Abstract
The compartmentation of metabolism in heterotrophic plant tissues is poorly understood due to the lack of data on metabolite distributions and fluxes between subcellular organelles. The main reason for this is the lack of suitable experimental methods with which intracellular metabolism can be measured. Here, we describe a nonaqueous fractionation method that allows the subcellular distributions of metabolites in developing potato (Solanum tuberosum L. cv Desiree) tubers to be calculated. In addition, we have coupled this fractionation method to a recently described gas chromatography-mass spectrometry procedure that allows the measurement of a wide range of small metabolites. To calculate the subcellular metabolite concentrations, we have analyzed organelle volumes in growing potato tubers using electron microscopy. The relative volume distributions in tubers are very similar to the ones for source leaves. More than 60% of most sugars, sugar alcohols, organic acids, and amino acids were found in the vacuole, although the concentrations of these metabolites is often higher in the cytosol. Significant amounts of the substrates for starch biosynthesis, hexose phosphates, and ATP were found in the plastid. However, pyrophosphate was located almost exclusively in the cytosol. Calculation of the mass action ratios of sucrose synthase, UDP-glucose pyrophosphorylase, phosphoglucosisomerase, and phosphoglucomutase indicate that these enzymes are close to equilibrium in developing potato tubers. However, due to the low plastidic pyrophosphate concentration, the reaction catalyzed by ADP-glucose pyrophosphorylase was estimated to be far removed from equilibrium.
Compartmentation is one of the distinguishing characteristics of plant metabolism (ap Rees, 1987). A true understanding of the nature and regulation of plant metabolic networks can only be achieved when the metabolic interactions between subcellular compartments have been charted and subjected to analysis through experimental procedures. Because of the profound difficulties associated with measuring enzymes, metabolites, and fluxes in specific subcellular compartments, our understanding of plant metabolism has lagged far behind that of animal and microbial systems.
Although methods have been developed for the assay of subcellular metabolite levels in leaf tissue (Stitt et al., 1989), and the interactions between plastidial and cytosolic metabolism during photosynthesis have been partially characterized (Stitt, 1997), little is known about the metabolic networks in heterotrophic cells. There are two main reasons for this. First, there is a lack of suitable methods for organelle isolation, which is a particularly difficult problem in heterotrophic cells because these often contain large starch grains that cause extra damage to the organelles during fractionation. Second, although leaf metabolism is highly conserved between different species (Heineke et al., 1997), heterotrophic tissues usually form differentiated organs with specific functions and therefore studies can be extrapolated between organs only with extreme caution.
Advances in plant molecular biology have allowed components of specific subcellular compartments to be rapidly cloned and characterized. The now-routine tools and procedures for the genetic manipulation of plants have also allowed the precise manipulation of the activity of proteins or enzymes associated with particular subcellular compartments.
However, the extent to which transgenic approaches have been able to deepen understanding of metabolism, particularly in heterotrophic tissues, have been severely limited by the ability to measure metabolism at the subcellular level.
The work presented here focuses on potato (Solanum tuberosum L. cv Desiree) tubers. The subcellular organization of tubers is poorly understood particularly in comparison with other heterotrophic tissues such as pea (Pisum sativum) roots, maize (Zea mays) roots, or cauliflower (Brassica oleracea var. botrytis) buds (Neuhaus and Emes, 2000). Import studies on isolated potato tuber amyloplasts have led to inconclusive results on the nature of the fluxes across the amyloplast membrane, probably due to the extreme experimental difficulty (Schott et al., 1995; Naeem et al., 1997; Wischmann et al., 1999). Further, due to the bulkiness of tuber tissue, NMR methods are also difficult to apply. Because of these restrictions, most of the information on subcellular metabolism in tubers has come indirectly from the use of transgenic plants (e.g. Müller-Röber et al., 1992; Stark et al., 1992; Sweetlove et al., 1996; Tjaden et al., 1998; Trethewey et al., 1999; Tauberger et al., 2000).
In this study, we addressed three major open questions related to subcellular metabolism in potato tuber cells: the location of hexoses and Suc (Trethewey et al., 1998, 1999), the availability of substrates for starch biosynthesis in the amyloplasts (Tjaden et al., 1998; Tauberger et al., 2000), and the distribution of pyrophosphate (PPi; Farré et al., 2000). A nonaqueous fractionation method, based upon the procedure that has been successfully applied to the study of subcellular leaf metabolites (Stitt et al., 1989; Heineke et al., 1997), was selected and adapted for the fractionation of tuber tissue. With this method, enzyme and metabolite stability during the fractionation procedure is achieved by maintaining a water-free and nonpolar environment. Metabolites in the different subcellular fractions were measured in part with a recently established gas chromatography-mass spectrometry (GC-MS) technique (Roessner et al., 2000).
To calculate subcellular metabolite concentrations, the volume of the specific compartment must be known. We have determined the volumes of subcellular compartments of growing potato tubers using electron microscopy techniques.
RESULTS
Separation of Tuber Material into Subcellular Compartments
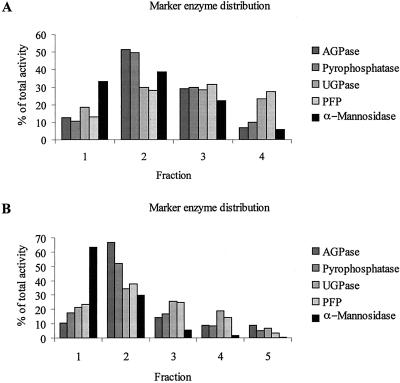
The most important requirements for a fractionation procedure to study subcellular metabolite distributions are the fast quenching and inactivation of any biological activity of the plant material and the avoidance of metabolite redistribution during the separation procedure. The nonaqueous fractionation method described here meets both of these criteria. The principle of this method is the separation of lyophilized tissue particles in a nonaqueous medium. The central assumption made is that the metabolites and proteins in a particular region of the cell aggregate together as the plant material is lyophilized. The size of the particles applied to the nonaqueous fractionation gradient ideally should be as small as possible to reduce the possibility of including material from different organelles. The use of a ball mill followed by 2 min of sonication led to an average particle size of 2 to 3 μm, as estimated by light microscopy (data not shown). ADP-Glc pyrophosphorylase (AGPase) (E.C. 2.2.7.27; Kim et al., 1989) and pyrophosphatase (E.C. 3.6.1.1; Weiner et al., 1987) were chosen as markers for the plastid, and α-mannosidase was selected for the vacuolar compartment (E.C. 3.2.1.24; Boller and Kende, 1979). PPi-dependent phosphofructokinase (PFP; E.C. 2.7.1.90; MacDonald and Preiss, 1986) and UDP-Glc pyrophosphorylase (UGPase; E.C. 2.7.7.9; Kleckowski, 1994, and references therein) were both used as cytosolic markers. Phosphoenolpyruvate (PEP)-carboxylase (E.C. 4.1.1.31) has often been used as a marker for the cytosol (Stitt et al., 1978); however, activity is low in tubers and its measurement is therefore highly error prone. Initial experiments showed that the distribution of PEP-carboxylase correlated exactly with the activities of PFP and UGPase (data not shown). Because the latter two enzymes are highly active in growing tubers and therefore can be easily measured, we decided to use them as routine markers.
Figure 1 shows the marker enzyme distributions in the fractionated material. The separation of compartments is comparable with many other published examples of nonaqueous fractionation (see Fig. 1, Gerhardt and Heldt, 1984; see Tables II and III, Weiner et al., 1987; see Fig. 1, Sharkey and Vanderweer, 1989; see Table III, Dancer et al., 1990; see Fig. 1, Riens et al., 1991). The separation of the organelle fractions is not complete, but is sufficent for calculating the metabolite distributions in the three main compartments (cytosol, plastid, and vacuole) using the deconvolution approach described by Riens et al. (1991). We chose to take four to five fractions instead of the six to seven taken by Riens et al. (1991) to have enough material in each fraction to be able to measure the marker enzyme distribution and metabolites as accurately as possible. We used the mean percentage distribution of pyrophosphatase and AGPase activities as plastidial marker and the mean distribution of UGPase and PFP as cytosolic marker to reduce the error due to the variability of single enzyme measurements in each fraction.
Figure 1.
Typical marker enzyme distribution of the fractionated material from developing tubers. Developing tuber samples were taken from 10-week-old plants grown in 2.5-L pots in the greenhouse. The tissue was fractionated using a nonaqueous fractionation procedure and the activities of marker enzymes were determined in the different fractions, four in A and five in B. Data represent the percentage of activity in each fraction.
Table II.
Subcellular distribution of metabolites in potato tubers
| Metabolite | % of Total Tissue Content
|
||
|---|---|---|---|
| Amyloplast | Cytosol | Vacuole | |
| Cluster A | 3.8 ± 1.9 | 1.3 ± 0.6 | 95.0 ± 2.5 |
| Cluster B | 16.5 ± 6.1 | 8.3 ± 3.7 | 75.3 ± 7.7 |
| Cluster C | 58.8 ± 9.3 | 39.3 ± 7.8 | 2.0 ± 1.7 |
| Cluster D | 46.0 ± 9.0 | 32.3 ± 11.7 | 21.7 ± 7.3 |
| Suc | 5.0 ± 3.3 | 17.7 ± 3.1 | 77.3 ± 0.7 |
| Glc | 8.7 ± 3.7 | 14.3 ± 5.9 | 77.0 ± 5.7 |
| Fru | 15.7 ± 6.7 | n.d. | 84.3 ± 6.7 |
| Pro | 11.3 ± 6.9 | 39.3 ± 6.2 | 49.3 ± 6.7 |
| Leu | 12.0 ± 5.7 | 35.7 ± 3.4 | 52.3 ± 3.0 |
| Iso-Leu | 12.7 ± 5.4 | 20.3 ± 8.6 | 67.0 ± 7.6 |
| Met | 12.3 ± 2.8 | 3.3 ± 2.7 | 84.3 ± 2.3 |
| Tyr | 18.3 ± 2.6 | 1.3 ± 1.1 | 80.3 ± 1.8 |
| Asn | 34.7 ± 6.0 | 1.7 ± 1.0 | 63.7 ± 5.5 |
| Gln | 19.5 ± 11.3 | 32.0 ± 18.5 | 48.5 ± 7.2 |
| Homo-Ser | 26.7 ± 8.0 | 5.3 ± 4.4 | 68.0 ± 3.7 |
| Trp | 37.0 ± 16.8 | n.d. | 63.0 ± 16.8 |
Developing tuber samples were taken from 10-week-old plants grown in 2-L pots in the greenhouse. The tissue was fractionated using a nonaqueous procedure. Metabolites in each fraction were measured in methanol extracts using GC-MS. Cluster analysis was performed on the percentage distribution in the fractions of the gradient. Cluster A, Quinate, Lys, isocitrate, fumarate, malate, Man, and citrate. Cluster B, Ala, Gly, Ser, Thr, Tyr, Phe, Val, 5-oxo-Pro, Orn, mannitol, inositol, shikimate, and succinate. Cluster C, Fru-6-P and Glu-6-P. Cluster D, Asp and Glu. The subcellular distributions were calculated by comparing the metabolite and marker enzyme distributions using a three-compartment calculation program. The results represent the means ± se of measurements on three different fractionations with different tuber samples. n.d., Not detected.
Table III.
Distribution of metabolites belonging to cluster A in the different fractions of one gradient
| Fraction | % in Fraction
|
Mean ± sd | ||||||
|---|---|---|---|---|---|---|---|---|
| Lys | Man | Citrate | Malate | Fumarate | Quinate | Isocitrate | ||
| 1 | 46.5 | 52.1 | 49.8 | 51.6 | 52.2 | 48.1 | 50.2 | 50.1 ± 2.0 |
| 2 | 38.3 | 36.6 | 42.3 | 36.5 | 34.1 | 39.1 | 35.8 | 37.5 ± 2.5 |
| 3 | 10.9 | 8.4 | 0.1 | 8.5 | 9.5 | 8.8 | 9.6 | 8.0 ± 3.3 |
| 4 | 4.3 | 3.0 | 7.9 | 3.4 | 4.2 | 4.0 | 4.4 | 4.5 ± 1.5 |
Developing tuber samples were taken from 10-week-old plants grown in 2-L pots in the greenhouse. The tissue was fractionated using a nonaqueous procedure. Metabolites in each fraction were measured in methanol extracts using GC-MS. The data represent the percentage of each metabolite in the different fractions of a gradient.
The marker enzyme distribution along the gradient is similar to that found in leaves (Gerhardt and Heldt, 1984). The vacuolar material is found in the more dense fractions and the cytosolic material is equally distributed along the gradient, but with a relative enrichment in the higher part of the gradient. Plastidial material was mainly found in the second fraction as a narrow band at a density of approximately 1.54 g cm−3.
When analyzing the distribution of citrate synthase (E.C. 4.1.3.7), a marker enzyme for the mitochondrial matrix (Stitt et al., 1989), an analogous distribution to the cytosolic markers in wild-type tuber was observed (data not shown). Therefore, for the following calculations, the “cytosol” represents a “cytosolic + mitochondrial” compartment. This is similar to the situation with leaf tissue, where no appropriate separation of the mitochondrial compartment has been achieved using a nonaqueous fractionation method (Gerhardt and Heldt, 1984).
Stability of Metabolites during the Fractionation Procedure
In the protocol upon which this work is based (Stitt et al., 1989), the majority of the fractionation procedure was carried out at 4°C. Because it is difficult to reliably meet this condition while keeping the material water free during the whole experimental procedure, we decided to test whether or not metabolites were stable during fractionation at room temperature, where water-free handling can be more precisely guaranteed. To this end, metabolite levels per milligram protein of frozen tuber discs were compared with levels found after fractionation at room temperature. Even notoriously unstable metabolites such as pyruvate showed no difference between the two procedures (data not shown).
Variability in the Determination of Metabolite Distributions
Three sets of plants were used for the analysis of different metabolites. Plants were grown under the same conditions in all three experiments and the tubers used were of the same developmental stage. The first set was used to measure phosphorylated intermediates and nucleotides (Table I). GC-MS measurements were performed with the second set (Table II) and PPi was determined in the third set of plants (Table I). This experimental setup was necessary because it was impossible to complete all measurements with the same set of plants, given the amount of material required for each analysis procedure.
Table I.
Subcellular distributions and concentrations of phosphorylated intermediates in potato tubers
| Metabolite | Total Tissue Content | % of Total Tissue Content (mm)
|
||
|---|---|---|---|---|
| Amyloplast | Cytosol | Vacuole | ||
| nmol gfresh wt−1 | ||||
| 3-P-Glycerate | 82 | 65.5 ± 4.4 (0.46) | 34.5 ± 4.4 (0.30) | n.d. |
| PEP | 20 | 72.0 ± 2.9 (0.122) | 27.5 ± 2.6 (0.057) | 0.8 ± 0.4 (<0.001) |
| Pyruvate | 5 | 90.5 ± 6.6 (0.038) | 6.8 ± 4.3 (0.004) | 2.8 ± 2.4 (<0.001) |
| Glc-6-P | 140 | 77.8 ± 5.2 (0.97) | 22.3 ± 5.2 (0.34) | n.d. |
| Fru-6-P | 46 | 65.0 ± 10.2 (0.26) | 35.0 ± 10.2 (0.17) | n.d. |
| Glc-1-P | 21 | 43.5 ± 14.8 (0.08) | 22.5 ± 11.4 (0.051) | 34.0 ± 10.7 (0.014) |
| UDP-Glc | 180 | 57.3 ± 3.5 (0.901) | 42.8 ± 3.5 (0.83) | n.d. |
| UTP | 49 | 19.8 ± 7.6 (0.084) | 76.8 ± 6.6 (0.40) | 3.5 ± 2.0 (0.003) |
| UDP | 9 | 40.0 ± 5.3 (0.031) | 60.0 ± 5.3 (0.058) | n.d. |
| ATP | 77 | 74.3 ± 2.8 (0.495) | 25.8 ± 2.8 (0.21) | n.d. |
| ADP | 29 | 94.3 ± 2.9 (0.24) | 5.8 ± 2.9 (0.018) | n.d. |
| PPi | 3 | 14.3 ± 12.0 (0.003) | 79.0 ± 10.1 (0.023) | 6.7 ± 5.4 (<0.001) |
Developing tuber samples were taken from 10-week-old plants grown in 2-L pots in the greenhouse. The tissue was fractionated using a nonaqueous procedure. Metabolites in each fraction were measured in trichoracetic acid (TCA) extracts by an enzyme-coupled test or by HPLC. The subcellular distributions were calculated by comparing the metabolite and marker enzyme distributions using a three-compartment calculation program. The results represent the means ± se of measurements on four different fractionations with different tuber samples. Concentrations were calculated using the estimation of subcellular volumes shown in Fig. 3. PPi was determined in a separate set of plants than the other metabolites and its total tissue content was estimated from Farré et al. (2000). n.d., Not detected.
Two ways of calculating subcellular metabolite distributions from nonaqueous fractionation data have been used: a two-compartment analysis as described by Gerhardt and Heldt (1984), and a three-compartment analysis; for example, the one described by Riens et al. (1991). The first method is based on correlation curves between marker enzymes and metabolite distribution in the different fractions, and makes the assumption that the metabolite studied is found exclusively in two compartments. The second assumes a distribution between three compartments. Because most of the metabolites we have analyzed are predicted to be distributed between three compartments, the second analysis was preferred, and the calculations of the subcellular distributions of metabolites were carried out as described by Riens et al. (1991). This calculation essentially follows a deconvolution approach. It is based on the assumption that the metabolites are confined to the plastidial, cytosolic, and vacuolar compartment as designated by the corresponding marker enzymes. The evaluation is done by a computer program testing all possible cases for the distribution of a certain metabolite between the three compartments at intervals of 1%; for example: (a) plastid 100%, cytosol 0%, and vacuole 0%; (b) plastid 99%, cytosol 1%, and vacuole 0%; and (c) plastid 99%, cytosol 0%, and vacuole 1%.
There are 5,151 possibilities for the distribution of a metabolite between the three compartments, and this procedure calculates which of the possibilities agrees most closely with the experimental results.
The data represent the mean values based upon three to four independent fractionations each with different tuber samples. As already observed for leaf tissue, this method gives highly reproducible results for metabolites almost exclusively located in one compartment (e.g. malic acid or hexoses that are predominantly located in the vacuole). A higher variation is found when metabolites are located in more than one organelle. The variability is greatest when the proportion found in a particular compartment is low (less than 20% of the total). Given this variability, we estimate that the limit of detection of a compound in a particular organelle is around 5% of the total amount in the tissue.
The Combination of GC-MS and Nonaqueous Fractionation Allows the Measurement of the Subcellular Distribution of a Large Number of Compounds
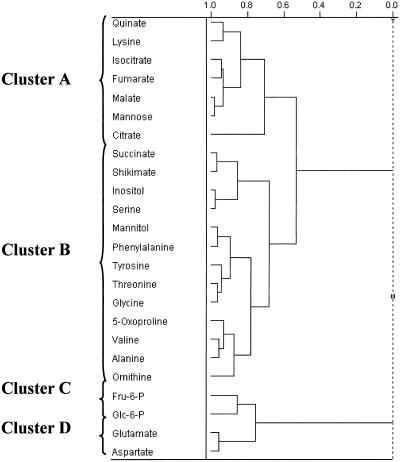
A GC-MS based method that allows the analysis of a large number of metabolites in parallel was developed recently in our laboratory (Roessner et al., 2000). This method was combined with the nonaqueous fractionation technique to study metabolite compartmentation. To calculate their subcellular distributions, detectable amounts of the compounds in each fraction are needed. Therefore, the total number of compounds that can be analyzed is reduced in comparison to the high number that can be identified in a tuber total extract. Due to the large amount of data generated, hierarchical cluster analysis was used to group metabolites that showed a similar fractionation pattern along the gradient. The mean fractionation pattern (percentage distribution in the fractions) of all the compounds belonging to one cluster was used to calculate the subcellular distribution of that cluster in a particular gradient. Two main clusters, A and B, of seven and 13 metabolites, respectively, were identified (Fig. 2, Table II). The sd from the mean between different compounds belonging to one cluster was lower than 10% in most fractions. Only fractions that contained less than 10% of the total content in the gradient had higher deviations (Table III). Apart from the two main clusters A and B, two further small clusters were repeatedly detected. Cluster C contained Glc-6-P and Fru-6- and Cluster D contained the amino acids Glu and Asp (Fig. 2, Table II). The remaining compounds did not have distributions similar enough to be grouped within separate clusters.
Figure 2.
Dendogram obtained following hierarchical cluster analysis of metabolite distributions along a density gradient. Only metabolites are shown that have a similar clustering behavior in at least three out of four gradients. The complete linkage method was used in the assignment of clusters.
Determination of Subcellular Volumes
To determine the volumes of the subcellular compartments, over 60 electron micrographs were taken from representative regions of the potato tissue. From these photographs, the relative volumes (percent of total) were calculated according to the principle of Delesse (1847). For the calculation of the mitochondrial volume, the relative volume of the mitochondria as percentage of the cytosol in the high magnification photographs was multiplied by the mean volume of the cytosol determined in the low magnification photographs.
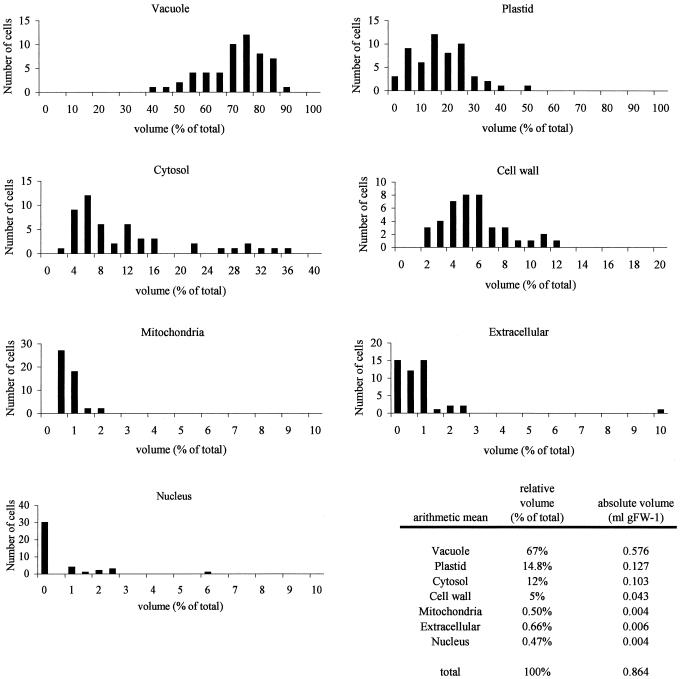
The mean volume (percent of total) of the different compartments of potato tuber tissue is shown in Figure 3. The histograms of volume distributions show the range of volumes obtained for each compartment. The relative volumes (percent of total) were converted to absolute volumes per unit of mass by taking into account that under our growth conditions the specific density of the tubers was 1.16 g fresh weight mL−1.
Figure 3.
Volume of subcellular compartments of growing potato tuber tissue. Potato tuber tissue was visualized through transmission electron microscopy. From these photographs, the relative volumes (percent of total) were calculated according to the principle of Delesse (1847). The histograms of volume distributions show the range of volumes obtained for each compartment. The relative volumes (percent of total) were converted to absolute volumes per unit of mass by taking into account that under our growth conditions the density of 1.16 g fresh weight mL−1.
Most compartments show a gaussian distribution with a clear single maximum. The values for the cytosol show a broad bimodal distribution as would be expected from two different types of tissue: a small number of meristematic cells with a higher percentage, and a high number of differentiated storage cells with a lower percentage of cytosol. It is unfortunate that with this method it is not possible to separate the meristematic from storage cells. In potato tuber, the major compartment was the vacuole, with 67% of the cell volume (0.58 mL g fresh weight−1), followed by the plastid (15%, 0.13 mL g fresh weight−1) and cytosol (12%, 0.1 mL g fresh weight−1), whereas other compartments had a much smaller volume (Fig. 3).
The estimation of the actual aqueous volume of amyloplasts is problematic because the starch granule fills most of the plastid volume in potato tubers. Little is known about the in vivo water content of starch granules, the characteristics of the granule water, and the diffusion capacity of metabolites through the starch granule (Tang et al., 2000). Only the extreme situations can be estimated; the actual in vivo situation will lie somewhere between these two estimates. If we assume that metabolites can diffuse freely through the starch granule, and that plastids occupy about 15% of the cell volume, the absolute volume would be 0.127 mL g fresh weight−1. If it is assumed that metabolites are restricted to the free diffusible water space, which does not seem to be higher than 10% of the total amyloplast volume (Kosegarten et al., 1995), the volume estimations in this case would be 0.013 mL g fresh weight−1.
Calculation of the Subcellular Concentrations
Tables I and II show the percentage distribution of metabolites between different subcellular compartments. These data, together with measurements of the total tissue contents and the calculation of the subcellular volumes described in the previous section, were used to estimate the metabolite concentrations in the amyloplast, cytosol, and vacuole (Tables I and IV). Because we did not achieve a separation between the mitochondria and the cytosol, the volume of the cytosolic compartment used for the calculations was 0.11 mL g fresh weight−1.
Table IV.
Estimation of subcellular metabolite concentrations in developing potato tubers
| Metabolite | Total Tissue Content | Amyloplast | Cytosol | Vacuole |
|---|---|---|---|---|
| nmol g fresh wt−1 | mm | |||
| Citratea | 18,860 | 5.6 | 2.1 | 31.1 |
| Fumaratea | 200 | 0.059 | 0.022 | 0.329 |
| Isocitratea | 170 | 0.050 | 0.019 | 0.280 |
| Malatea | 5,390 | 1.6 | 0.6 | 8.9 |
| Quinatea | 15,670 | 4.6 | 1.7 | 25.8 |
| Shikimatea | 370 | 0.48 | 0.27 | 0.48 |
| Succinatea | 970 | 1.26 | 1.27 | 1.27 |
| Frub | 900 | 1.11 | n.d. | 1.32 |
| Glca | 23,840 | 16 | 31 | 32 |
| Mana | 140 | 0.04 | 0.02 | 0.23 |
| Suca | 25,910 | 10 | 41 | 35 |
| Fru-6-Pb | 40 | 0.19 | 0.14 | 0.001 |
| Glc-6-Pa | 210 | 0.97 | 0.74 | 0.01 |
| Inositola | 60 | 0.078 | 0.044 | 0.078 |
| Mannitola | 60 | 0.078 | 0.044 | 0.078 |
| Alaa | 1,680 | 2.2 | 1.2 | 2.2 |
| Aspa | 1,270 | 4.6 | 3.7 | 0.5 |
| Glub | 1,800 | 6.5 | 5.2 | 0.7 |
| Glya | 230 | 0.30 | 0.17 | 0.30 |
| Iso-Leua | 940 | 0.94 | 1.71 | 1.09 |
| Leua | 430 | 0.41 | 1.37 | 0.39 |
| Lysa | 1,060 | 0.31 | 0.12 | 1.75 |
| Meta | 650 | 0.63 | 0.19 | 0.95 |
| Phea | 590 | 0.77 | 0.43 | 0.77 |
| Proa | 180 | 0.16 | 0.63 | 0.15 |
| Sera | 1,340 | 1.7 | 1.0 | 1.7 |
| Thrb | 1,100 | 1.4 | 0.8 | 1.4 |
| Tyrb | 900 | 1.17 | 0.66 | 1.17 |
| Vala | 2,510 | 3.3 | 1.8 | 3.3 |
Calculations were done using data from Table II and the estimated subcellular volumes shown in Figure 3. n.d., Not detected.
Calculations were done using the measurements of the total tissue content from Roessner et al. (2000).
Calculations were done using the measurements of the total tissue content from Trethewey et al. (1998).
The calculations of plastidial concentrations in Tables I and IV were done assuming unrestricted diffusion of metabolites in the starch granule. However, in “Results” and “Discussion,” the plastidial concentrations given in parentheses represent the values calculated assuming the case in which diffusion across the granule is restricted.
Phosphorylated Intermediates Are Partitioned between the Cytosol and the Plastid
More than one-half of the Glc-6-P and Fru-6-P was located within the plastid (Table I). These results were confirmed in the second experiment (Table II). From these values, the estimated concentrations were: 1 mm (10 mm) for Glc-6-P in the plastid, 0.34 mm for Glc-6-P in the cytosol, 0.26 mm (2.6 mm) for Fru-6-P in the plastid, and 0.17 mm for Fru-6-P in the cytosol (Table I). The distribution of Glc-1-P along the gradient was unexpected. In general, Glc-1-P was present at a higher percentage than the other hexose phosphates in fraction 1 and a lower percentage in fractions 3 and 4; therefore, up to 34% correlated with the vacuolar marker (Table I). This particularly high correlation with the vacuole was also found in other experiments (data not shown).
The glycolytic intermediates 3-P-glycerate, PEP, and pyruvate were located mainly in the plastid (Table I). The concentration of 3-P-glycerate was estimated to be 0.30 mm in the cytosol and 0.46 mm (4.6 mm) within the plastid (Table I). These concentrations are lower than those in potato leaves. Leidreiter et al. (1995) estimated a concentration of 2 mm for 3-P-glycerate in the stroma and 1.7 mm for 3-P-glycerate in the cytosol.
Adenine and Uridine Nucleotides Show Different Partitioning between the Cytosol and the Plastid
The majority of the uridine nucleotides were found in the cytosol (Table I), at similar levels to those found in leaves (Dancer et al., 1990). More than 70% of the UTP and 60% of the UDP were found in the cytosol. This leads to estimated cytosolic concentrations for UTP of 0.40 mm and for UDP of 0.058 mm, which are very similar to the concentrations calculated for leaf tissue (Dancer et al., 1990). However, we found an unexpected subcellular distribution for UDP-Glc, where only 42% was located in the cytosol, with an estimated concentration of 0.83 mm (Table I) and a significant amount found in the plastid, in disagreement with the distribution found in leaf tissue where the location was almost exclusively cytosolic (Dancer et al., 1990).
On the contrary, adenine nucleotides were found mainly associated with the plastidial marker (Table I). The estimated concentrations for ATP in the plastid and in the cytosol were 0.49 (4.9 mm) and 0.2 mm, respectively, and for ADP 0.24 mm (2.4 mm) and 0.018 mm (Table I), respectively. The ADP-Glc content in potato tubers is very low; in our experiment, we measured 3.6 ± 0.1 nmol g fresh weight−1, which is in agreement with previous studies (Geigenberger et al., 1994; Farré et al., 2000). We could only detect ADP-Glc in the fraction enriched for amyloplasts (data not shown) and therefore were not able to calculate the exact metabolite distribution. However, this indicates that in potato tubers, ADP-Glc is probably exclusively located in the plastid with a concentration of approximately 28 μm.
PPi Is Almost Exclusively Present in the Cytosol
As shown in Figure 1, alkaline pyrophosphatase activity appeared to correlate with AGPase activity and therefore was exclusively located in the plastid. On the other hand, PPi was mainly located in the cytosol, with an estimated concentration of about 23 μm (Table I). Although this concentration is lower than that estimated for leaves, the distributions of soluble inorganic pyrophosphatase activity and PPi between the cytosolic and plastidial compartments are identical to the distribution in green tissues (Weiner et al., 1987).
Glc, Fru, and Suc Accumulate in the Vacuole
Most of the sugars were found in the vacuole. About 77% of both Suc and Glc were located in this compartment (Table II), giving an estimated concentration of 35 mm for Suc and 32 mm for Glc (Table IV). Seventeen percent of Suc was found to be located in the cytosol (Table II). Other experiments that we have performed (E.M. Farré and L. Willmitzer, unpublished data) corroborate this finding. Therefore, we estimated that the cytosolic concentration of Suc is around 40 mm (Table IV). In the case of Glc, 8.7% was found in the plastid (Table II), with an estimated concentration of about 16 mm (160 mm; Table IV). In the case of Fru, 84% was found in the vacuole and 15% in the plastid, whereas the amount of Fru present in the cytosol was below the detection limit (Table II).
The Vacuole Contains a Large Pool of Different Organic Acids, Amino Acids, and Sugar Alcohols
Most amino acids, organic acids, and sugar alcohols were grouped in cluster A and cluster B and were mainly located in the vacuole (Table II). When the estimates of subcellular volumes were taken into consideration, all compounds in cluster A had higher vacuolar than cytosolic concentrations and compounds in cluster B had similar concentrations in the vacuole and the cytosol (Table IV). A high percentage of most amino acids were found associated with the vacuole. The exceptions were Asp and Glu (Cluster D), which were mainly located in the plastid and the cytosol. This distribution is similar to that found for leaves, where a high percentage of these two amino acids was found in the stroma (Leidreiter et al., 1995). A substantial amount of Asn was also associated with the plastidial markers. The amino acids Pro, Leu, and iso-Leu displayed a higher amount that appeared correlated with the cytosolic compartment.
Estimated Mass Action Ratios for Key Enzymes in Potato Tuber Carbohydrate Metabolism
From data on the subcellular distributions of sugars, hexose phosphates, nucleotides, and PPi shown in Tables I and IV, it is possible to calculate the mass action ratios of different cytosolic and plastidial reactions involved in carbon metabolism. These ratios are independent of the actual volume of a particular compartment. For the calculation of the mass action ratio of the AGPase, we made the assumption that ADP-Glc is exclusively located in the amyloplast because we could only detect it in those fractions enriched for plastidial markers. The mass action ratios of phosphoglucoisomerase, phosphoglucomutase, and UGPase are close to their Keq, whereas the mass action ratio of the AGPase reaction is 500 times lower than the Keq (Table V).
Table V.
Calculation of the molar mass action ratio of different reactions in the cytosol and plastid of potato tubers
| Reaction | Equation Used to Estimate the Molar Mass Action Ratio | In Vivo Molar Mass Action Ratio
|
Theoretical Keq | |
|---|---|---|---|---|
| Cytosol | Plastid | |||
| Phosphoglucoisomerase | 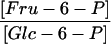 |
0.5 | 0.26 | 0.51a |
| Phosphoglucomutase | 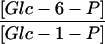 |
6.8 | 12.0 | 19a |
| Suc synthase | 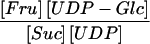 |
0.18 | – | 0.15–0.56a |
| UGPase | 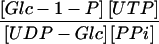 |
0.96 | – | 3.2a |
| AGPase | 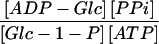 |
– | 0.002 | 1b |
The values of the mass action ratios were calculated using the metabolite concentrations in Tables I and IV. Cytosolic Fru content is assumed to be 5% of the total. ADP-Glc is assumed to be located exclusively in the amyloplast and with a tissue content of 3.6 nmol g fresh wt−1.
Keq for AGPase was calculated with AGo′ = 2.88 (Kruger, 1997).
The level of Fru in the cytosol is below the limit of detection of our method, which we have estimated to be 5% of the total content of a compound. In Table V, we calculated the mass action ratio of Suc synthase assuming 5% of Fru is located in the cytosol, which corresponds to a cytosolic concentration of 0.5 mm.
DISCUSSION
Despite the central importance of the compartmentation of metabolic pathways in plant cells, until now there have been few studies exploring this issue and most of these studies deal exclusively with photosynthetically active tissue. We have used a nonaqueous fractionation method to study subcellular metabolite distributions in potato tuber. To the best of our knowledge, this is the first report on metabolite distributions between the vacuolar, cytosolic, and plastidial compartments of a heterotrophic tissue. Although Liu and Shannon described a nonaqueous fractionation method for the isolation of maize endosperm starch granules and their associated metabolites, their method used glycerol and 3-chloro-1,2-propandiol, which they later showed to lead to the inactivation of enzyme activities, thus preventing a comparison with the distribution of marker enzymes (Liu and Shannon, 1981a; Shannon et al., 1998). We have chosen a method that uses nonpolar solvents (heptane and tetrachlorethylene) because the stability of both metabolites and enzymes in these media allows the measurement of marker enzyme activities and therefore the identification of the isolated fractions.
The Relative Subcellular Volumes in Growing Potato Tubers Are Similar to the Ones in Leaves
Cell and amyloplast sizes change during tuber development as shown in the work of Tauberger et al. (1999) and therefore we determined the subcellular volumes in growing tubers of the same developmental stage as the ones used for biochemical analysis. The subcellular volumes of source leaves from different species have been determined already (for review, see Heineke et al., 1997). In mesophyll cells, although the absolute volumes show considerable differences between the species, the relative compartmentation is rather similar (Heineke et al., 1997). In leaves, the percent of total cell volume taken up by various organelles ranges from 73% to 79% for vacuoles, 16% to 19% for chloroplasts, 3.8% to 7% for cytoplasm, and 0.5% to 1% mitochondria. These values are similar to the values determined for growing potato tubers (Fig. 3).
Hexose Phosphates and Glycolytic Intermediates Are Distributed Differently between the Cytosol and the Amyloplast
Most of the phosphorylated intermediates analyzed were distributed between the cytosol and the plastid and were absent from the vacuole. Liu and Shannon (1981b) also found many different phosphorylated intermediates associated with starch granules in maize endosperm.
The cloning of a Glc-6-P/phosphate translocator that is highly expressed in potato tubers (Kammerer et al., 1998) and the reduction of starch synthesis due to the specific inhibition of a plastidial phosphoglucomutase (Tauberger et al., 2000) has led to the conclusion that Glc-6-P is taken up by the plastids as substrate for starch synthesis in potato tubers. Both Glc-6-P and Fru-6-P were located in the amyloplast in significant amounts. The estimated cytosolic concentration of Glc-6-P is 0.34 to 0.8 mm (Tables I and IV), which is similar to the apparent Km of the plastidial Glc-6-P/phosphate translocator from pea roots (0.7 mm, Kammerer et al., 1998), and also to the concentration of 1 mm at which Hill and Smith (1991) observed saturation of the rate of starch synthesis from Glc-6-P in isolated amyloplasts from developing pea embryos. The phosphoglucoisomerase mass action ratio does not differ significantly between the cytosol and the plastid, although the cytosolic reaction seems to be slightly closer to the theoretical equilibrium constant (Keq; Table V). Therefore, it is apparent that the phosphoglucoisomerase reaction is close to equilibrium in both compartments in potato tubers. In leaves, however, the chloroplastic mass action ratio differs from the cytosolic one, the latter being closer to equilibrium (Gerhardt et al., 1987; Schleucher et al., 1999).
Glc-1-P shows an unexpected distribution: A significant percentage was found associated with the vacuolar marker. Because vacuoles have a high unspecific phosphatase activity (De, 2000), it is unlikely that Glc-1-P is actually located in this organelle. It is possible that the Glc-1-P is associated with a further compartment that cofractionates with the vacuole; for example, the Golgi or endoplasmatic reticulum system. The Glc-6-P/Glc-1-P ratio in the cytosol (6.8) was lower than the one in the plastid (12.0), although due to the high variability in the Glc-1-P measurements, these values are not significantly different. The in vivo molar mass action ratio of the phosphoglucomutase reaction therefore is close to the Keq (Table V).
The 3-P-glycerate distribution in tubers resembles the distribution found in leaves, in which a high percentage of this compound was associated with the chloroplast (Heineke et al., 1997). The plastidial 3-P-glycerate concentration ranged between 0.46 mm (assuming unrestricted diffusion) and 4.6 mm (if diffusion is restricted) and thus lies in the range of the A0.5 of AGPase from potato tubers, which has been determined to be 0.4 mm (Sowokinos and Preiss, 1982). However, due to the lack of information about the concentration of the AGPase inhibitor orthophosphate (Sowokinos and Preiss, 1982), it is difficult to draw any conclusions concerning the in vivo activity of this enzyme in potato tubers. The different pools of 3-P-glycerate, cytosolic and plastidial, may change independently as they have been shown to do in leaves (Gerhardt et al., 1987). This could explain why correlations between 3-P-glycerate and starch synthesis have only been shown in some cases (Hajirezaei et al., 1994; Geigenberger et al., 1997; Preiss, 1997; Farré et al., 2001), and not in others (Geigenberger et al., 1994; Geiger et al., 1998; Trethewey et al., 1998, 1999; Fernie et al., 2001).
The high percentage of PEP and pyruvate associated with the amyloplast leading to similar concentrations in the plastid and the cytosol is surprising. PEP is needed in the plastid for the shikimic acid pathway leading to aromatic amino acids and other secondary metabolites, and pyruvate is used for fatty acid biosynthesis. It is still unclear whether potato tuber amyloplasts have a complete sequence of glycolytic enzymes and are able to synthesize PEP and pyruvate from hexose phosphates. Although a PEP/phosphate translocator has recently been found in several photosynthetic and non-photosynthetic plastids (Fischer et al., 1997), its presence in potato tubers has not yet been shown.
Adenine and Uridine Nucleotides, with the Exception of UDP-Glc, Have a Similar Distribution to Those Found in Leaves
Estimated cytosolic ATP and ADP concentrations (0.21 and 0.018 mm, respectively) were similar to the concentrations found in Ricinus communis phloem sap, which is free from organelles and despite its specific transport function is often considered as cytosolic (Geigenberger et al., 1993), and in darkened spinach (Spinacia oleracea) leaf cytosol (Heineke et al., 1991), but were lower than concentrations found for leaves in the light (Heineke et al., 1991). The cytosolic ADP/ATP ratio (0.08) was lower than the plastidial ratio (0.49). This situation is analogous to that observed in photosynthetic tissue (wheat [Triticum aestivum] leaf protoplasts, Stitt et al., 1982; spinach leaves, Heineke et al., 1991). The amyloplast membrane in potato tubers contains an ATP/ADP transporter with a Km for both nucleotides of around 20 μm (Tjaden et al., 1998). This transporter probably catalyzes a counterexchange of ATP and/or ADP (Trentmann et al., 2000). The differences in the ratio of both nucleotides might favor a net import of ATP, which in turn could secure the supply of ATP for starch biosynthesis in potato amyloplasts.
We could only detect ADP-Glc in the fractions enriched for amyloplasts and therefore were not able to calculate the subcellular distribution of this compound. However, this finding agrees with the exclusive location of ADP-Glc in the plastid of potato tubers.
The similarity between the cytosolic ADP/ATP (0.08) and UDP/UTP (0.15) ratios supports the hypothesis formulated by Dancer et al. (1990) that the ATP and UTP energy systems are equilibrated via a cytosolic nucleotide-diphosphate kinase. Uridine nucleotides (UTP and UDP) are almost exclusively located in the cytosol in tubers, similar to the situation found in leaf tissue (Dancer et al., 1990). Low plastidial concentrations of UTP and UDP are, perhaps, a general characteristic. Previous studies have concluded that UDP-Glc is absent from chloroplasts (Gerhardt et al., 1987; Bligny et al., 1990; Dancer et al., 1990). Therefore, it is surprising that a significant amount of UDP-Glc was found to be associated with plastidial markers in potato tubers. Based on the current knowledge, it can be excluded that UDP-Glc is a major substrate for starch synthesis in potato tubers (Kossman and Lloyd, 2000, and references therein). UDP-Glc had been shown to act as substrate of amylogenin a protein that was thought to act as primer for starch synthesis. However, it seems that amylogenin is not located in the amyloplasts but rather in the Golgi apparatus and has been proposed to be involved in cell wall biosynthesis (Dhugga et al., 1997; Bocca et al., 1999). Because UDP-Glc is the substrate for cellulose synthesis, the high amount of UDP-Glc colocalized with the amyloplast might also be interpreted as indicating the presence of cell wall fractions comigrating with this compartment. UDP-Glc in the plastid might be involved in the biosynthesis of sulfolipids. The enzyme SQD1, which catalyzes the transfer of SO3− to UDP-Glc, is thought to be involved in the biosynthesis of sulfoquinovosyl headgroup in plant sulfolipids, and is localized in the plastids (Essigmann et al., 1998).
Although More Than 75% of the Suc Is Located in the Vacuole, the Concentration of Suc in the Cytosol and the Vacuole Is Similar
Most of the Suc, Glc, and Fru are located in the vacuole. However, due to the small volume of the cytosol relative to the vacuole, cytosolic concentrations (approximately 40 mm for Suc and 33 mm for Glc) are similar to those in the vacuole (approximately 35 mm for Suc and 32 mm for Glc; Table IV). Therefore, it appears that sugars do not accumulate against a concentration gradient in potato tuber vacuoles. The same distribution has been reported for leaves of different species (Heineke et al., 1997; De, 2000). This agrees with experiments showing facilitated diffusion of Suc across the tonoplast (for review, see Martinoia et al., 2000). Until now, only one putative tonoplast Suc transporter has been cloned, that from sugar beet (Beta vulgaris; Chiou and Bush, 1996).
Potato tubers are characterized by low invertase activities during the starch accumulation phase. It is still unclear which proportion of the Suc is cleaved by the acid or the alkaline invertases and where this cleavage occurs. Isla et al. (1998) showed that cleavage of Suc occurs in isolated vacuoles from potato tubers. However, the antisense repression of a soluble acid invertase in potato did not lead to a change in the Suc/hexose ratio in growing tubers, only in cold-stored tubers (Zrenner et al., 1996). Because the apoplast probably contains significant amounts of Suc (Trethewey et al., 1999; Fernie et al., 2000), it is also possible that cleavage occurs via acidic invertases in the extracellular space. The resulting hexoses may be taken into intracellular compartments via membrane or vesicular transport. We found a high vacuolar Glc to Fru ratio and a cytosolic concentration of Fru that was below the level of detection (less than 5% of the total amount). Because potato tubers are characterized by low hexokinase and high fructokinase activities (Renz and Stitt, 1993), the Fru produced either in the cytosol or vacuole must in any case be accessible to the cytosolic fructokinase.
We reproducibly found a low amount of Suc (10 mm [100 mm], Table IV) associated with the plastid. This is not in agreement with the current textbook position of Suc being absent from plastids. However, the possibility of Suc accumulating in the plastid has been previously described. Chloroplasts from cold-hardened cabbage (Brassica oleracea var. capitata) contained up to 20% of the cellular Suc (Santarius and Milde, 1977) and tobacco (Nicotiana tabacum) plants expressing a cytosolic invertase accumulate Suc in the chloroplast up to a concentration of 20 mm (Heineke et al., 1994), similar to the concentration that we estimate in tubers. Furthermore, Liu and Shannon (1981b) found Suc associated with maize endosperm starch granules. The production of fructans in plastids (Gerrits et al., 2001) can be seen as strong indirect evidence for the presence of Suc in this organelle. Further studies are needed to analyze if plastids have the capacity to import or synthesize Suc.
The AGPase Reaction Is Irreversible in Vivo, Whereas Suc Synthase and UGPase Reactions Are Near Equilibrium But Slightly Biased toward Suc Breakdown
We found that the distributions of PPi and soluble inorganic pyrophosphatase resemble the distributions found in photosynthetic tissues. Therefore, the plastidial PPi concentration is very low, 3 μm (30 μm; Table I). The calculation of the mass action ratio of the AGPase reaction shows that it differs significantly from the theoretical equilibrium constant of 1 (Table V). Therefore, this reaction is likely to be effectively irreversible in vivo, supporting a long standing view of the role of AGPase as a central regulatory enzyme in starch biosynthesis (Preiss, 1997). The presence of detectable quantities of PPi in the plastid, even given the large variability observed in its concentration, leaves open the possibility that PPi is rapidly recycled from the plastid to the cytosol to support Suc breakdown (Farré et al., 2000).
Suc synthase and UGPase represent the first two steps of Suc metabolism in potato tubers. When their mass action ratios are compared with the Keq (Table V), it is clear that both reactions are close to equilibrium, although slightly biased toward Suc and UDP-Glc breakdown, which is in close agreement with the data of Geigenberger and Stitt (1993).
We were not able to detect Fru in the cytosol in either of our experiments. Therefore, for the calculation of the mass action ratio of Suc synthase, we had to estimate the cytosolic concentration of Fru. We have assumed it to be 5% of the total Fru content, which is the estimated limit of detection of our method (see comments in “Results”). Even if this assumption would disagree with the real value by a factor of 5, our estimate of the mass action ratio for Suc synthase would still be close to its Keq. The calculated concentrations of Suc (40 mm), UDP (0.06 mm), and UDP-Glc (0.83 mm) are similar to the Kms of the Suc synthase reaction, which are 50 to 100, 0.1 to 0.7, and 1 to 2 mm, respectively (Avigad, 1982).
Vacuoles Accumulate a Large Range of Sugars, Sugar Alcohols, Organic Acids, and Amino Acids
The GC-MS measurements show that the vacuole accumulates a large variety of different compounds: sugars, sugar alcohols, organic acids, and amino acids.
Most of the organic acids measured had higher vacuolar than cytosolic concentrations (they were grouped in cluster A, Table II). High amounts of organic acids in vacuoles have been reported from several species (De, 2000; Martinoia et al., 2000). It seems that there is a relatively small active organic acid pool located in the cytosol, mitochondria and/or amyloplast, and a large pool in the vacuole. It is interesting that not only do organic acids that are TCA cycle intermediates accumulate in vacuoles, but also shikimic acid and quinic acid, which are involved in the shikimate pathway.
Like the organic acids, most amino acids accumulate in the vacuole. However, for most amino acids, the vacuolar concentrations were similar to or lower than the cytosolic concentrations. As found in potato leaves (Leidreiter et al., 1995) and in other species (De, 2000), the total amino acid concentration in the vacuole is lower than in the cytosol although the total amino acid content is higher. An active extrusion of amino acids from vacuoles by translocators has been proposed (Winter et al., 1993; Martinoia et al., 2000). Little is known about amino acid synthesis in tubers. It is still unclear to what extent de novo synthesis occurs as compared with amino acids imported from the phloem or derived from lytic breakdown of proteins inside the vacuole. The large pool of amino acids in the vacuole could have two functions: storage of nitrogen and homeostasis of amino acid metabolism (De, 2000).
CONCLUSION
The development and application of a method with which to study subcellular metabolite distributions in potato tubers is described. The determination of a large number of compounds by combining traditional enzymatic measurements with GC-MS measurements reveals that many metabolites (including amino acids, organic acids, and uridine nucleotides) have a distribution similar to those in leaf tissue. Vacuoles contain most of the sugars, sugar alcohols, and organic and amino acids. The substrates for starch biosynthesis, hexose phosphates, and ATP were found in significant amounts in the amyloplast. Analogous to the leaf situation, soluble inorganic pyrophosphatase activity was exclusively associated with the plastid and PPi was mainly located in the cytosol.
MATERIALS AND METHODS
Materials
Potato (Solanum tuberosum L. cv Desiree) was supplied by Saatzucht Lange AG (Bad Schwartau, Germany). Plants were grown from stem cuttings. The plants used for biochemical analysis were raised in the greenhouse in 2-L pots under a 16-h-light, 8-h-dark regime at 22°C with supplementary light to ensure a minimum of 250 μmol photons m−2 s−1; plants were 10 weeks old and completely green when the tubers were harvested. Tubers (20–40 g fresh weight) were still growing when harvested. This developmental stage of tubers (cv Desiree) is commonly used for the study of growing tuber metabolism and comparison of metabolite data between tubers harvested from plants between 8 and 12 weeks old is readily possible (Trethewey et al., 1999; Farré et al., 2000, 2001; Roessner et al., 2000; Tauberger et al., 2000). To sample tuber material, a cylinder (12-mm diameter) was cut perpendicular to the stolon-apex axis in the middle of the tuber (Merlo et al., 1993). For biochemical analysis, tuber slices 1 mm thick were cut from the cylinder and immediately frozen in liquid nitrogen and stored at −80°C until use. All enzymes were purchased from Boehringer Mannheim (Mannheim, Germany), with the exception of PFP from Propionibacterium freudenreichii shermanii, which was purchased from Sigma (Deisenhofen, Germany). All chemicals were obtained from either Sigma or Merck (Darmstadt, Germany).
Nonaqueous Fractionation of Tuber Tissue
The method described here was originally based upon the procedure of Stitt et al. (1989) for the analysis of leaf subcellular metabolism. The material (approximately 4 g fresh weight per gradient) was homogenized using a ball mill precooled with liquid nitrogen. The frozen powder was resuspended in liquid nitrogen, placed in a plastic beaker, and then dried at 4 Pa for 48 h in a lyophilizer, which had been precooled to −30°C. The temperature in the lyophilizer was left to rise to room temperature after the vacuum had been reached. The lyophilizer was ventilated with dry N2 gas and the plastic beakers were quickly closed, placed in boxes containing silica gel, and stored in plastic bags at −20°C. The dry tuber powder in the plastic beaker was resuspended in 20 mL of a tetrachlorethylene-heptane mixture (66:34 [v/v]; density = 1.3 g cm −3; the solvents were dried and stored over molecule sieve beads, all from Merck) and ultrasonicated for a total of 120 s, with 10-s pulses and 10-s breaks (Bandolin Sonoplus HD 200, MS 73/D, Bandolin, Berlin). To prevent overheating, the plastic beaker was placed on ice and closed with a foam seal. The suspension was then poured through a polyester monolen sieve with a pore size <30 μm, diluted 3-fold with heptane, and centrifuged for 10 min at 2,200g (CS 6KR, Beckmann, Munich). The clear supernatant was discarded and the sediment was resuspended in 3 mL of a tetrachlorethylene-heptane mixture (1.3 g cm−3). Two 200-μL aliquots were withdrawn (for determination of enzyme activity and metabolites in the unfractionated material), and the remaining material was transferred to a 30-mL Teflon centrifuge tube (Nalge Nunc Int., Hereford, UK). The gradient was underlayed using an 11-cm-long needle. A linear gradient (20 mL between 1.43 and 1.62 g cm−3) was made using a gradient former connected to a peristaltic pump (Econo Pump, Bio-Rad, Munich) followed by a 5-mL cushion of tetrachlorethylene (1.62 g cm−3). The gradients were centrifuged for 1 h at 10,000g at 7°C (swing-out rotor AS4.13, ultracentrifuge Centrikon T-124, Kendro, Berlin). The centrifuge tube contents were removed from the bottom in four or five fractions (1–2 mL for fraction 2 and 2–5 mL for other fractions) using the needle. Each of these fractions was divided into two unequal portions, consisting of one-third of the volume for assay of marker enzymes and two-thirds of the volume for assay of metabolites. The divided portions and the two aliquots taken from the material applied to the gradient were all diluted 3-fold with heptane and centrifuged for 10 min at 2,200g (Beckmann CS 6KR). After discarding the supernatant, the samples were dried in a desiccator containing a silica gel drying agent for 12 h, and then extracted for assay of enzymes or metabolites.
Enzyme Assays
Extracts were prepared as described by Geigenberger and Stitt (1993) with the exception that bovine serum albumin was not added to the extraction buffer. Enzymes were assayed according to the following references: ADP-Glc-pyrophosphorylase (AGPase; E.C. 2.2.7.27) activity was measured as described by Müller-Röber et al. (1992); inorganic alkaline pyrophosphatase (E.C.3.6.11) was measured using the assay described by Jelitto et al. (1992) except that the reaction buffer contained 20 mm MgCl2, and termination and detection of phosphate was carried out as described by Gross and ap Rees (1986); UGPase (E.C. 2.7.7.9) was measured as described by Zrenner et al. (1993); PFP (E.C. 2.7.1.90) was assayed as described by Burrell et al. (1994); α-mannosidase (E.C. 3.2.1.24) was determined as detailed by Stitt et al. (1989) with the exception that the reaction was stopped with 1 m NaCO3; and citrate synthase (E.C. 4.1.3.7) was measured as described by Stitt et al. (1989). Total protein was determined by the method of Bradford (1976).
Determination of Metabolic Intermediates
TCA extracts were prepared as described by Trethewey et al. (1998). Carbohydrates were measured as detailed by Trethewey et al. (1998) and hexose phosphates, 3-P-glycerate, PEP, and pyruvate were determined in the extracts photometrically as described by Stitt et al. (1989). PPi was measured according to Farré et al. (2000). Pseudoextracts (without tissue) were also prepared to confirm the absence of significant PPi contamination in all the solutions and vessels used in the procedure.
Nucleotides were measured in the same TCA extracts using an HPLC method (Fernie et al., 2001). The reliability of the TCA extraction and assay protocol has been confirmed previously (e.g. Trethewey et al., 1998; Veramendi et al., 1999; Farré et al., 2000).
GC-MS analysis was carried out with methanol extracts as described by Roessner et al. (2000).
Data Analysis
A three-compartment calculation program (Bestfit) that has been described in detail by Riens et al. (1991) was used to evaluate the subcellular metabolite distribution. The results in Tables I and II represent the means ± se of measurements on four (Table I) or three (Table II) different fractionations each from a different tuber sample. Hierarchical cluster analysis was performed with the software Pirouette 2.6 (Infometrix, Woodinville, WA). The complete linkage method was then used in the assignment of clusters. The hierarchical cluster analysis uses the Euclidean distance matrix.
Electron Microscopy
Tuber tissue was fixed in 2% (w/v) glutaraldehyde (in phosphate buffer, pH 7.4) for 1 h, washed five times in phosphate buffer, and then contrasted in 2% (w/v) osmium tetraoxide (OsO4) for 1 h and in 1:10 diluted osmium solution overnight. The samples were washed five times in double distilled water and dehydrated by subsequent incubation in higher concentrations of acetone (30%, 50%, 70%, 90%, and 100% [w/v]). The samples were then incubated in an acetone/Spurr mix (Spurr, 1969) (increasing concentrations of Spurr 33%, 66%, and 100% [w/v] for 1 h per concentration, and overnight at 100% [w/v]). The samples were then placed in Spurr and dried for a week at 60°C. Sample blocks were then trimmed with a razor and sectioned in an ultra-microtome (Leica Microsystems, Wetz-ler, Germany) with a glass knife. The slices (50–80 nm) were placed onto copper grids and dried. Grids were then stained by incubation in uranil-acetate solution for 6 min and 6 min in lead-citrate solution. The samples were visualized with a transmission electron microscope (Carl Zeiss, Göttingen, Germany).
Determination of Subcellular Volumes
Electron micrographs of thin sections from fixed material were used for the evaluation of subcelluar volumes according the principle of Delesse (1847): “the areal density of profiles on sections is an unbiased estimate of the volume density of structures” (Weibel and Bolender, 1973; Winter et al., 1993). Over 60 electron micrographs were taken from material from four independent fixation procedures and from eight different tubers. Low magnification pictures (×700) in which several cells could be visualized were preferentially used to determine the volumes of most organelles. High magnification pictures (>1,000×) were used to determine the volumes of mitochondria that were not always visible in the low magnification pictures.
The relative volumes (percent of total) were converted to absolute volumes per unit of mass by taking into account that under our growth conditions a tuber slice of 130 mg fresh weight contained 1 mg protein and had a volume of 113 μL (0.86 mL g fresh weight−1).
ACKNOWLEDGMENTS
We would like to thank Frank Huhn for careful supervision of greenhouse plants, Cornelia Wagner for the introduction to GC-MS, Doreen Brust for the help with the cluster analysis, Megan McKenzie for careful editing of the manuscript, Prof. Dieter Heineke for the Bestfit software and helpful discussion, Prof. Werner Herth for the introduction to electron microscopy, and Prof. Mark Stitt for critical comments on the manuscript. The work of P.G. and A.T. was supported by DFG grant Ge 878/1–1.
Footnotes
This work was supported by the Max-Planck-Gesellschaft (grant to E.M.F.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010280.
LITERATURE CITED
- ap Rees T. Compartmentation of plant metabolism. In: Davies DD, editor. The Biochemistry of Plants. Vol. 12. New York: Academic Press; 1987. pp. 87–115. [Google Scholar]
- Avigad G. Sucrose and other disaccharides. In: Loewus FA, Tanner W, editors. Encyclopedia of Plant Physiology. Berlin: Springer-Verlag; 1982. pp. 217–347. [Google Scholar]
- Bligny R, Gardestrom P, Roby C, Douce R. 31P NMR studies of spinach leaves and their chloroplasts. J Biol Chem. 1990;265:1319–132. [PubMed] [Google Scholar]
- Bocca SN, Kissen R, Rojas-Beltran JA, Noel F, Gebhardt C, Moreno S, du Jardin P, Tandecarz JS. Molecular cloning and characterization of the enzyme UDP-glucose: protein transglucosylase from potato. Plant Physiol Biochem. 1999;37:809–819. doi: 10.1016/s0981-9428(99)00117-5. [DOI] [PubMed] [Google Scholar]
- Boller T, Kende H. Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol. 1979;63:1123–1132. doi: 10.1104/pp.63.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein using the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burrell MM, Mooney PS, Blundy MDC, Wilson F, Green J, Blundy KS, ap Rees T. Genetic manipulation of 6-phosphofructokinase in potato tubers. Planta. 1994;194:95–101. [Google Scholar]
- Chiou T-J, Bush DR. Molecular cloning, immunochemical localization to the vacuole, and expression in transgenic yeast and tobacco of a putative sugar transporter from sugar beet. Plant Physiol. 1996;110:511–520. doi: 10.1104/pp.110.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancer J, Neuhaus HK, Stitt M. Subcellular compartmentation of uridine nucleotides and nucleoside-5′-diphosphate kinase in leaves. Plant Physiol. 1990;92:637–641. doi: 10.1104/pp.92.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De DN. Plant Cell Vacuoles: An Introduction. Collingwood, Australia: CSIRO Publishing; 2000. [Google Scholar]
- Delesse MA. Procédé mecanique pour determiner la composition des roches. C R Acad Sci (Paris) 1847;25:544. [Google Scholar]
- Dhugga KS, Tiwari SC, Ray PM. A reversibly glycosylated polypetide (RGP1) possibly involved in plant cell wall synthesis: purification, gene cloning, and trans-Golgi localization. Proc Natl Acad Sci USA. 1997;94:7679–7684. doi: 10.1073/pnas.94.14.7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essigmann B, Güler S, Narang RA, Linke D, Benning C. Phosphate availability affects the thylakoid lipid composition and the expression of SQD1, a gene required for sulfolipid biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1998;95:1950–1955. doi: 10.1073/pnas.95.4.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farré EM, Bachmann A, Willmitzer L, Trethewey RN. Acceleration of potato tuber sprouting by the expression of a bacterial pyrophosphatase. Nature Biotechnol. 2001;19:268–272. doi: 10.1038/85726. [DOI] [PubMed] [Google Scholar]
- Farré EM, Geigenberger P, Willmitzer L, Trethewey RN. A possible role for pyrophosphate in the coordination of cytosolic and plastidial carbon metabolism within the potato tuber. Plant Physiol. 2000;123:681–688. doi: 10.1104/pp.123.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie AR, Riesmeier JW, Martini A, Ramalingan S, Willmitzer L, Trethewey RN. Consequences of the expression of a bacterial glucokinase in potato tubers, both in combination with and independently of a yeast-derived invertase. Aust J Plant Physiol. 2000;27:827–833. [Google Scholar]
- Fernie AR, Roscher A, Ratcliffe RG, Kruger NJ. Fructose 2,6-bisphosphate activates pyrophosphate: fructose-6-phosphate 1-phosphotransferase and increase triose phosphate to hexose phosphate cycling in heterotrophic cells. Planta. 2001;212:250–263. doi: 10.1007/s004250000386. [DOI] [PubMed] [Google Scholar]
- Fischer K, Kammerer B, Gutensohn M, Arbinger B, Weber A, Häusler RE, Flügge UI. A new class of plastidic phosphate translocators: a putative link between primary and secondary metabolism by the phosphoenolpyruvate/phosphate antiporter. Plant Cell. 1997;9:453–462. doi: 10.1105/tpc.9.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenberger P, Langenberger S, Wilke I, Heineke D, Heldt HW, Stitt M. Sucrose is metabolised by sucrose synthase and glycolysis within the phloem complex of Ricinus communis L. seedlings. Planta. 1993;190:446–453. [Google Scholar]
- Geigenberger P, Merlo L, Reimholz R, Stitt M. When growing potato tubers are detached from their mother plant there is a rapid inhibition of starch synthesis, involving inhibition of ADP-glucose pyrophosphorylase. Planta. 1994;193:486–493. [Google Scholar]
- Geigenberger P, Reimholz R, Geiger M, Merlo L, Canale V, Stitt M. Regulation of sucrose and starch metabolism in potato tubers in response to short-term water deficit. Planta. 1997;201:502–518. [Google Scholar]
- Geigenberger P, Stitt M. Sucrose synthase catalyzes a readily reversible reaction in vivo in developing potato tubers and other plant tissues. Planta. 1993;190:440–450. doi: 10.1007/BF00194429. [DOI] [PubMed] [Google Scholar]
- Geiger M, Stitt M, Geigenberger P. Metabolism in potato tuber slices responds differently after addition of sucrose and glucose. Planta. 1998;206:245–252. [Google Scholar]
- Gerhardt R, Heldt HW. Measurement of subcellular metabolite levels in leaves by fractionation of freeze-stopped material in nonaqueous media. Plant Physiol. 1984;75:542–547. doi: 10.1104/pp.75.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt R, Stitt M, Heldt HW. Subcellular metabolite levels in spinach leaves. Plant Physiol. 1987;83:399–407. doi: 10.1104/pp.83.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrits N, Turk SCHJ, van Dun KPM, Hulleman SHD, Visser RGF, Weisbeek PJ, Smeekens SCM. Sucrose metabolism in plastids. Plant Physiol. 2001;125:926–934. doi: 10.1104/pp.125.2.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross P, ap Rees T. Alkaline inorganic pyrophosphatase and starch synthesis in amyloplasts. Planta. 1986;167:140–145. doi: 10.1007/BF00446381. [DOI] [PubMed] [Google Scholar]
- Hajirezaei M, Sonnewald U, Viola R, Carlisle S, Dennis D, Stitt M. Transgenic potato plants with strongly decreased expression of pyrophosphate:fructose-6-phosphate phosphotransferase show no visible phenotype and only minor changes in metabolic fluxes in their tubers. Planta. 1994;192:16–30. [Google Scholar]
- Heineke D, Lohaus G, Winter H. Compartmentation of C/N Metabolism. In: Foyer CH, Quick WP, editors. A Molecular Approach to Primary Metabolism in Higher Plants. London: Tailor & Francis Ltd; 1997. pp. 205–217. [Google Scholar]
- Heineke D, Riens B, Grosse H, Hoferichter P, Peter U, Flügge UI, Heldt HW. Redox transfer across the inner chloroplast envelope membrane. Plant Physiol. 1991;95:1131–1137. doi: 10.1104/pp.95.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heineke D, Willdenberger K, Sonnewald U, Willmitzer L, Heldt HW. Accumulation of hexoses in leaf vacuoles: studies with transgenic tobacco plants expressing yeast-derived invertase in the cytosol, vacuole or apoplasm. Planta. 1994;194:29–33. [Google Scholar]
- Hill LM, Smith AM. Evidence that glucose 6-phosphate is imported as the substrate for starch synthesis by the plastids of developing pea embryos. Planta. 1991;185:91–96. doi: 10.1007/BF00194519. [DOI] [PubMed] [Google Scholar]
- Isla MI, Vattuone MA, Sampietro AR. Hydrolysis of sucrose within isolated vacuoles from Solanum tuberosum L. tubers. Planta. 1998;205:601–605. doi: 10.1007/s004250050362. [DOI] [PubMed] [Google Scholar]
- Jelitto T, Sonnewald U, Willmitzer L, Hajirezeai M, Stitt M. Inorganic pyrophosphate content and metabolites in potato and tobacco plants expressing E.coli pyrophosphate in their cytosol. Planta. 1992;188:238–244. doi: 10.1007/BF00216819. [DOI] [PubMed] [Google Scholar]
- Kammerer B, Fischer K, Hilpert B, Schubert S, Gutesohn M, Weber A, Flügge UI. Molecular characterization of a carbon transporter in plastids from heterotrophic tissues: the glucose 6-phosphate/phosphate antiporter. Plant Cell. 1998;10:105–117. doi: 10.1105/tpc.10.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WT, Franceschi VR, Okita TW, Robinson NL, Morell M, Preiss J. Immunocytochemical localization of ADPglucose pyrophosphorylase in developing potato tuber cells. Plant Physiol. 1989;91:217–220. doi: 10.1104/pp.91.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckowski L. Glucose activation and metabolism through UDP-glucose pyrophosphorylase in plants. Phytochemistry. 1994;37:1507–1515. [Google Scholar]
- Kosegarten H, Zetsche K, Mengel K. Isolation of intact storage tissue amyloplasts from suspension-cultured potato cells (Solanum tuberosum) and determination of their intermembrane and stroma volumes. J Appl Bot. 1995;69:211–214. [Google Scholar]
- Kruger NJ. Carbohydrate synthesis and degradation. In: Dennis D, Turpin DH, Lefebvre DD, Layzell DB, editors. Plant Metabolism. Harlow: Addison Wesley Longman; 1997. pp. 83–104. [Google Scholar]
- Kossman J, Lloyd J. Understanding and influencing starch biochemistry. Crit Rev Plant Sci. 2000;19:171–226. [PubMed] [Google Scholar]
- Leidreiter K, Kruse A, Heineke D, Robinson DG, Heldt HW. Subcellular volumes and metabolic concentrations in potato (Solanum tuberosum cv. Désirée) leaves. Bot Acta. 1995;108:439–444. [Google Scholar]
- Liu TL, Shannon JC. A nonaqueous procedure for isolating starch granules with associated metabolites from maize (Zea mays L.) endosperm. Plant Physiol. 1981a;67:518–524. doi: 10.1104/pp.67.3.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T-TY, Shannon JC. Measurement of metabolites associated with nonaqueously isolated starch granules from immature Zea mays L. endosperm. Plant Physiol. 1981b;67:525–529. doi: 10.1104/pp.67.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald FD, Preiss J. The subcellular location and characteristics of pyrophosphate-fructose-6-phosphate phosphotransferase from suspension-cultured cells of soybean. Planta. 1986;167:240–245. doi: 10.1007/BF00391421. [DOI] [PubMed] [Google Scholar]
- Martinoia E, Massonneau A, Frangne N. Transport processes of solutes across the vacuolar membrane of higher plants. Plant Cell Physiol. 2000;41:1175–1186. doi: 10.1093/pcp/pcd059. [DOI] [PubMed] [Google Scholar]
- Merlo L, Geigenberger P, Hajirezaei M, Stitt M. Changes in carbohydrates metabolites and enzyme activities in potato tubers during development, and within a single tuber along a stolon-apex gradient. J Plant Physiol. 1993;142:392–402. [Google Scholar]
- Müller-Röber B, Sonnewald U, Willmitzer L. Inhibition of the ADP-pyrophosphorylase in transgenic portatoes leads to sugar-storing tubers and influences tuber formation and expression of tuber storage protein genes. EMBO J. 1992;11:1229–1238. doi: 10.1002/j.1460-2075.1992.tb05167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeem M, Tetlow IJ, Emes MJ. Starch synthesis in amyloplasts purified from developing potato tubers. Plant J. 1997;11:1095–1103. [Google Scholar]
- Neuhaus HE, Emes MJ. Nonphotosynthetic metabolism in plastids. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:111–140. doi: 10.1146/annurev.arplant.51.1.111. [DOI] [PubMed] [Google Scholar]
- Preiss J. Modulation of starch synthesis. In: Foyer CH, Quick WP, editors. A Molecular Approach to Primary Metabolism in Higher Plants. London: Tailor & Francis Ltd; 1997. pp. 81–104. [Google Scholar]
- Renz A, Stitt M. Substrate specificity and product inhibition of different forms of fructokinase and hexokinases in developing potato tubers. Planta. 1993;190:166–175. [Google Scholar]
- Riens B, Lohaus G, Heineke D, Heldt HW. Amino-acid and sucrose content determined in the cytosolic, chloroplastic, and vacuolar compartments and in the phloem sap of spinach leaves. Plant Physiol. 1991;97:227–233. doi: 10.1104/pp.97.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessner U, Wagner C, Kopka J, Trethewey RN, Willmitzer L. Simultaneous analysis of metabolites in potato tuber by gas chromatography-mass spectrometry. Plant J. 2000;23:131–142. doi: 10.1046/j.1365-313x.2000.00774.x. [DOI] [PubMed] [Google Scholar]
- Santarius KA, Milde H. Sugar compartmentation in frost-hardy and partially deharded cabbage leaf cells. Planta. 1977;136:163–166. doi: 10.1007/BF00396193. [DOI] [PubMed] [Google Scholar]
- Schleucher J, Vanderveer P, Markley JL, Sharkey TD. Intramolecular deuterium distributions reveal disequilibrium of chloroplast phosphoglucose isomerase. Plant Cell Environ. 1999;22:525–533. [Google Scholar]
- Schott K, Borchert S, Müller-Röber B, Heldt HW. Transport of inorganic phosphate and C3- and C4-sugar phosphates across the envelope membranes of potato tuber amyloplasts. Planta. 1995;196:647–652. [Google Scholar]
- Shannon J, Pien F, Cao H, Liu K. Brittle-1, and adenylate translocator facilitates transfer of extraplastidial synthesized ADP-glucose into amyloplasts of maize endosperms. Plant Physiol. 1998;117:1235–1252. doi: 10.1104/pp.117.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Vanderveer PJ. Stromal phosphate concentration is low during feedback limited photosynthesis. Plant Physiol. 1989;91:679–684. doi: 10.1104/pp.91.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowokinos JR, Preiss J. Pyrophosphorylases in Solanum tuberosum: III. Purification, physical, and catalytic properties of ADP-glucose pyrophosphorylase in potatoes. Plant Physiol. 1982;69:1459–1466. doi: 10.1104/pp.69.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr AR. A low viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969;26:31. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Stark DM, Timmerman KP, Barry GF, Preiss J, Kishore GM. Regulation of the amount of starch in plant tissues by ADP-glucose pyrophosphorylase. Science. 1992;258:287–291. doi: 10.1126/science.258.5080.287. [DOI] [PubMed] [Google Scholar]
- Stitt M, Bulpin PV, ap Rees T. Pathway of starch breakdown in photosynthetic tissue of Pisum sativum. Biochim Biophys Acta. 1978;544:200–214. doi: 10.1016/0304-4165(78)90223-4. [DOI] [PubMed] [Google Scholar]
- Stitt M, Lilley RM, Heldt HW. Adenine nucleotide levels in the cytosol, chloroplasts, and mitochondria of wheat leaf protoplasts. Plant Physiol. 1982;70:971–977. doi: 10.1104/pp.70.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M, Lilley RM, Gerhardt R, Heldt HW. Metabolite levels in specific cells and subcellular comparments of plant leaves. Methods Enzymol. 1989;174:518–550. [Google Scholar]
- Stitt M. The flux of carbon between the chloroplast and cytoplasm. In: Dennis DT, Layzell DB, Lefebvre DD, Turpin DH, editors. Plant Metabolism. Singapore: Longman Singapore Publishers (Pte) Ltd; 1997. pp. 382–400. [Google Scholar]
- Sweetlove LJ, Burrell MM, ap Rees T. Starch metabolism in tubers of transgenic potato (Solanum tuberosum) with increased ADPglucose pyrophosphorylase. Biochem J. 1996;320:493–498. doi: 10.1042/bj3200493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H-R, Godward J, Hills B. The distribution of water in native starch granules: a multinuclear NMR study. Carbohydr Polymers. 2000;43:375–387. [Google Scholar]
- Tauberger E, Fernie AR, Emmermann M, Renz A, Kossman J, Willmitzer L, Trethewey RN. Antisense inhibition of plastidial phosphoglucomutase provides compelling evidence that potato tuber amyloplasts import carbon from the cytosol in the form of glucose-6-phosphate. Plant J. 2000;23:43–53. doi: 10.1046/j.1365-313x.2000.00783.x. [DOI] [PubMed] [Google Scholar]
- Tauberger E, Hoffmann-Benning S, Fleisher-Notter H, Willmitzer L, Fisahn J. Impact of invertase overexpression on cell size, starch granule formation and cell wall properties during tuber development in potatoes with modified carbon allocation patterns. J Exper Bot. 1999;50:477–486. [Google Scholar]
- Tjaden J, Möhlman T, Kampfenkel K, Henrichs G, Neuhaus HE. Altered plastidic ATP/ADP-transporter activity influences potato (Solanum tuberosum L.) tuber morphology, yield and composition of tuber starch. Plant J. 1998;16:531–540. [Google Scholar]
- Trentmann O, Decker C, Winkler HH, Neuhaus HE. Charged amino-acid residues in transmembrane domains of the plastidic ATP/ADP transporter from Arabidopsis are important for transport efficiency, substrate specificity, and counter exchange properties. Eur J Biochem. 2000;267:4098–4105. doi: 10.1046/j.1432-1033.2000.01468.x. [DOI] [PubMed] [Google Scholar]
- Trethewey RN, Geigenberger P, Riedel K, Hajirezaei M-R, Sonnewald U, Stitt M, Riesmeier JW, Willmitzer L. Combined expression of glucokinase and invertase in potato tubers leads to a dramatic reduction in starch accumulation and a stimulation of glycolysis. Plant J. 1998;15:109–118. doi: 10.1046/j.1365-313x.1998.00190.x. [DOI] [PubMed] [Google Scholar]
- Trethewey RN, Geigenberger P, Sonnewald U, Hennig A, Müller-Röber B, Willmitzer L. Induction of the activity of glycolytic enzymes correlates with enhanced hydrolysis of sucrose in the cytosol of transgenic potato tubers. Plant Cell Environ. 1999;22:71–79. [Google Scholar]
- Veramendi J, Roessner U, Renz A, Willmitzer L, Trethewey RN. Antisense repression of hexokinase 1leads to an overaccumulation of starch in leaves of trans-genic potato plants but not to significant changes in tuber carbohydrate metabolism. Plant Physiol. 1999;121:1–11. doi: 10.1104/pp.121.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel ER, Bolender RP. Stereological techniques for electron microscopic morphometry. In: Hayat MA, editor. Principles and Techniques of Electron Microscopy, Biological Applications. New York: Van Nostrand Reinhold Company; 1973. pp. 237–296. [Google Scholar]
- Weiner H, Stitt M, Heldt HW. Subcellular compartmentation of pyrophosphate and alkaline pyrophosphatase in leaves. Biochim Biophys Acta. 1987;893:13–21. [Google Scholar]
- Winter H, Robinson DG, Heldt HW. Subcellularvolumes and metabolite concentrations in barley leaves. Planta. 1993;191:180–190. [Google Scholar]
- Wischmann B, Nielsen TH, Moller BL. In vitro biosynthesis of phosphorylated starch in intact potato amyloplasts. Plant Physiol. 1999;119:455–462. doi: 10.1104/pp.119.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrenner R, Schüler K, Sonnewald U. Soluble acid invertase determines the hexose-to-sucrose ratio in cold-stored potato tubers. Planta. 1996;198:246–252. doi: 10.1007/BF00206250. [DOI] [PubMed] [Google Scholar]
- Zrenner R, Willmitzer L, Sonnewald U. Analysis of the expression of potato uridinediphosphate-glucose pyrophosphorylase and its inhibition by antisense RNA. Planta. 1993;190:247–252. doi: 10.1007/BF00196618. [DOI] [PubMed] [Google Scholar]