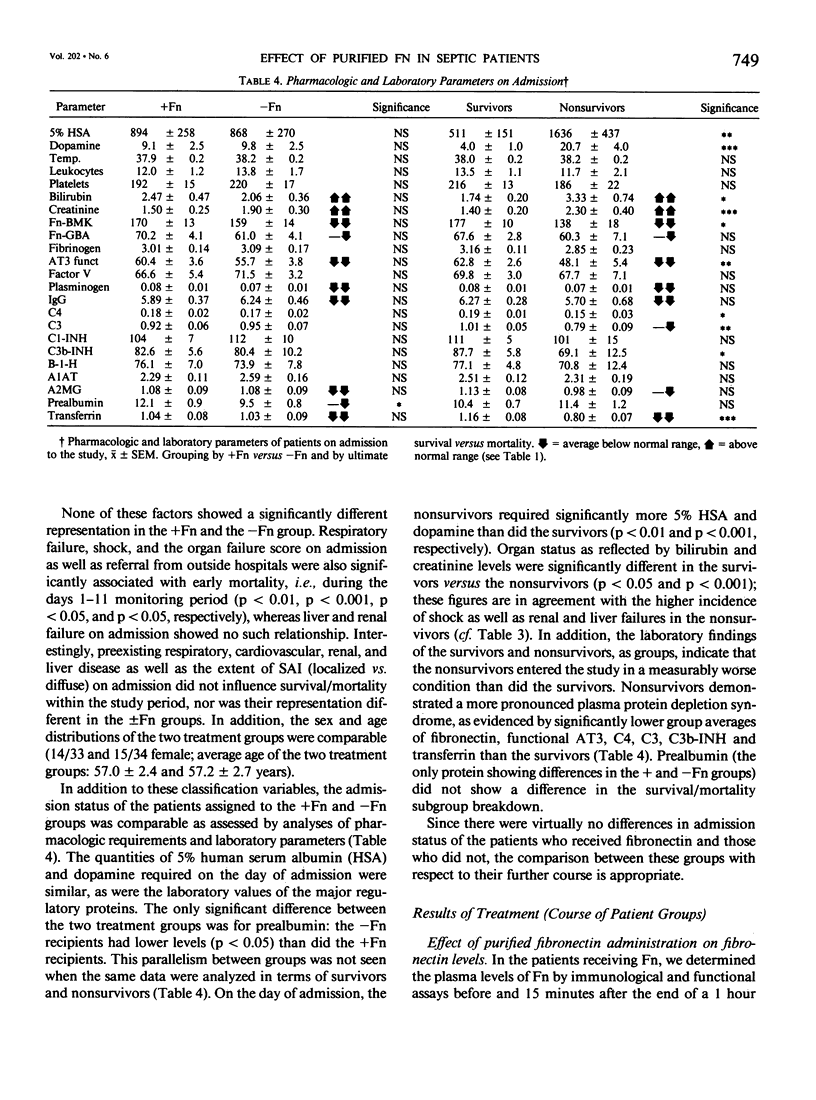
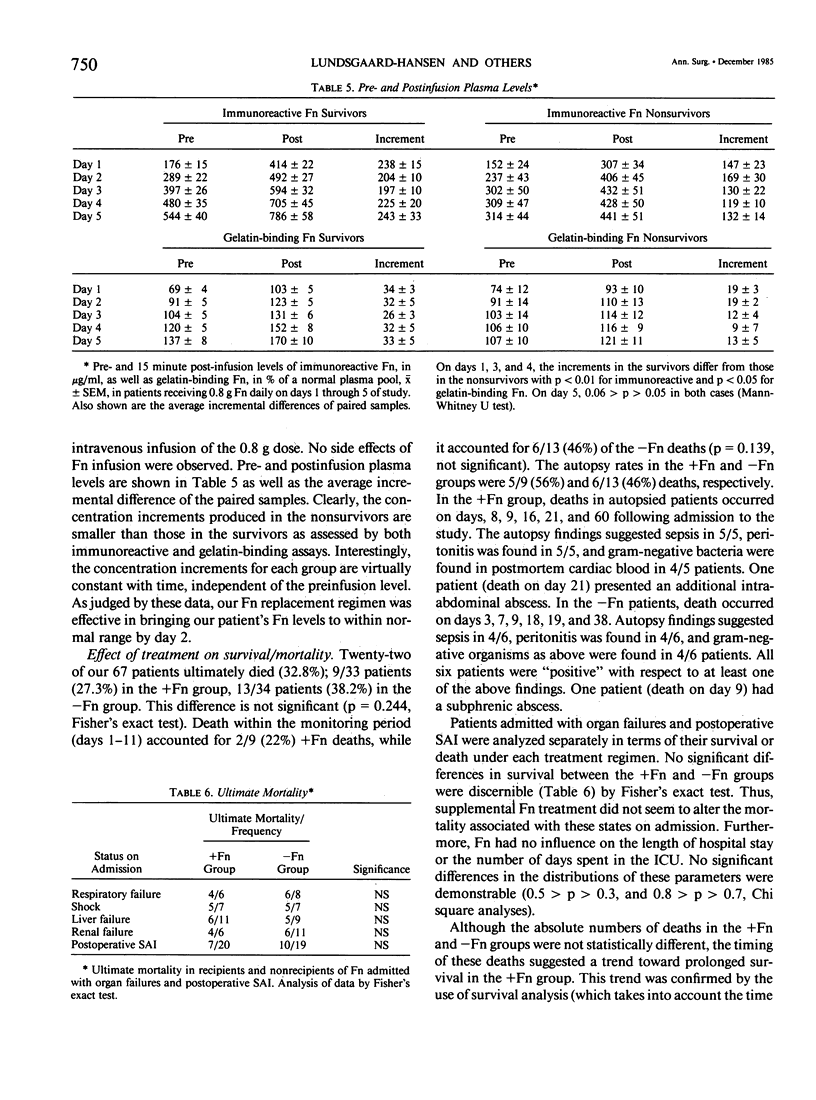
Abstract
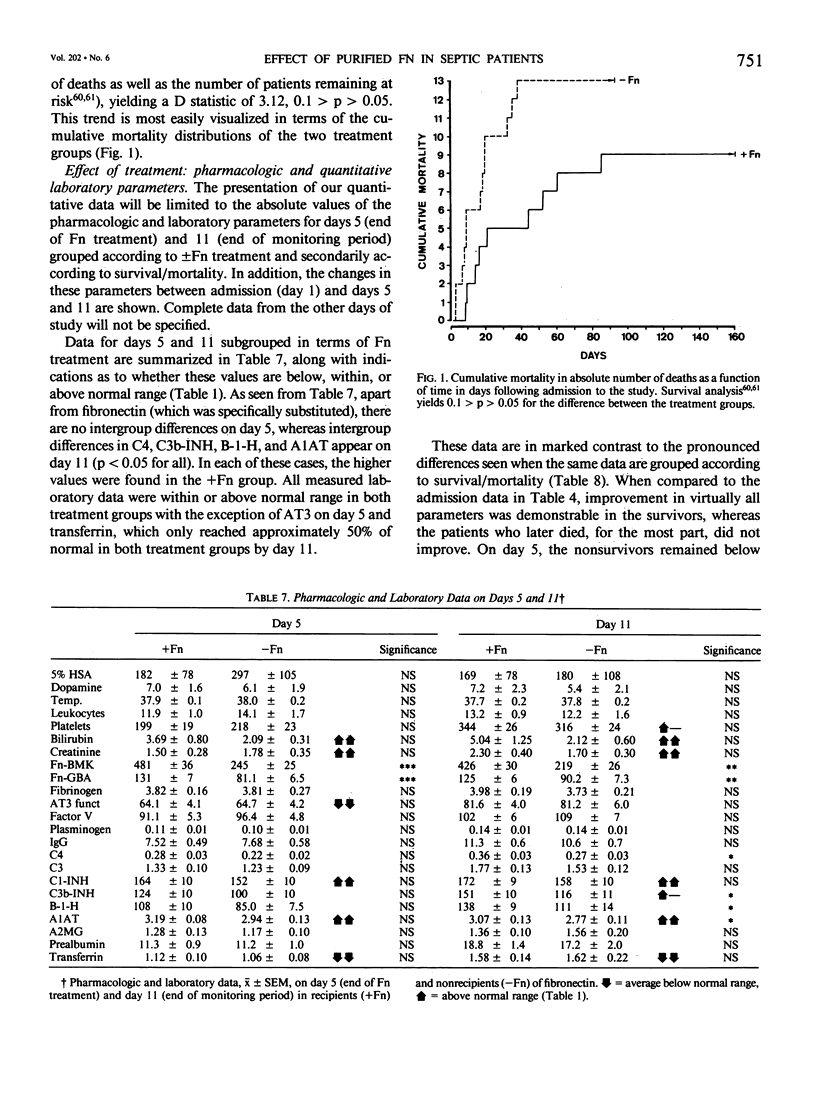
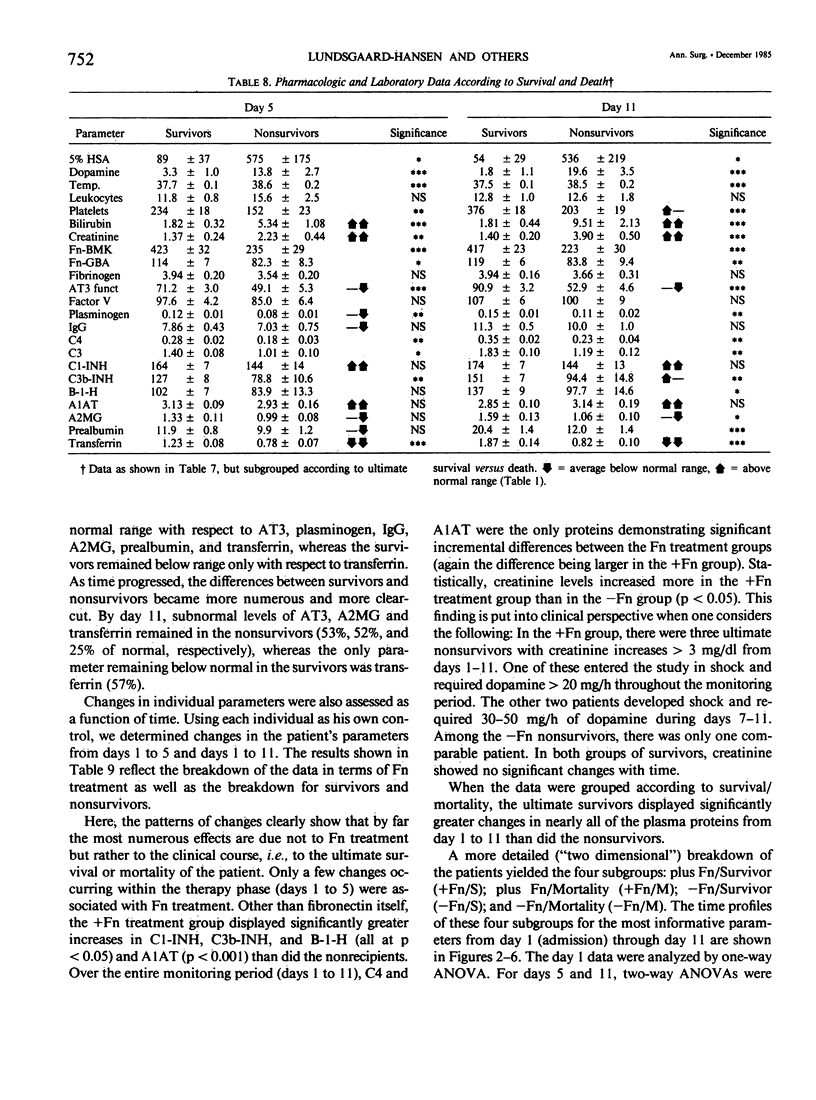
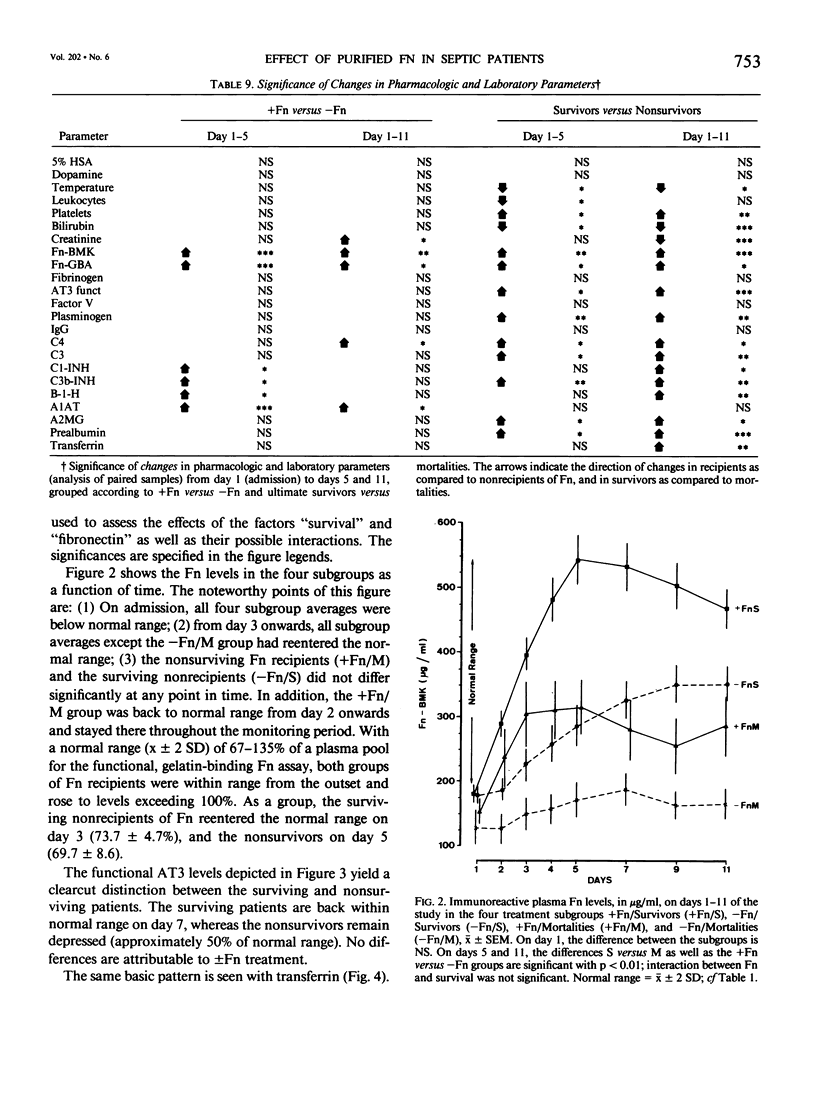
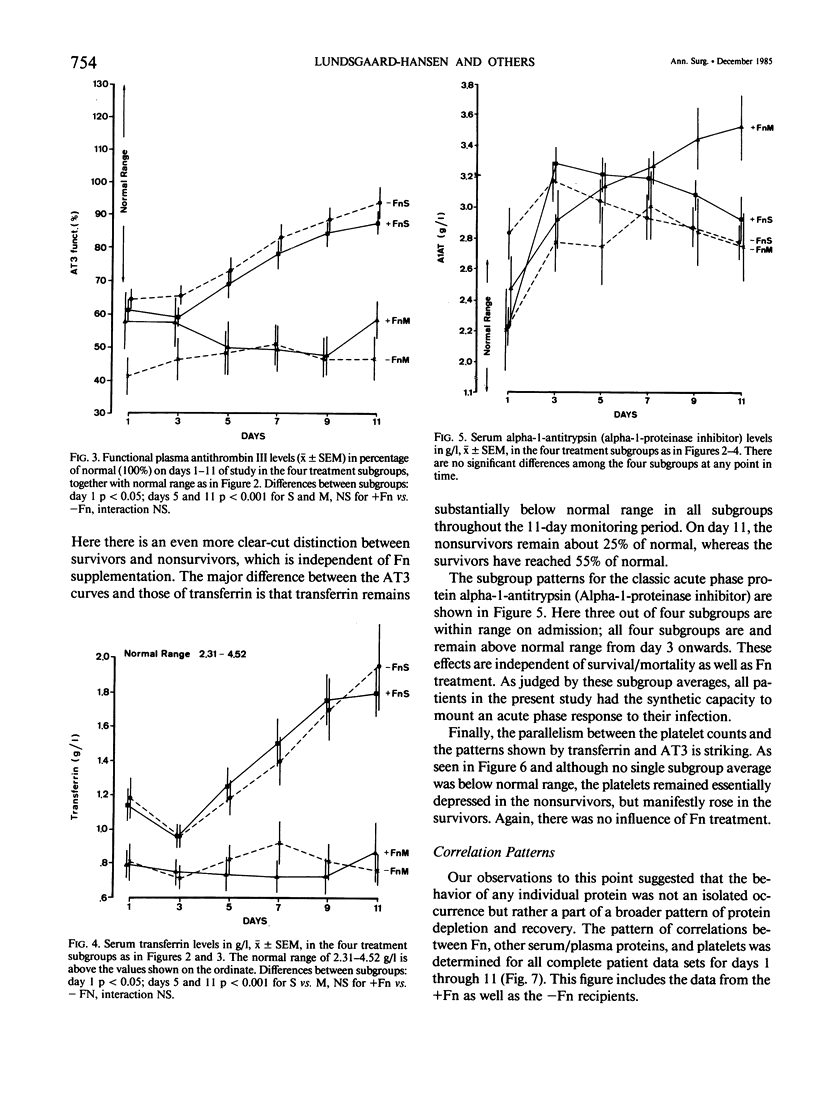
Subnormal plasma fibronectin (Fn) levels are found in patients with severe abdominal infections (SAI). The repletion of Fn has been postulated to have therapeutic benefit by virtue of its opsonic, reticuloendothelial system (RES) stimulating effects. A controlled, prospective trial of Fn administration was performed in patients with SAI to assess its use as an adjunct to standard procedures of intensive care. Thirty-three SAI patients were given daily doses of 0.8 g of purified Fn on days 1-5 following admission to the ICU, whereas 34 control patients received no Fn. All patients received the clinical care, antibiotics, and pharmacologic agents appropriate to their individual needs. The admission status and laboratory profiles of the two patient groups (+ and -Fn) were comparable on admission to the study. No side effects of the Fn preparation were observed. As judged by subgroup averages, the Fn replacement regimen was effective in elevating Fn levels to within normal range from day 2 onwards, as measured by immunological and functional assays. The estimated intravascular recovery of Fn averaged 82% in those patients who survived, yet only 52% in the nonsurvivors. Ultimate hospital mortality was 9/33 (27.3%) in the +Fn group versus 13/34 (38.2%) in the -Fn group (p = 0.244, Fisher's exact test). Although ultimate mortality was not significantly changed by the administration of Fn, the Fn treated patients appeared to survive longer than did the control patients. This trend was confirmed through the analysis of expected survival curves (D = 3.12, 0.1 greater than p greater than 0.05). When compared to the survivors, the ultimate nonsurvivors entered the study with statistically higher group averages of bilirubin and creatinine concomitant with lower averages of Fn, antithrombin III, C4, C3, C3b-INH, and transferrin. These differences persisted throughout the 11-day monitoring period; differences between survivors and nonsurvivors with respect to platelets, plasminogen, B-1-H, alpha-2-macroglobulin, and prealbumin appeared during the same period. Dramatic differences between the +Fn and -Fn treatment groups were not seen. Other than Fn, the Fn recipients only developed higher levels of the acute phase reactants C4, C3b-INH, B-1-H and alpha-1-antitrypsin (p less than 0.05) than did their non-Fn treated counterparts. In the present study, we again found a highly significant pattern of correlations between the absolute levels as well as the changes of Fn and other plasma proteins.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aasen A. O., Dale J., Ohlsson K., Gallimore M. Effects of slow intravenous administration of endotoxin on blood cells and coagulation in dogs. Eur Surg Res. 1978;10(3):194–205. doi: 10.1159/000128008. [DOI] [PubMed] [Google Scholar]
- Aasen A. O., Ohlsson K., Larsbraaten M., Amundsen E. Changes in plasminogen levels, plasmin activity and activity of antiplasmins during endotoxin shock in dogs. Eur Surg Res. 1978;10(1):63–72. doi: 10.1159/000127993. [DOI] [PubMed] [Google Scholar]
- Abildgaard U., Lie M., Odegård O. R. Antithrombin (heparin cofactor) assay with "new" chromogenic substrates (S-2238 and Chromozym TH). Thromb Res. 1977 Oct;11(4):549–553. doi: 10.1016/0049-3848(77)90208-0. [DOI] [PubMed] [Google Scholar]
- Alexander J. W., McClellan M. A., Ogle C. K., Ogle J. D. Consumptive opsoninopathy: possible pathogenesis in lethal and opportunistic infections. Ann Surg. 1976 Dec;184(6):672–678. doi: 10.1097/00000658-197612000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annest S. J., Scovill W. A., Blumenstock F. A., Stratton H. H., Newell J. C., Paloski W. H., Saba T. M., Powers S. R. Increased creatinine clearance following cryoprecipitate infusion in trauma and surgical patients with decreased renal function. J Trauma. 1980 Sep;20(9):726–732. doi: 10.1097/00005373-198009000-00003. [DOI] [PubMed] [Google Scholar]
- Bick R. L., Bick M. D., Fekete L. F. Antithrombin III patterns in disseminated intravascular coagulation. Am J Clin Pathol. 1980 Apr;73(4):577–583. doi: 10.1093/ajcp/73.4.577. [DOI] [PubMed] [Google Scholar]
- Blauhut B., Necek S., Vinazzer H., Bergmann H. Substitution therapy with an antithrombin III concentrate in shock and DIC. Thromb Res. 1982 Aug 1;27(3):271–278. doi: 10.1016/0049-3848(82)90074-3. [DOI] [PubMed] [Google Scholar]
- Bohnen J., Boulanger M., Meakins J. L., McLean A. P. Prognosis in generalized peritonitis. Relation to cause and risk factors. Arch Surg. 1983 Mar;118(3):285–290. doi: 10.1001/archsurg.1983.01390030017003. [DOI] [PubMed] [Google Scholar]
- Büller H. R., Bolwerk C., ten Cate J., Roos J., Kahlé L. H., ten Cate J. W. Postoperative hemostatic profile in relation to gram-negative septicemia. Crit Care Med. 1982 May;10(5):311–315. [PubMed] [Google Scholar]
- Cembrowski G. S., Mosher D. F. Plasma fibronectin concentration in patients with acquired consumptive coagulopathies. Thromb Res. 1984 Dec 1;36(5):437–445. doi: 10.1016/0049-3848(84)90300-1. [DOI] [PubMed] [Google Scholar]
- Ceulemans F., Mahieu P., Kets E., Wouters R., Schneider Y. J., Trouet A. Follow-up of the plasma concentration of fibronectin in two intensive care patients; effect of administration of plasma cryoprecipitate. Acta Clin Belg. 1983;38(5):309–314. doi: 10.1080/22953337.1983.11718951. [DOI] [PubMed] [Google Scholar]
- Coulaud J. M., Jacqueson A., Labrousse J., Allard D., Beyne P., Rapin J., Tenaillon A., Lissac J. Insuffisances respiratoires et circulatoires aiguës. Valeur pronostique du taux de fibronectine plasmatique. Presse Med. 1983 Jan 29;12(4):213–216. [PubMed] [Google Scholar]
- Coulaud J. M., Jacqueson A., Labrousse J., Allard D. Fibronectine plasmatique et syndrome de détresse respiratoire de l'adulte. Presse Med. 1983 Jul 9;12(29):1825–1825. [PubMed] [Google Scholar]
- Doran J. E., Callaway B. D., Reese A. C., Wynn J. J., Mansberger A. R. A competitive inhibition assay for gelatin binding fibronectin. Vox Sang. 1983;45(3):243–251. doi: 10.1111/j.1423-0410.1983.tb01910.x. [DOI] [PubMed] [Google Scholar]
- Doran J. E., Mansberger A. R., Reese A. C. Cold insoluble globulin-enhanced phagocytosis of gelatinized targets by macrophage monolayers: a model system. J Reticuloendothel Soc. 1980 May;27(5):471–483. [PubMed] [Google Scholar]
- Ekindjian O. G., Marien M., Wassermann D., Bruxelle J., Cazalet C., Konter E., Yonger J. Plasma fibronectin time course in burned patients: influence of sepsis. J Trauma. 1984 Mar;24(3):214–219. doi: 10.1097/00005373-198403000-00005. [DOI] [PubMed] [Google Scholar]
- Fourrier F., Chopin C., Steenhouwer F., Masurier C. Fibronectine plasmatique au cours des syndromes de détresse respiratoire aiguë septique. Presse Med. 1983 Jun 11;12(25):1611–1611. [PubMed] [Google Scholar]
- Hammerschmidt D. E., Weaver L. J., Hudson L. D., Craddock P. R., Jacob H. S. Association of complement activation and elevated plasma-C5a with adult respiratory distress syndrome. Pathophysiological relevance and possible prognostic value. Lancet. 1980 May 3;1(8175):947–949. doi: 10.1016/s0140-6736(80)91403-8. [DOI] [PubMed] [Google Scholar]
- Hutchinson D. R., Halliwell R. P., Smith M. G., Parke D. V. Serum "prealbumin" as an index of liver function in human hepatobiliary disease. Clin Chim Acta. 1981 Jul 18;114(1):69–74. doi: 10.1016/0009-8981(81)90229-1. [DOI] [PubMed] [Google Scholar]
- Jacob H. S. Complement-mediated leucoembolization: a mechanism of tissue damage during extracorporeal perfusions, myocardial infarction and in shock--a review. Q J Med. 1983 Summer;52(207):289–296. [PubMed] [Google Scholar]
- Jochum M., Duswald K. H., Neumann S., Witte J., Fritz H. Proteinases and their inhibitors in septicemia - basic concepts and clinical implications. Adv Exp Med Biol. 1984;167:391–404. doi: 10.1007/978-1-4615-9355-3_34. [DOI] [PubMed] [Google Scholar]
- Jochum M., Witte J., Schiessler H., Selbmann H. K., Ruckdeschl G., Fritz H. Clotting and other plasma factors in experimental endotoxemia: inhibition of degradation by exogenous proteinase inhibitors. Eur Surg Res. 1981;13(2):152–168. doi: 10.1159/000128181. [DOI] [PubMed] [Google Scholar]
- Kaplan J. E., Saba T. M. Humoral deficiency and reticuloendothelial depression after traumatic shock. Am J Physiol. 1976 Jan;230(1):7–14. doi: 10.1152/ajplegacy.1976.230.1.7. [DOI] [PubMed] [Google Scholar]
- Kaplan J. E., Saba T. M. Immunoreactive serum opsonic alpha 2 sb glycoprotein as a noninvasive index of RES systemic defense after trauma. Adv Shock Res. 1979;2:83–92. [PubMed] [Google Scholar]
- Lanser M. E., Saba T. M., Scovill W. A. Opsonic glycoprotein (plasma fibronectin) levels after burn injury. Relationship to extent of burn and development of sepsis. Ann Surg. 1980 Dec;192(6):776–782. doi: 10.1097/00000658-198012000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W., Umfleet R. A. The cold-insoluble globulin of human plasma. I. Purification, primary characterization, and relationship to fibrinogen and other cold-insoluble fraction components. J Biol Chem. 1970 Nov 10;245(21):5728–5736. [PubMed] [Google Scholar]
- Mosher D. F., Grossman J. E. Clinical use of fibronectin. (Potential applications). Ric Clin Lab. 1983 Jan-Mar;13(1):43–54. doi: 10.1007/BF02904744. [DOI] [PubMed] [Google Scholar]
- Mosher D. F., Williams E. M. Fibronectin concentration is decreased in plasma of severely ill patients with disseminated intravascular coagulation. J Lab Clin Med. 1978 May;91(5):729–735. [PubMed] [Google Scholar]
- Owen C. A., Jr, Bowie E. J. Generation of plasmatic coagulation factors by the isolated rat liver perfused with completely synthetic blood substitute. Thromb Res. 1981 May 1;22(3):259–266. doi: 10.1016/0049-3848(81)90118-3. [DOI] [PubMed] [Google Scholar]
- Owen M. C., Brennan S. O., Lewis J. H., Carrell R. W. Mutation of antitrypsin to antithrombin. alpha 1-antitrypsin Pittsburgh (358 Met leads to Arg), a fatal bleeding disorder. N Engl J Med. 1983 Sep 22;309(12):694–698. doi: 10.1056/NEJM198309223091203. [DOI] [PubMed] [Google Scholar]
- Owens M. R., Cimino C. D. Synthesis of fibronectin by the isolated perfused rat liver. Blood. 1982 Jun;59(6):1305–1309. [PubMed] [Google Scholar]
- Owens M. R., Miller L. L. Net biosynthesis of antithrombin III by the isolated rat liver perfused for 12--24 hours. Compared with rat fibrinogen and alpha-2 (acute-phase) globulin, antithrombin III is not an acute phase protein. Biochim Biophys Acta. 1980 Jan 3;627(1):30–39. doi: 10.1016/0304-4165(80)90120-8. [DOI] [PubMed] [Google Scholar]
- Peto R., Pike M. C., Armitage P., Breslow N. E., Cox D. R., Howard S. V., Mantel N., McPherson K., Peto J., Smith P. G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer. 1976 Dec;34(6):585–612. doi: 10.1038/bjc.1976.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto R., Pike M. C., Armitage P., Breslow N. E., Cox D. R., Howard S. V., Mantel N., McPherson K., Peto J., Smith P. G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977 Jan;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards W. O., Scovill W. A., Shin B. Opsonic fibronectin deficiency in patients with intra-abdominal infection. Surgery. 1983 Aug;94(2):210–217. [PubMed] [Google Scholar]
- Robbins A. B., Doran J. E., Reese A. C., Mansberger A. R., Jr Clinical response to cold insoluble globulin replacement in a patient with sepsis and thermal injury. Am J Surg. 1981 Nov;142(5):636–638. doi: 10.1016/0002-9610(81)90443-8. [DOI] [PubMed] [Google Scholar]
- Rubli E., Büssard S., Frei E., Lundsgaard-Hansen P., Pappova E. Plasma fibronectin and associated variables in surgical intensive care patients. Ann Surg. 1983 Mar;197(3):310–317. doi: 10.1097/00000658-198303000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba T. M., Albert W. H., Blumenstock F. A., Evanega G., Staehler F., Cho E. Evaluation of a rapid immunoturbidimetric assay for opsonic fibronectin in surgical and trauma patients. J Lab Clin Med. 1981 Oct;98(4):482–491. [PubMed] [Google Scholar]
- Saba T. M., Blumenstock F. A., Scovill W. A., Bernard H. Cryoprecipitate reversal of opsonic alpha2-surface binding glycoprotein deficiency in septic surgical and trauma patients. Science. 1978 Aug 18;201(4356):622–624. doi: 10.1126/science.675246. [DOI] [PubMed] [Google Scholar]
- Saba T. M., Cho E. Reticuloendothelial (RE) response to surgery as modified by intravenous administration of plasma cryoprecipitate or cold-insoluble globulin (plasma fibronectin) purified by affinity chromatography. Adv Shock Res. 1980;3:251–271. [PubMed] [Google Scholar]
- Saba T. M., Cho E. Reticuloendothelial systemic response to operative trauma as influenced by cryoprecipitate or cold-insoluble globulin therapy. J Reticuloendothel Soc. 1979 Aug;26(2):171–186. [PubMed] [Google Scholar]
- Saba T. M. Physiology and physiopathology of the reticuloendothelial system. Arch Intern Med. 1970 Dec;126(6):1031–1052. [PubMed] [Google Scholar]
- Saba T. M. Prevention of liver reticuloendothelial systemic host defense failure after surgery by intravenous opsonic glycoprotein therapy. Ann Surg. 1978 Aug;188(2):142–152. doi: 10.1097/00000658-197808000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipper H. G., Roos J., vd Meulen F., ten Cate J. W. Antithrombin III deficiency in surgical intensive care patients. Thromb Res. 1981 Jan 1;21(1-2):73–80. doi: 10.1016/0049-3848(84)90034-3. [DOI] [PubMed] [Google Scholar]
- Scovill W. A., Annest S. J., Saba T. M., Blumenstock F. A., Newell J. C., Stratton H. H., Powers S. R. Cardiovascular hemodynamics after opsonic alpha-2-surface binding glycoprotein therapy in injured patients. Surgery. 1979 Aug;86(2):284–293. [PubMed] [Google Scholar]
- Scovill W. A., Saba T. M., Blumenstock F. A., Bernard H., Powers S. R., Jr Opsonic alpha2 surface binding glycoprotein therapy during sepsis. Ann Surg. 1978 Oct;188(4):521–529. doi: 10.1097/00000658-197810000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte J., Jochum M., Scherer R., Schramm W., Hochstrasser K., Fritz H. Disturbances of selected plasma proteins in hyperdynamic septic shock. Intensive Care Med. 1982;8(5):215–222. doi: 10.1007/BF01694524. [DOI] [PubMed] [Google Scholar]


