Abstract
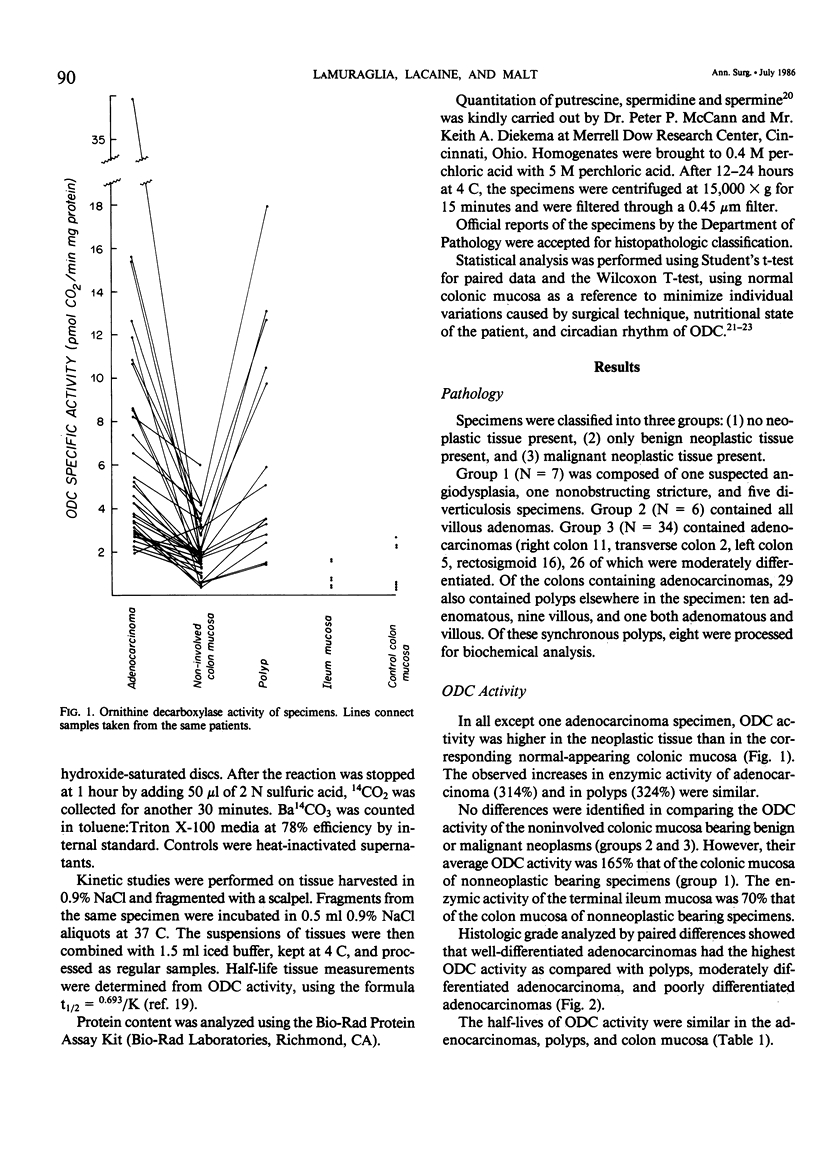
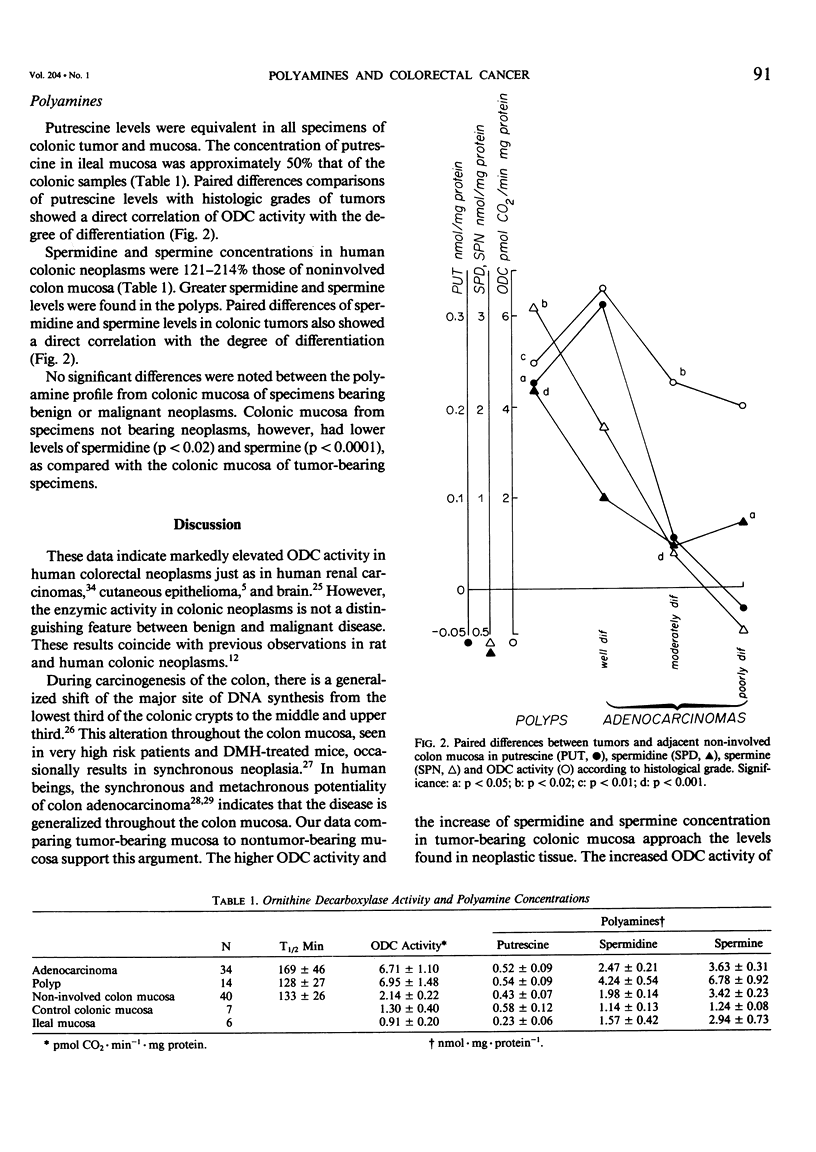
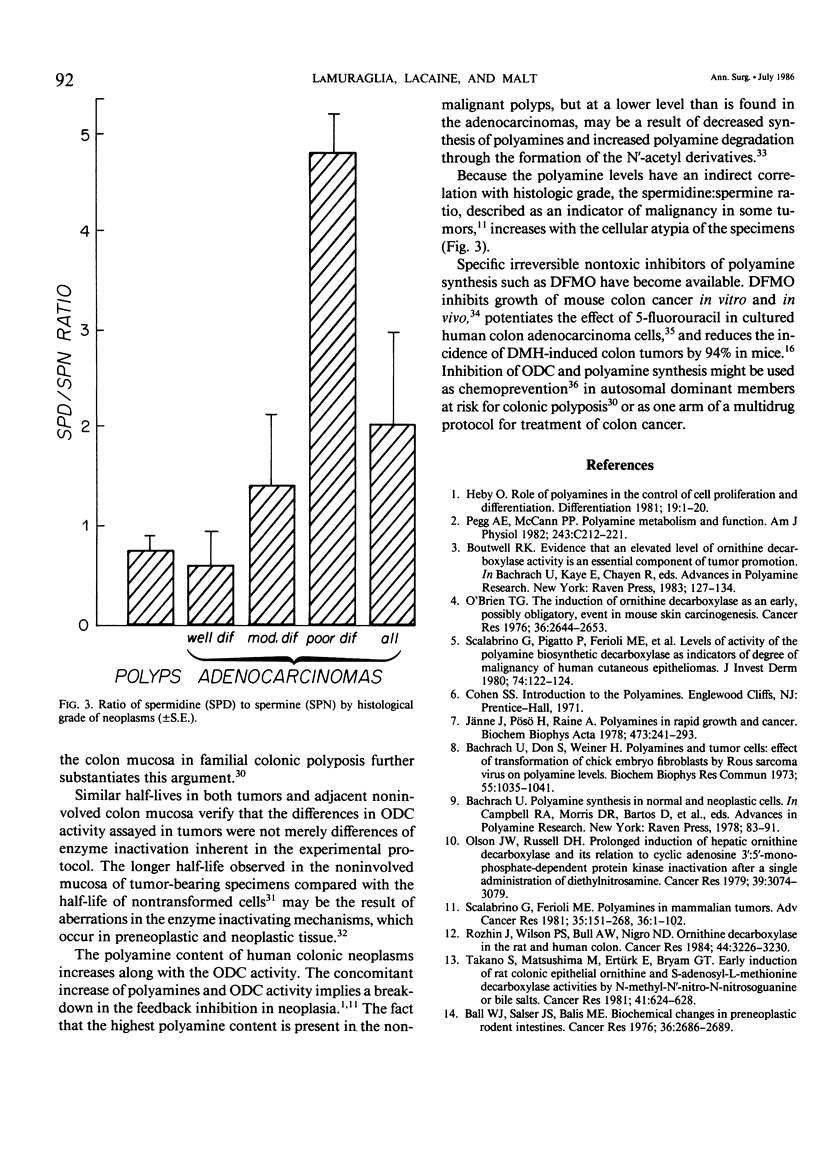
Polyamines are required for cell proliferation, and ornithine decarboxylase (ODC) is the first and probably rate-limiting enzyme in their synthesis. Tissue containing colonic or rectal adenocarcinomas (N = 34) or polyps (N = 6) and noninvolved paired colonic mucosa were obtained from fresh surgical specimens. ODC activity was elevated (mean: 320%) in both the cancer and polyps. In noninvolved colonic mucosa of tumor-bearing specimens, ODC activity was 165% that of colonic mucosa of non-neoplastic disease. Concentrations of polyamines in neoplasms were 121-214% increased, as compared with normal mucosa; those of spermidine and spermine varied inversely with the histological grade of the tumor. High levels of ODC activity and of polyamines were features of neoplasia, but not of malignancy alone. These characteristics of colonic neoplasia suggest its susceptibility to control by inhibition of ODC.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachrach U., Don S., Wiener H. Polyamines and tumor cells: effect of transformation of chick embryo fibroblasts by Rous sarcoma virus on polyamine levels. Biochem Biophys Res Commun. 1973 Dec 10;55(3):1035–1041. doi: 10.1016/0006-291x(73)91246-1. [DOI] [PubMed] [Google Scholar]
- Ball W. J., Jr, Salser J. S., Balis M. E. Biochemical changes in preneoplastic rodent intestines. Cancer Res. 1976 Jul;36(7 Pt 2):2686–2689. [PubMed] [Google Scholar]
- Fujimoto M., Kanaya A., Nakabou Y., Hagihira H. Circadian rhythm in the ornithine decarboxylase activity of rat small intestine. J Biochem. 1978 Jan;83(1):237–242. doi: 10.1093/oxfordjournals.jbchem.a131896. [DOI] [PubMed] [Google Scholar]
- Gravela E., Zuretti M. F., Papino F., Sartorio L. Relative in vitro stability of ornithine decarboxylase from liver preneoplastic nodules and hepatomas. Cancer Res. 1983 May;43(5):2298–2300. [PubMed] [Google Scholar]
- Harik S. I., Sutton C. H. Putrescine as a biochemical marker of malignant brain tumors. Cancer Res. 1979 Dec;39(12):5010–5015. [PubMed] [Google Scholar]
- Hayashi S., Aramaki Y., Noguchi T. Diurnal change in ornithine decarboxylase activity of rat liver. Biochem Biophys Res Commun. 1972 Jan 31;46(2):795–800. doi: 10.1016/s0006-291x(72)80211-0. [DOI] [PubMed] [Google Scholar]
- Heby O. Role of polyamines in the control of cell proliferation and differentiation. Differentiation. 1981;19(1):1–20. doi: 10.1111/j.1432-0436.1981.tb01123.x. [DOI] [PubMed] [Google Scholar]
- Jänne J., Pösö H., Raina A. Polyamines in rapid growth and cancer. Biochim Biophys Acta. 1978 Apr 6;473(3-4):241–293. doi: 10.1016/0304-419x(78)90015-x. [DOI] [PubMed] [Google Scholar]
- KIRSNER J. B., RIDER J. A., MOELLER H. C., PALMER W. L., GOLD S. S. Polyps of the colon and rectum. Statistical analysis of a long term follow-up study. Gastroenterology. 1960 Aug;39:178–182. [PubMed] [Google Scholar]
- Kingsnorth A. N., King W. W., Diekema K. A., McCann P. P., Ross J. S., Malt R. A. Inhibition of ornithine decarboxylase with 2-difluoromethylornithine: reduced incidence of dimethylhydrazine-induced colon tumors in mice. Cancer Res. 1983 Jun;43(6):2545–2549. [PubMed] [Google Scholar]
- Kingsnorth A. N., Russell W. E., McCann P. P., Diekema K. A., Malt R. A. Effects of alpha-difluoromethylornithine and 5-fluorouracil on the proliferation of a human colon adenocarcinoma cell line. Cancer Res. 1983 Sep;43(9):4035–4038. [PubMed] [Google Scholar]
- Lapointe D. S., Cohen R. J. A rapid and efficient microassay of ornithine decarboxylase. Anal Biochem. 1980 Dec;109(2):291–294. doi: 10.1016/0003-2697(80)90651-x. [DOI] [PubMed] [Google Scholar]
- Luk G. D., Baylin S. B. Ornithine decarboxylase as a biologic marker in familial colonic polyposis. N Engl J Med. 1984 Jul 12;311(2):80–83. doi: 10.1056/NEJM198407123110202. [DOI] [PubMed] [Google Scholar]
- Malt R. A., Kingsnorth A. N., Lamuraglia G. M., Lacaine F., Ross J. S. Chemoprevention and chemotherapy by inhibition of ornithine decarboxylase activity and polyamine synthesis: colonic, pancreatic, mammary, and renal carcinomas. Adv Enzyme Regul. 1985;24:93–102. doi: 10.1016/0065-2571(85)90071-8. [DOI] [PubMed] [Google Scholar]
- Marton L. J., Lee P. L. More sensitive automated detection of polyamines in physiological fluids and tissue extracts with omicron-phthalaldehyde. Clin Chem. 1975 Nov;21(12):1721–1724. [PubMed] [Google Scholar]
- Matsuda M., Osafune M., Kotake T., Sonoda T., Sobue K., Nakajima T. Concentrations of polyamines in renal cell carcinoma. Clin Chim Acta. 1978 Jul 1;87(1):93–99. doi: 10.1016/0009-8981(78)90062-1. [DOI] [PubMed] [Google Scholar]
- O'Brien T. G. The induction of ornithine decarboxylase as an early, possibly obligatory, event in mouse skin carcinogenesis. Cancer Res. 1976 Jul;36(7 Pt 2):2644–2653. [PubMed] [Google Scholar]
- Olson J. W., Russell D. H. Prolonged induction of hepatic ornithine decarboxylase and its relation to cyclic adenosine 3':5'-monophosphate-dependent protein kinase activation after a single administration of diethylnitrosamine. Cancer Res. 1979 Aug;39(8):3074–3079. [PubMed] [Google Scholar]
- Pegg A. E., McCann P. P. Polyamine metabolism and function. Am J Physiol. 1982 Nov;243(5):C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- Rozhin J., Wilson P. S., Bull A. W., Nigro N. D. Ornithine decarboxylase activity in the rat and human colon. Cancer Res. 1984 Aug;44(8):3226–3230. [PubMed] [Google Scholar]
- Scalabrino G., Pigatto P., Ferioli M. E., Modena D., Puerari M., Carú A. Levels of activity of the polyamine biosynthetic decarboxylases as indicators of degree of malignancy of human cutaneous epitheliomas. J Invest Dermatol. 1980 Mar;74(3):122–124. doi: 10.1111/1523-1747.ep12535012. [DOI] [PubMed] [Google Scholar]
- Schottenfeld D., Berg J. W., Vitsky B. Incidence of multiple primary cancers. II. Index cancers arising in the stomach and lower digestive system. J Natl Cancer Inst. 1969 Jul;43(1):77–86. [PubMed] [Google Scholar]
- Stanley B. A., Kazarinoff M. N. Induction of ornithine decarboxylase in colon and liver by starvation and refeeding: a comparison of effects on total and holoenzyme. Biochem Biophys Res Commun. 1982 Mar 30;105(2):773–777. doi: 10.1016/0006-291x(82)91501-7. [DOI] [PubMed] [Google Scholar]
- Takano S., Matsushima M., Ertürk E., Bryan G. T. Early induction of rat colonic epithelial ornithine and S-adenosyl-L-methionine decarboxylase activities by N-methyl-N'-nitro-N-nitrosoguanidine or bile salts. Cancer Res. 1981 Feb;41(2):624–628. [PubMed] [Google Scholar]
- Takenoshita S., Matsuzaki S., Nakano G., Kimura H., Hoshi H., Shoda H., Nakamura T. Selective elevation of the N1-acetylspermidine level in human colorectal adenocarcinomas. Cancer Res. 1984 Feb;44(2):845–847. [PubMed] [Google Scholar]
- Weir J. A. Colorectal cancer: metachronous and other associated neoplasms. Dis Colon Rectum. 1975 Jan-Feb;18(1):4–5. doi: 10.1007/BF02587229. [DOI] [PubMed] [Google Scholar]


